Abstract
Indole-3-carbinol (I3C), a constituent of commonly consumed Brassica vegetables, has been shown to have anticancer effects in a variety of preclinical models of lung cancer. However, it has shown only limited efficacy in clinical trials, likely due to its poor oral bioavailability. Intranasal administration of I3C has the potential to enhance the pulmonary accumulation of the drug, thereby improving its availability at the target site of action. In this study, we developed a liposomal formulation of I3C and evaluated its lung delivery and chemopreventive potential in tobacco smoke carcinogen [4-(methylnitro-samino)-1-(3-pyridyl)-1-butanone (NNK)]-treated mice. Intranasal administration of I3C liposomes led to a ~100-fold higher lung exposure of I3C than the oral route of administration. Further, intranasal delivery of liposomal I3C led to a significant reduction (37%; p<0.05) in the levels of the DNA adduct formation induced by NNK treatment. Liposomal I3C also significantly increased (by 10-fold) the expression of CYP1A1, a cytochrome P450 enzyme known to increase the detoxification of chemical carcinogens by enhancing their metabolism. Overall, our findings demonstrate that intranasal administration of liposomal I3C has the potential to significantly improve the efficacy of I3C for lung cancer chemoprevention.
Keywords: liposomes, indole-3-carbinol, 4-(methylnitro-samino)-1-(3-pyridyl)-1-butanone, cytochrome P450 1A1, DNA adduct, O6-Methylguanine DNA adduct
1. Introduction
Lung cancer is the leading cause of cancer-related deaths in the world (Siegal et al., 2012). Despite significant advances in the diagnosis and treatment of lung cancer, there have been only limited improvements in disease-related morbidity and mortality. Hence, there is considerable interest in developing strategies that can prevent or decrease the occurrence of lung cancer.
Chemoprevention involves the use of dietary or pharmaceutical products to suppress early stages of tumorigenesis. It has been found to be effective in populations at high risk for prostate, colon and breast cancers (Kinsinger et al., 2002; Thompson et al., 2003; Meyskens et al., 2008). Indole-3-carbinol (I3C), a breakdown product of indole glucosinolates found in commonly consumed Brassica vegetables, has shown promising activity in preclinical models of lung cancer (Morse et al., 1990). I3C prevents tumorigenesis by targeting multiple proteins required for the growth and survival of cancer cells, including Akt, NF-κB, caspases, cyclin-dependent kinases, estrogen receptors and endoplasmic reticulum stress-related proteins (Cover et al., 1998; Howells et al., 2002). I3C has also been shown to upregulate genes involved in the detoxification of carcinogens (Bradfield and Bjeldanes, 1984; Manson et al., 1997), thereby blocking carcinogenesis at a very early stage. However, instability of I3C in the gastrointestinal tract and its poor oral bioavailability are barriers to its clinical translation (Reed et al., 2006). Therefore, strategies that can improve bioavailability of I3C would potentially enhance its chemopreventive efficacy.
Intranasal administration of I3C has the potential to enhance the delivery of the drug to the lungs, the target site of action (Chien, 1989). Such a local delivery approach can also reduce the overall dose needed and potentially minimize off-target exposure of I3C. However, the use of this strategy is limited by the low dosing volume available to this route (~50 μL in mice) and the poor aqueous solubility of I3C (7 mg/ml). Liposomal delivery systems are widely used for the systemic administration of insoluble drugs (Torchilin, 2005) and can be a viable option for intranasal drug delivery (Caplen et al., 1995).
In this study, we developed a liposomal formulation of I3C and investigated whether intranasal administration of this novel formulation improves its availability in the lungs. We also determined whether increased pulmonary bioavailability of I3C with the liposomal formulation translates into enhanced biological activity by measuring the expression of cytochrome P450 enzymes and suppression of DNA adducts in a carcinogen-induced mouse model of lung cancer.
2. Materials and methods
2.1. Materials
I3C, cholesterol, 4-methoxy-indole, and carbitol were purchased from Sigma (St Louis, MO, USA). DIM was obtained from LKT Labs (St. Paul, MN, USA). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was acquired from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). O6-Methyl-Guanine and [CD3]O6-Methyl-Guanine were obtained from Midwest Research Institute (Kansas City, MO, USA) and Toronto Research Chemicals (Toronto Ontario, Canada), respectively. Cremophor EL and Captex 355 were purchased from Abitech (Janesville, WI, USA). All other solvents were acquired from Sigma (St Louis, MO, USA) or Fisher Scientific (Fairlawn, NJ, USA). The tobacco smoke carcinogen 4-(methylnitro-samino)-1-(3-pyridyl)-1-butanone (NNK) was synthesized as described previously (Hecht et al., 1983). D2-DIM was prepared as described elsewhere (Jackson et al., 1987).
