Abstract
Background & Aims
Chronic hepatitis B virus (HBV) infection is an important cause of cirrhosis and hepatocellular carcinoma worldwide; populations that migrate to the US and Canada might be disproportionately affected. The Hepatitis B Research Network (HBRN) is a cooperative network of investigators from the United States and Canada, created to facilitate clinical, therapeutic, and translational research in adults and children with hepatitis B. We describe the structure of the network and baseline characteristics of adults with hepatitis B enrolled in the network.
Methods
The HBRN collected data on clinical characteristics of 1625 adults with chronic HBV infection who are not receiving antiviral therapy from 21 clinical centers in North America.
Results
Half of the subjects in the HBRN are male, and the mean age is 42 years; 72% are Asian, 15% are Black, and 11% are White, with 82% born outside of North America. The most common HBV genotype was B (39%); 745 of subjects were negative for the hepatitis B e antigen. The median serum level of HBV DNA when the study began was 3.6 log10 IU/mL; 68% of male subjects and 67% of female subjects had levels of alanine aminotransferase above the normal range.
Conclusions
The HBRN cohort will be used to address important clinical and therapeutic questions for North Americans infected with chronic HBV and to guide health policies on HBV prevention and management in North America.
Keywords: HBeAg, Chronic hepatitis B virus infection, USA, ALT
Background
Chronic hepatitis B virus (HBV) infection is a common cause of morbidity and mortality in the United States (U.S.) and throughout the world.1 Globally, cirrhosis ranks as the twelfth leading cause of death2 and chronic HBV infection accounts for approximately a third of cases of cirrhosis and half of all hepatocellular carcinomas (HCC).3 Although conventional estimates have cited approximately 1.4 million persons in the U.S. to be HBV-infected,4 the number of infected persons may be as high as 3 million due to undersampling of immigrant populations.5
Despite the availability of an effective vaccine for prevention of hepatitis B and safe and effective therapy, many questions regarding the natural history and pathogenesis of chronic HBV infection remain unanswered. With substantial racial and ethnic diversity, the U.S. and Canadian populations are ideal to study the full breadth of the epidemiology, natural history and clinical virology of chronic HBV infection.
The Hepatitis B Research Network (HBRN) is a cooperative network of investigators from 28 geographically distinct clinical centers across the U.S. and in Canada (North America), a data coordinating center (DCC) and an immunology center funded by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). The goal of the HBRN is to facilitate and conduct epidemiological clinical virological and therapeutic research in acute and chronic hepatitis B in both adult and pediatric patients who reside in North America. This report describes the structure of the HBRN and the baseline characteristics of the participants in the Adult Cohort Study.
Methods
Establishment and governance of the HBRN
The HBRN sites were selected through a process of peer review in response to a Request for Application issued by the NIDDK. Each Clinical Center Grantee is comprised of 1-3 clinical sites resulting in a total of 21 adult and 7 pediatric clinical centers in North America. The HBRN also includes a DCC and an Immunology Center (Figure 1). The DCC is responsible for coordinating study operations and logistics, developing and implementing data and other systems, maintaining the database, and performing data analyses. The Immunology Center is responsible for designing and conducting immunologic studies to elucidate immune responses to HBV during different phases of CHB and in response to antiviral therapy. There are two central virology laboratories.
Figure 1.

Map indicating location of clinical sites (blue circles, orange diamonds and green square), DCC (red balloon), Immunology Center (yellow arrowhead) comprising the HBRN and the funding agency, NIDDK (same location as NIH clinical center).
The HBRN is funded through cooperative U01 grants with NIDDK officials participating in its design and execution. A Steering Committee comprised of the principal investigator (PI) of each U01 grant, the PI from the DCC, the PI from the Immunology Center, and the NIDDK project scientist is responsible for study oversight. Several subcommittees have been established to facilitate the work of the Steering Committee (Figure 2). An independent Data and Safety Monitoring Board (DSMB) was appointed by the NIDDK to review the protocols and monitor the progress of the studies and participant safety.
Figure 2.