2.2. Synthesis and physicochemical characterization of I3C and DIM liposomes
Liposomes were prepared by lipid film hydration technique (Ko et al., 2009). 7.5 mg DPPC, 2.5 mg cholesterol and 5 mg I3C were dissolved in 115 μL of chloroform-methanol (6:1). Subsequently, the solution of drugs and lipids was transferred to a round bottom flask and the solvents were evaporated using a rotovap at 100 RPM for 1 hour at room temperature. HEPES buffer (10 mM, pH 7.4) was added to the dried lipid film, and the round bottom flask was placed in a bath sonicator for 15 minutes. The resultant dispersion was sonicated using probe sonicator on an ice bath for 2 minutes at an output of 15 W (Sonicator XL, Misonix, Melville, NY, USA). Blank liposomes were synthesized by the same method without the addition of drug. Particle size and zeta potential of the liposomes were measured by dynamic light scattering using Delsa™ Nano C (Beckman Coulter Inc., Fullerton CA, USA).
To determine drug loading, a freshly prepared liposome suspension was centrifuged at 14,000 RPM for 15 minutes. The supernatant was discarded and the pellet was extracted with 500 μL of acetonitrile. The concentration of I3C and DIM in the extract was analyzed by using gradient HPLC. A 4.6 × 250 mm, 5 μm, C18 column was used as the stationary phase (Beckman Instruments, Fullerton, CA, USA). The mobile phase consisted of acetonitrile and distilled water at a flow rate of 0.8 mL/min. For the first 5 min, the mobile phase composition was held constant at 20% acetonitrile. The composition was changed to 60% acetonitrile linearly over the next minute, and held constant for 5 min. At 11 minutes, the composition was changed to 90% acetonitrile linearly over 1 minute and was held constant at 90% acetonitrile up to 20 minutes. At 20 min, the composition was changed back to 20% acetonitrile. Fifty μL of the sample was injected using an autoinjector (Model 508, Beckmann Instruments). The compounds were analyzed using a UV detector at 249 nm (System Gold 168 detector). The retention times for I3C and DIM were 9.5 min and 15.2 min, respectively.
2.3. Synthesis of oral formulations of I3C
Microemulsion form of I3C was prepared as described previously (Sane et al., 2013). Briefly, an oil phase consisting of Cremophor EL, Carbitol, and Captex 355 (6:3:1) was prepared. I3C was dissolved in the oil phase at a concentration of 50 mg/mL and the oil phase was diluted with saline before dosing. A suspension formulation of I3C was prepared by dispersing I3C in cotton seed oil immediately before dosing.
2.4. Assessment of pulmonary bioavailability of I3C in mice
The pulmonary bioavailability of I3C was studied in 5–6 weeks-old female A/J mice (The Jackson Laboratory, Bar Harbor, ME, USA). Mice were treated with an oral suspension (250 mg/kg), an oral microemulsion (250 mg/kg) or an intranasal liposomal formulation of I3C (2.5 mg/kg). For intranasal instillation, mice were sedated using isofluorane, and 50 μL of the liposomal dispersion was administered. Control mice received blank liposomes. Mice were sacrificed at predetermined time points using an overdose of carbon dioxide (3 mice/group at each time point). Lungs were harvested and immediately stored at −80 °C.
For extraction of I3C and its main metabolite DIM, lung tissues were homogenized in 1 mL of ice-cold phosphate buffered saline (0.15 mM, pH 7.4) in the presence of 4-methoxy-indole (200 pmole) and D2-DIM (100 pmole), which were used as internal standards for I3C and DIM, respectively. Samples were equilibrated on ice for 30 min. Four volumes of acetonitrile were added to each sample and vortex-mixed for 2 min. The samples were centrifuged and the supernatant was transferred to a fresh tube. The solvent was evaporated and the residue was re-suspended in 100 μL of 5% acetonitrile in 15 mM ammonium acetate and centrifuged again. The supernatant was used for HPLC-MS analysis.
HPLC-MS analysis was performed on a TSQ Quantum Discover Max instrument (Thermo Fisher Scientific, Waltham, MA, USA), in the positive ion-mode with nitrogen gas as the nebulizing and drying gas. Samples (8 μL) were injected from an autosampler using an Agilent 1100 capillary LC system (Agilent Technologies, Santa Clara, CA) equipped with a 5μm, 0.5 x 150 mm Zorbax SB-18 column (Agilent Technologies, Santa Clara, CA, USA). The flow rate was 16 μL/min for 1min and then 10 μL/min with a gradient from 10% methanol in 15 mM ammonium acetate to 50% methanol in 30 min and to 95% methanol in 5 min and held for additional 10 min. The mass transitions monitored were m/z 130.0 to 103.0 and 130.0 to 77 for I3C, m/z 148.08 to 118.0 and 148.08 to 103 for 4-methoxyindole (internal standard for I3C), m/z 247.12 to 130.07 for DIM and m/z 249.12 to 132.07 for D2-DIM (internal standard for DIM). Sample concentrations were determined as described previously (Fujioka et al., 2014).
The area under the curve (AUC) of I3C and DIM in the lungs was determined using the trapezoidal rule.
2.5. Analyses of NNK-induced DNA adducts and expression of cytochrome P450 enzymes in lung tissues of mice
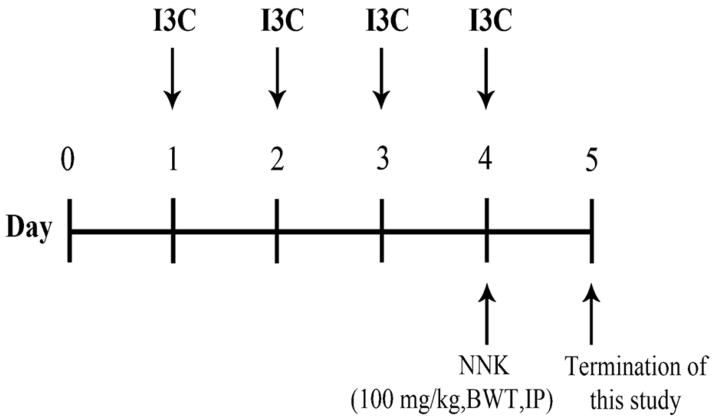
Female A/J mice (5–6 weeks old) were treated with an oral suspension of I3C (250 mg/kg), oral liposomal I3C (2.5 mg/kg), or intranasal liposomal I3C (2.5 mg/kg) once every day for 4 days. On day 4, the mice received an intraperitoneal injection of NNK (100 mg/kg). Mice were sacrificed 24 hours later, and lungs were excised. The study design is shown in Figure 1.
Figure 1.
Experimental design for the treatment of mice with I3C and NNK. Mice received different forms of I3C orally or intranasally every day for four days. On the fourth day, mice were treated intraperitoneally with NNK (100 mg/kg). The mice were sacrificed 24 hours later and lung tissues were collected for the analyses of NNK-induced DNA adducts and expression of CYP 1A1 and CYP1A2.
For DNA adduct analyses, DNA samples were extracted from lung tissues, hydrolyzed and purified as described previously (Upadhyaha et al., 2009). A stable-isotope liquid chromatography–nanoelectrospray high-resolution tandem mass spectrometry–parallel reaction monitoring (LC–NSI-HRMS/MS–PRM) method was used to analyze the DNA samples as described elsewhere (Lao et al., 2006; Balbo et al., 2013). Deuterated analogues of the analytes were used as internal standards for quantitation.
Real-time PCR was performed to measure the levels of CYP1A1 and CYP1A2 expression in the lungs. Total RNA was extracted from lung tissues using Trizol reagent (Life Technologies, Grand Island, NY, USA). Following treatment with DNase I (Qiagen, Valencia, CA, USA), the quality and quantity of the RNA were assessed using NanoDrop1000 spectrophotometer (NanoDrop Technologies Inc, Wilmington, DE, USA) at 260/280 nm. Subsequently, the cDNA was used as the template for real-time PCR quantification of CYP1A1 and CYP1A2. The forward and reverse mRNA primers for CYP1A1 were 5′-TGTGAACCAGTGGCAGGTTA-3′, and 5′-GGCCAGGAAGAGAAAGACCT-3′, respectively. The forward and reverse mRNA primers for CYP1A2 were 5′-AGGGACACCTCACTGAATGG-3′, and 5′-GTCGATGGCCGAGTTGTTAT-3′, respectively. The forward and reverse primers for β-actin (control for real-time PCR), were 5′-TGTTACCAACTGGGACGACA-3 and 5′-GGGGTGTTGAAGGTCTCAAA-3′, respectively. Real-time PCR was performed on an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA) with SYBR green fluorescent label. Differences in mRNA levels of the genes in the different groups of mice were evaluated by comparing Ct values of the target mRNAs. Quantification of mRNA expression levels were normalized relative to the median expression level of β-actin. ΔCt and ΔΔCt values were calculated according to standard protocols and ΔΔCt values were converted to approximate fold differences in gene expression by assuming 100% primer efficiency and using the equation 2−(ΔΔCt).