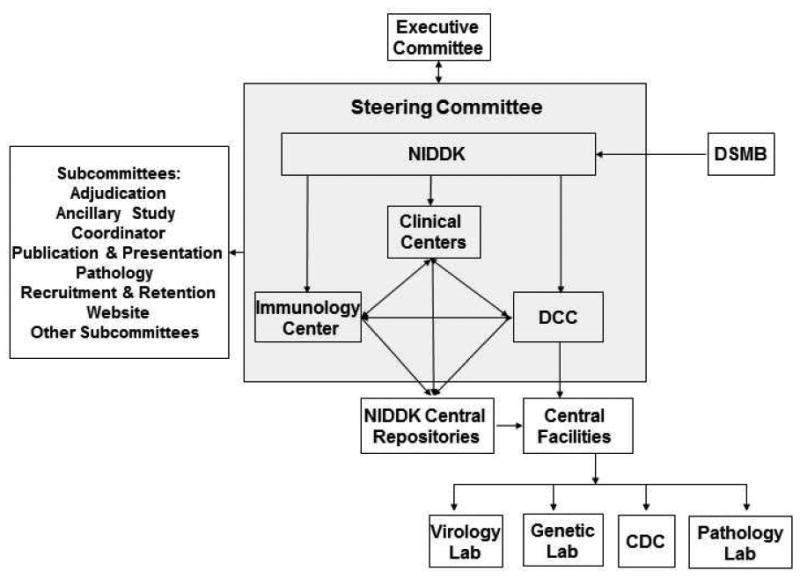
Organizational Structure of HBRN. The Hepatitis B Research Network (HBRN) consists of 21 clinical sites recruiting adults, a data coordinating center, an immunology center, and the Liver Disease Branch at NIDDK. A Steering Committee is responsible for study oversight. Several committees were established and an independent Data and Safety Monitoring Board (DSMB), appointed by NIDDK, reviewed the protocols and the progress of the studies.
The HBRN studies include two Cohort Studies (one for adults and another for children), two clinical trials of antiviral therapy in immune tolerant participants (one for adults and one for children), and a randomized clinical trial of antiviral therapy in immune active participants (adults only). The designs of the cohort studies and the clinical trials in immune tolerant participants for adults and children are similar and are available on the HBRN website: http://www.hepbnet.org/. Currently, there are 14 approved ancillary studies. All protocols were approved by the Steering Committee and the Institutional Review Boards (IRB) (Research Ethics Board [REB] in the case of the Toronto site) of the participating sites, and all participants provided written informed consent.
Adult Cohort Study Design
This is an observational study of adult patients with chronic HBV infection who are not on antiviral treatment. The primary aims of the study are to describe the characteristics of participants in a prospectively accrued cohort of chronic HBV infection in North America and to identify predictors of disease activation and progression. A secondary goal is to identify potential participants for the HBRN clinical trials. HBsAg-positive patients 18 years or older were eligible for enrollment after providing written informed consent. Patients with a history of hepatic decompensation, HCC, solid organ or bone marrow transplantation, or human immunodeficiency virus (HIV) coinfection, and those who were receiving antiviral therapy (with the exception of women who were pregnant at the time of enrollment), were excluded. Participants were evaluated at enrollment, Week 12, Week 24, and every 24 weeks thereafter. Participants experiencing hepatitis flares, HBeAg or HBsAg loss, or who were pregnant were seen at more frequent intervals.
Baseline and Follow-up Evaluation
The baseline evaluation includes a detailed medical history, physical examination, and laboratory tests. Information on risk factors for HBV infection, family history of HBV, prior antiviral therapy, results of recent imaging tests, medical comorbidities, health behavior and socio-economic indicators were recorded. Recommended laboratory tests include complete blood count, liver panel, creatinine, INR, alpha fetoprotein (AFP), quantitative HBV DNA level, HBsAg, antibody against HBsAg (anti-HBs), hepatitis B e antigen (HBeAg), antibody against HBeAg (anti-HBe), antibody against HIV (anti-HIV), antibody against hepatitis C virus (anti-HCV), antibody against hepatitis D virus (anti-HDV), autoimmune markers, and fasting glucose and lipid panel. Evaluations at follow up visits include an updated medical history, use of HBV antiviral therapy which may have been initiated after entry into the cohort, physical examination, laboratory testing for HBV DNA and a liver panel, and results of recent imaging tests.