2.6. Statistical analyses
Differences in the pulmonary accumulation of I3C, DNA adduct levels and CYP1A1 and CYP1A2 expression in the lung were analyzed for statistical significance by ANOVA using Graphpad Prism 5 software (Graphpad, La Jolla, CA, USA). Data are reported as mean ± standard deviation of triplicate determinations. *, P< 0.05; **, P < 0.01; ***, P < 0.001.
3. RESULTS
3.1. Physicochemical characterization of I3C and DIM liposomes
The liposomal formulation of I3C was sub-micron in size and had a negative zeta potential (Table 1). There was rapid degradation of I3C into several oligomeric products during the synthesis of liposomes. This led to a low encapsulation efficiency of I3C in the liposomes. Overall, about 15% of I3C was converted to DIM (the major active metabolite of I3C) during the liposome fabrication.
Table 1.
Physicochemical characterization of I3C liposomesa
| Physicochemical character | I3C liposomes |
|---|---|
| Particle size (nm) | 664.4 ± 212.3 |
| Polydispersity index | 0.277 ± 0.078 |
| Zeta potential (mV) | −6.7 ± 1.06 |
| Encapsulation efficiency (%) | 7.9 ± 4.0 |
Results are expressed as mean ± SD, n=3
3.2. Pulmonary bioavailability of liposomal I3C
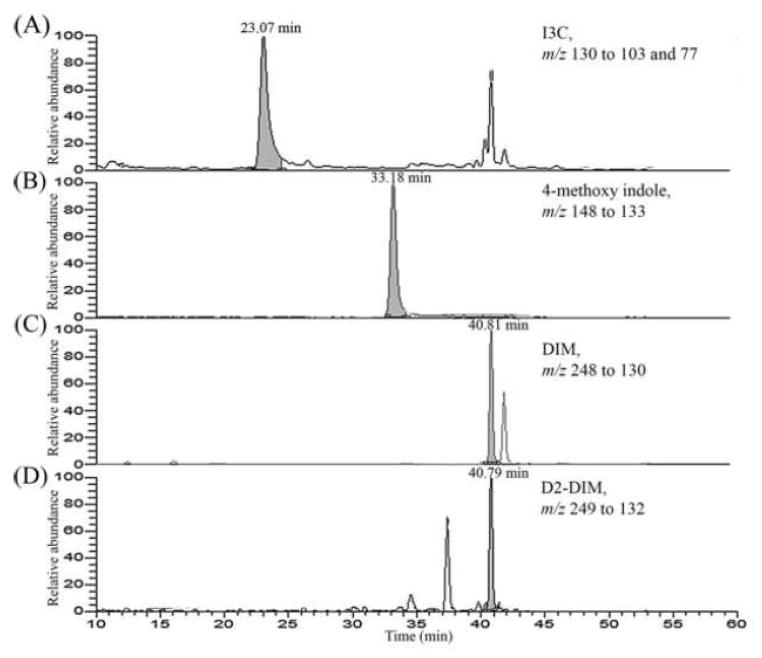
Figure 2 shows representative LC-ESI-MS/MS-SRM chromatograms of I3C and DIM obtained upon analysis of homogenates of lungs from mice treated intranasally with liposomal I3C and sacrificed after 30 minutes. Using this methodology, I3C and DIM could be measured in tissues with a sensitivity of 1 pg/ml.
Figure 2.
Representative LC-ESI-MS/MS-SRM chromatogram obtained upon analysis of lung homogenates of mice treated intranasally with liposomal I3C and sacrificed at 30 minutes. LC-ESI-MS/MS-SRM analysis of the samples was performed as described in the Materials and Methods section.
The lung concentrations of I3C and DIM upon oral and intranasal administration are shown in Figure 3. A very small fraction of the orally administered I3C was found in the lungs. When administered as a suspension formulation, only 0.0004% of the administered dose was found in the lungs at 30 minutes post dose. The concentration of I3C was 2-fold higher when it was administered in a microemulsion formulation. However, at 4 h and 24 h, the levels of I3C rapidly declined and were comparable for both the suspension and microemulsion formulations. On the other hand, when administered intranasally in liposomal formulation, ~0.04% of the dose was found in the lungs at 30 minutes. The decline in I3C concentration over 24 h was not as rapid as that with the oral formulations (Fig. 3A). The concentration of DIM in the lungs was also significantly higher upon intranasal dosing (Fig. 3B).
Figure 3.
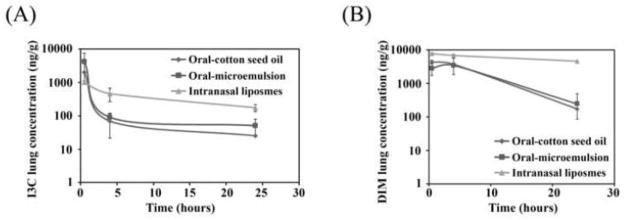
Lung concentration of I3C (A) and DIM (B) following various routes of administration. I3C was administered orally (250 mg/kg) as a suspension or a microemulsion, or by the intranasal route (2.5 mg/kg). Data as mean ± S.D. (n=3).*P<0.05, **P<0.01, ***P<0.001
The AUCs of I3C and DIM in the lungs are listed in Table 2. Administration of I3C in the form of an oral suspension showed the lowest AUC. When administered as an oral microemulsion, the lung AUC increased two-fold, suggesting that poor solubility of I3C may interfere with its absorption and thereby reducing its bioavailability. However, administration of a 100-fold lower dose of liposomal I3C intranasally led to a comparable lung AUC as that of the oral microemulsion formulation.
Table 2.
Area under the curve (AUC) for I3C and DIM in the lungsa
| Route of administration and formulation | I3C (ng x h / g) | DIM (ng x h / g) |
|---|---|---|
| Oral suspension | 5088.3 | 54537.5 |
| Oral microemulsion | 10119.1 | 48962.4 |
| Intranasal liposomes | 9472.7 | 144676.9 |
AUC was calculated using Equation 1.
3.3. Inhibition of NNK-induced DNA adducts by liposomal I3C in the lung tissues of mice
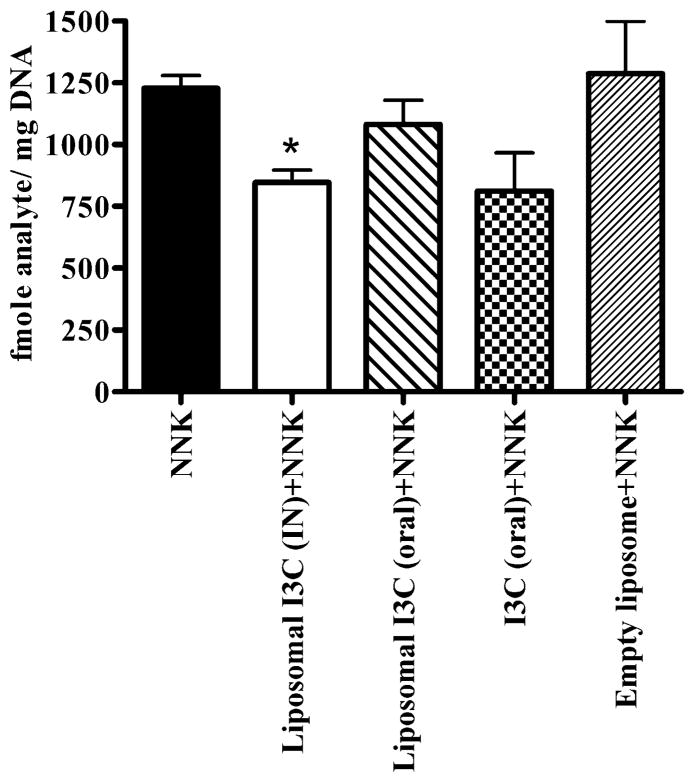
We studied the efficacy of I3C in suppressing the formation of O6-Methylguanine (O6-Methyl-G) DNA adduct, a critical DNA adduct in NNK-induced mouse lung tumorigenesis (Peterson et al., 1991).
Figure 4 shows the effect of I3C on the levels of O6-methyl-G. Intranasal administration of I3C liposomes (2.5 mg/kg) led to a ~31% reduction in DNA adducts as compared to the untreated group (P<0.05). Oral administration of a relatively high dose of I3C (250 mg/kg) decreased the level of O6-methyl-G, although the effect was not statistically significant (Fig. 4). However, oral liposomal I3C (2.5 mg/kg), (Fig. 4) did not modulate the level of O6-Methyl-G in the lungs.
Figure 4.