Collection and Storage of Biospecimens
Serum and plasma samples for future research are drawn at each visit, stored frozen at each clinical center and sent regularly to the NIDDK central repository where they are stored at -80°C. Samples are sent by the repository in batches to central laboratories for testing. One sample of whole blood for extracting host DNA is collected from all consenting participants for genetic testing. Liver biopsy slides, either historical or from a standard of care biopsy, and a sample of liver tissue stored in RNALater if a biopsy was performed during the study, are collected from all consenting participants. Data are collected at each clinical site and entered into a password protected electronic database by trained personnel. The DCC regularly monitors the data to assess the accuracy and timeliness of data collection. Eleven of the 21 clinical sites enrolling adults are also engaged in ancillary immunology studies. Participants eligible for the immunology studies are enrolled after providing a separate written consent.
Enrollment Process and Criteria
The study set out to enroll consecutive, eligible HBsAg-positive patients at each clinical site with an aggregate target sample size of 2,500 subjects. At each site, a screening log is kept to record reasons for non-enrollment. The first participant was enrolled on January 14, 2011. After enrolling approximately 1,500 participants, the Steering Committee reviewed revised power calculations and changed from consecutive to targeted enrollment focusing on the following groups: (1) potential candidates for the clinical trials, (2) patients who were pregnant, (3) individuals who experienced flares of chronic hepatitis B, (4) patients with acute hepatitis B, (5) those with known HDV infection, and (6) patients who are eligible for the immunology study. The protocol amendment was approved at all sites in 2013. Only participants with chronic HBV infection enrolled prior to switching to targeted enrollment are included in the current analysis.
Monitoring and Testing
Standardized cutoffs for upper limit of normal (ULN) for ALT of 30 U/L for men and 20 U/L for women are used, regardless of the normal range at the laboratory from which results are obtained. HBV DNA levels from the Central Virology Laboratory are based on results of the Roche real-time PCR assay (COBAS Ampliprep/COBAS Taqman HBV Test, v2.0), which has a lower and upper limit of quantification of 20 IU/mL and 1.7 × 108 IU/mL, respectively. HBV genotype data are based on mass spectrometry, performed at the Molecular Epidemiology and Bioinformatics Laboratory in the Division of Viral Hepatitis, Centers for Disease Control and Prevention (CDC).6 If there are insufficient research blood samples for baseline HBV DNA testing or HBV genotyping to be done in the central laboratories, historical results from the medical record are used, if available. Because this is an observational study with study visits conducted in conjunction with clinical practice, laboratory tests are not always done on the same day as the study visits. Often, they are done prior to the visit so a window of up to 12 months is accepted for baseline ALT and HBV DNA and a window of up to 24 months is accepted for HBeAg status. However, reported results are typically more recent, i.e., 99% of ALT and HBV DNA is obtained within 3 months of entry and for HBeAg, 91% are within 12 months of entry.
Assignment of CHB Phenotype
Investigators are asked to assign a phenotype for each participant on the day of enrollment. The guidelines are:
Immune tolerant: HBeAg-positive, ALT levels ≤ULN on ≥2 occasions at least 6 months apart and HBV DNA levels ≥106 IU/mL
-
Immune active chronic hepatitis B (CHB)
HBeAg-positive: ALT levels >2x ULN on ≥2 occasions at least 6 months apart and HBV DNA levels ≥104 IU/mL; or
HBeAg-negative: ALT levels >2x ULN on ≥2 occasions at least 6 months apart and HBV DNA levels ≥103 IU/mL
Inactive carrier: HBeAg-negative, ALT levels ≤ULN and HBV DNA levels <103 IU/mL on ≥2 occasions at least 6 months apart.
Indeterminate: Individuals who did not fit into one of these categories.
Statistical Analysis
Baseline characteristics were summarized via frequencies and percentages for categorical variables and medians and quartiles for continuous variables. To test whether distributions of categorical variables differed across racial groups, the Pearson's Chi-square test or its exact version were used, as appropriate. Because the results are descriptive, pairwise comparisons were not adjusted for multiple testing. The same method was used to examine the association between genotype distributions and birthplace. The Kruskal-Wallis test was used to test whether the distributions of continuous variables differed across racial groups. For all statistical tests, the significance level of 0.05 was used to establish statistical significance. The statistical analyses were conducted in SAS (version 9.2, SAS Institute).