Effect of I3C on the formation of O6-Methyl-Guanine adduct in the lung tissues of NNK-treated mice. I3C was administered intranasally (IN) or orally (oral) for four consecutive days. On the fourth day mice were treated with the tobacco smoke carcinogen NNK and sacrificed 24 hours later. Lungs were harvested and used for the analysis of O6-Methyl-Guanine DNA adduct. Data as mean ± S.D.; n=3; *P<0.05.
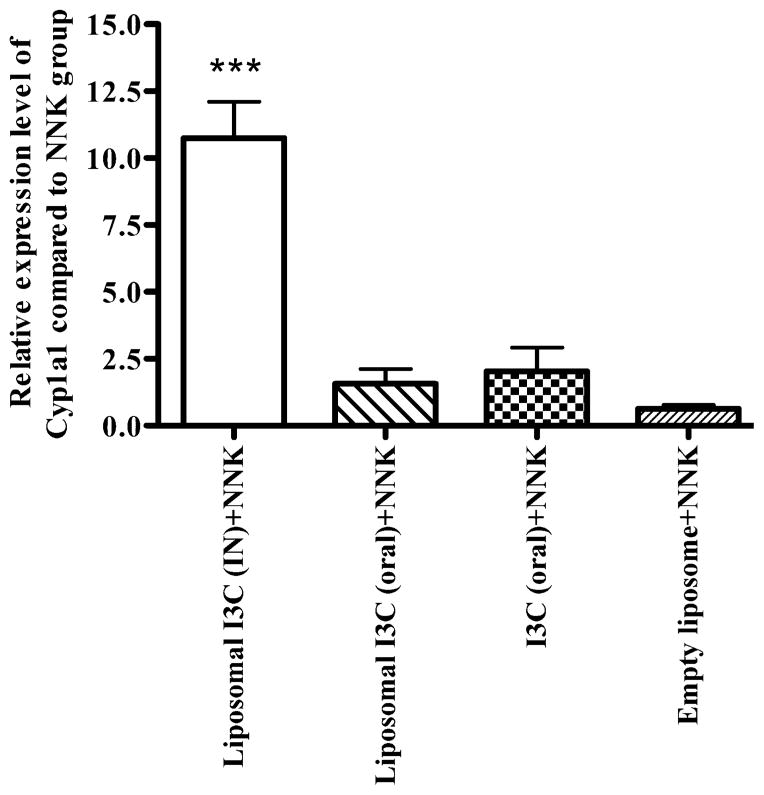
3.4. Effect of liposomal I3C on the expression of CYP1A1 and CYP1A2 in lungs
I3C has been shown to reduce the formation of carcinogen-induced DNA adducts through CYP1A1 and CYP1A2-mediated detoxification of the carcinogens (He et al., 2000). We sought to determine modulation of the expression of these enzymes by I3C. Intranasal administration of liposomal I3C led to a 10-fold increase in the lung expression of CYP1A1 as compared to untreated mice (Fig. 5). None of the other treatments resulted in a significant change in the expression of CYP1A1. The expression level of CYP1A2 was not significantly modulated by any of the treatments (data not shown).
Figure 5.
qRT-PCR analysis of cytochrome P450 1A1 in the lung tissues of mice treated with liposomal or native I3C. Mice were treated with I3C as described in the experimental design and lung tissues were used for the analysis of mRNA levels of CYP1A1. Data as mean ± S.D.; n=3; ***P<0.001.
4. DISCUSSION
I3C is a promising chemopreventive agent that has been shown to inhibit early stages of tumorigenesis. However, the clinical potential of this compound has not been fully realized yet because of its poor oral bioavailability. In this study, we show that intranasal administration of liposomal I3C improves its delivery to the lungs. The enhanced lung bioavailability of I3C led to a decrease in the formation of O6-Methyl-G in the lung tissues of NNK-treated mice. Additionally, I3C treatment led to a 10-fold upregulation of a detoxification enzyme, CYP1A1.
The poor oral bioavailability of I3C has been reported in several pre-clinical and clinical studies. In CD-1 mice treated orally with I3C (250 mg/kg), the drug was detected in the plasma only during the first 45 minutes post dose, while DIM was detected, albeit at a very low level, up to 24 h after administration (Anderton et al., 2004). In a phase I clinical study, an oral dose of 1200 mg resulted in no detectable parent drug in the plasma while plasma concentrations of DIM reached a maximum of ~600 ng/mL (Reed et al., 2006). The low bioavailability of I3C in both studies was attributed to rapid degradation of the drug in the acidic pH of the stomach (Anderton et al., 2004; Reed et al., 2006). Consistent with these reports, we found that oral administration of I3C led to low levels of the parent drug in the lungs. Intranasal administration of a 100-fold lower dose of I3C led to equivalent concentrations of the drug in the lungs. Moreover, the decline in lung concentrations of I3C and DIM were much slower than that for the oral formulations. The increased availability can be attributed to the direct introduction of the drug into the respiratory tract, which circumvents gastrointestinal degradation of the compound and first pass metabolism.
The tobacco-specific nitrosamine NNK is a potent animal carcinogen and classified by the International Agency for Research on Cancer, as a known human carcinogen (IARC, 2007). Once activated, it forms several forms of DNA adducts, including O6-Methyl-G, which is considered as one of the most critical DNA adducts in NNK-induced mouse lung tumorigenesis (Peterson et al., 1991). Earlier studies have shown that oral administration of I3C inhibited NNK-induced O6-Methyl-G in a dose and time-dependent manner (Morse et al., 1990). In our study, intranasal administration of I3C inhibited the formation of O6-Methyl-G significantly, whereas oral administration of a higher dose of I3C caused some reduction, albeit the effects did not reach statistical significance. The effect of I3C on the formation of O6-Methyl-G was weaker than that observed in previous studies, where oral administration of I3C (180 mg/kg) led to ~50% reduction of DNA adduct formation (Morse et al., 1990). This could be attributed to the differences in the time delay between administration of NNK and sacrifice of the mice. In contrast to previous studies where mice were sacrificed 2–6 hours post NNK administration, in our study mice were sacrificed 24 hours post NNK dosing. This time delay could lead to a reduction in the pulmonary levels of I3C resulting in loss of activity and/or allow for the repair of the DNA adducts through DNA repair pathway.
Our studies also showed intranasal liposomal I3C upregulated the expression of CYP1A1. Consistent with our findings, I3C has previously been reported to preferentially induce CYP1A1 over CYP1A2 (Hecht, 1998); CYP1A1 plays an important role in the metabolism of NNK in mouse lungs (Xu et al., 1996). Hence, one potential mechanism for I3C-mediated reduction in O6-Methyl-G levels could be increased metabolism of NNK by CYP1A1. Indeed, it has been shown that induction of CYP1A1 by I3C can lead to an increase in the metabolism of the cooked food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and reduced levels DNA adducts in the colon of rats (He et al., 2000).
Although I3C consistently showed cancer preventive effects when administered before or concurrently with the carcinogen, a number of reports have found that I3C promoted the development of cancer when administered chronically after the carcinogen, in particular in the liver (Kim et al., 1994; Stoner et al., 2002). The underlying mechanism for the tumor promoting activities of I3C is not known. However, the estrogenic property of the compound (Oganesian et al., 1999), the toxicity of the relatively high dose of I3C (Kim et al., 1994; Stoner et al., 2002) and the ensuing cell proliferation in response to cell injury are possible reasons. These untoward effects of I3C could be overcome, at least for lung cancer chemoprevention, through direct delivery of the drug to the lung by intranasal administration.
Overall, our results showed that intranasal administration of liposomal form of I3C is promising strategy for the chemoprevention of lung tumorigenesis. This approach led to improved pulmonary availability of I3C and reduction of O6-Methyl-G, probably through enhanced metabolism of the procarcinogen NNK. The effectiveness of intranasal liposomal I3C in preventing lung tumor formation will be assessed in future studies.
Acknowledgments
We thank Bob Carlson for his help in formatting the manuscript. This study was funded by NIH/NCI grant RO1 CA-128801 to F.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderton MJ, Manson MM, Vershoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- Balbo S, Upadhyaya P, Villalta PW, Qian X, Kassie F. DNA adducts in aldehyde dehydrogenase-positive lung stem cells of A/J mice treated with the tobacco specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Chem Res Toxicol. 2013;26:511–513. doi: 10.1021/tx400054s. [DOI] [PubMed] [Google Scholar]
- Bradfield CA, Bjeldanes LF. Effect of dietary indole-3-carbinol on intestinal and hepatic monooxygenase, glutathione S-transferase and epoxide hydrolase activities in the rat. Food Chem Toxicol. 1984;22:977–982. doi: 10.1016/0278-6915(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffery PK, Hodson ME, Coutelle C, Huang L, Porteous DJ, Williamson R, Geddes DM. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- Chien YW. Nasal systemic drug delivery. In: Chien YW, editor. Drugs and the Pharmaceutical Sciences. Marcel Dekker; New York: 1989. pp. 1–26. [Google Scholar]
- Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- Fujioka N, Ainslie-Waldman CE, Upadhyaya P, Carmella SG, Fritz VA, Rohwer C, Fan Y, Rauch D, Le C, Hatsukami DK, Hecht SS. Urinary 3,3′-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarkers Prev. 2014;23:282–287. doi: 10.1158/1055-9965.EPI-13-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YH, Friesen MD, Ruch RJ, Schut HA. Indole-3-carbinol as a chemopreventive agent in 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) carcinogenesis: inhibition of PhIP-DNA adduct formation, acceleration of PhIP metabolism, and induction of cytochrome P450 in female F344 rats. Food Chem Toxicol. 2000;38:15–23. doi: 10.1016/s0278-6915(99)00117-9. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Lin D, Castonguay A. Effects of α-deuterium substitution on the mutagenicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 1983;4:305–310. doi: 10.1093/carcin/4.3.305. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Howells LM, Gallacher-Horley B, Houghton CE, Manson MM, Hudson EA. Indole-3-carbinol inhibits protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol Cancer Ther. 2002;1:1161–1172. [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, France: 2007. Smokeless tobacco and tobacco-specific nitrosamines; p. 553. [PMC free article] [PubMed] [Google Scholar]
- Jackson AH, Prasitpan N, Shannon PVR, Tinker AC. Electrophilic substitution in indoles. part 15 The reaction between methylenedi-indoles and p-nitrobenzenediazonium fluoroborate. J Chem Soc Perkin Trans. 1987;1:2543–2551. [Google Scholar]
- Kim DJ, Lee KK, Han BS, Ahn B, Bae JH, Jang JJ. Biphasic modifying effect of indole-3-carbinol on diethylnitrosamine-induced preneoplastic glutathione S-transferase placental form-positive liver cell foci in Sprague-Dawley rats. Jpn J Cancer Res. 1994;85:578–583. doi: 10.1111/j.1349-7006.1994.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN. Chemoprevention of breast cancer: a summary of the evidence. Ann Intern Med. 2002;137:59–69. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- Ko YT, Falcao C, Torchilin VP. Cationic liposomes loaded with proapoptotic peptide D-(KLAKLAK)(2) and Bcl-2 antisense oligodeoxynucleotide G3139 for enhanced anticancer therapy. Mol Pharm. 2009;6:971–977. doi: 10.1021/mp900006h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao Y, Villalta PW, Sturla SJ, Wang M, Hecht SS. Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2006;19:674–682. doi: 10.1021/tx050351x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson MM, Ball HWL, Barrett MC, Clark HL, Judah DJ, Williamson G, Neal GE. Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin B-1 metabolism. Carcinogenesis. 1997;18:1729–1738. doi: 10.1093/carcin/18.9.1729. [DOI] [PubMed] [Google Scholar]
- Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse MA, LaGreca SD, Amin SG, Chung FL. Effects of indole-3-carbinol on lung tumorigenesis and DNA methylation induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and on the metabolism and disposition of NNK in A/J mice. Cancer Res. 1990;50:2613–2617. [PubMed] [Google Scholar]
- Oganesian A, Hendricks JD, Pereira CB, Orner GA, Bailey GS, Williams DE. Potency of dietary indole-3-carbinol as a promoter of aflatoxin B1-induced hepatocarcinogenesis: Results from a 9000 animal tumor study. Carcinogenesis. 1999;20:453–458. doi: 10.1093/carcin/20.3.453. [DOI] [PubMed] [Google Scholar]
- Peterson LA, Hecht SS. O6-Methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- Sane R, Mittapalli RK, Elmquist WF. Development and evaluation of a novel microemulsion formulation of elacridar to improve its bioavailability. J Pharm Sci. 102:1343–1354. doi: 10.1002/jps.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Stoner G, Casto B, Ralston S, Roebuck B, Pereira C, Bailey G. Development of a multi-organ rat model for evaluating chemopreventive agents: Efficacy of indole-3-carbinol. Carcinogenesis. 2002;23:265–272. doi: 10.1093/carcin/23.2.265. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Upadhyaya P, Lindgren BR, Hecht SS. Comparative levels of O6-methylguanine, pyridyloxobutyl-, and pyridylhydroxybutyl-DNA adducts in lung and liver of rats treated chronically with the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Drug Metab Dispos. 2009;37:1147–1151. doi: 10.1124/dmd.109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bailey AC, Hernaez JF, Taoka CR, Schut HA, Dashwood RH. Protection by green tea, black tea, and indole-3-carbinol against 2-amino-3-methylimidazo[4,5-f]quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]