Results
Baseline Demographics and Clinical Characteristics of the Adult Cohort
Among 7,118 adults screened for entry into the observational study prior to targeted enrollment, 1,653 (23%) were enrolled between January 2011 and August 2013. The major reasons for non-enrollment included current antiviral therapy (n=3,013), refusal to consent (n=924), inability to comply with follow-up (n=419), and ineligibility due to HIV co-infection (n=194), HCC (n=188), hepatic decompensation (n=180), liver transplantation (n=62), or other reasons (n=951). Twenty-eight subjects with acute hepatitis B are excluded from the current analyses resulting in 1625 subjects with chronic HBV infection in the cohort. The median age was 42 years (range 18-80 years), 51% were male, and median physician-estimated duration of infection was 31 years (range 1-76 years). As shown in Table 1, the majority of subjects were Asian (72%), followed by Black (15%), White (11%), and mixed or other race (2%). Less than one-fifth (18%) of the subjects were born in North America. For the 1,210 (74%) participants for whom a route of transmission was determined by the investigator, it was broadly categorized as vertical in 60% and horizontal in 40%. The distribution of HBV genotypes were A 18%, B 39%, C 33%, D 8%, E 3% and other 1%. At baseline, 74% of the subjects were negative for HBeAg. Median serum HBV DNA level was 3.6 log10 IU/ml with 36% <103 IU/mL, 21% 103-104 IU/mL, 25% 104-107 IU/mL and 18% ≥107 IU/mL. The distribution of serum ALT levels was ≤1x ULN in 33%, >1 to ≤2x ULN in 43% and >2x ULN in 24%. Physician assigned phenotype at study entry was reported as immune tolerant in 10%, HBeAg positive CHB in 14%, HBeAg negative CHB in 26%, inactive carrier in 37% and indeterminate in 13%. Most participants (86%) had never received treatment for HBV infection.
Table 1.
Demographic And Clinical Characteristics By Race.
| Characteristics | All N=1625 |
White N=184 |
Black N=237 |
Asian N=1158 |
Mixed/Other N=38 |
p-value |
|---|---|---|---|---|---|---|
| Age (yrs)A | N=1623 | N=184 | N=237 | N=1157 | N=38 | <.001 |
| 42(33,52) | 48(35,58) | 43(34,54) | 41(32,51) | 39(28,47) | ||
|
| ||||||
| Age group (yrs)B | N=1623 | N=184 | N=237 | N=1157 | N=38 | |
| 18-<30 | 264(16.3) | 27(14.7) | 31(13.1) | 194(16.8) | 11(28.9) | |
| 30-<40 | 452(27.8) | 37(20.1) | 64(27) | 341(29.5) | 8(21.1) | |
| 40-<50 | 405(25) | 37(20.1) | 67(28.3) | 287(24.8) | 14(36.8) | |
| 50-<60 | 324(20) | 48(26.1) | 44(18.6) | 227(19.6) | 3(7.9) | |
| 60+ | 178(11) | 35(19) | 31(13.1) | 108(9.3) | 2(5.3) | |
|
| ||||||
| SexB | N=1625 | N=184 | N=237 | N=1158 | N=38 | <.001 |
| Male | 829(51) | 111(60.3) | 132(55.7) | 569(49.1) | 13(34.2) | |
|
| ||||||
| HispanicB | N=1612 | N=183 | N=235 | N=1150 | N=38 | <.001 |
| Yes | 18(1.1) | 10(5.5) | 1(0.4) | 4(0.3) | 1(2.6) | |
|
| ||||||
| Place of birthB | N=1615 | N=184 | N=237 | N=1150 | N=37 | <.001 |
| US/Canada | 294(18.2) | 118(64.1) | 57(24.1) | 97(8.4) | 22(59.5) | |
| Other N.A. and S.A. | 27(1.7) | 10(5.4) | 11(4.6) | 3(0.3) | 1(2.7) | |
| Europe | 47(2.9) | 44(23.9) | 1(0.4) | 0(0.0) | 1(2.7) | |
| Asia/Australia | 1077(66.7) | 10(5.4) | 2(0.8) | 1048(91.1) | 13(35.1) | |
| Africa | 170(10.5) | 2(1.1) | 166(70) | 2(0.2) | 0(0.0) | |
|
| ||||||
| Presumed mode of transmissionB | N=1210 | N=120 | N=179 | N=875 | N=33 | <.001 |
| Vertical | 724(59.8) | 27(22.5) | 40(22.3) | 626(71.5) | 29(87.9) | |
| Horizontal/Household | 325(26.9) | 20(16.7) | 97(54.2) | 205(23.4) | 2(6.1) | |
| Sexual | 73(6) | 33(27.5) | 24(13.4) | 15(1.7) | 1(3) | |
| Medical exposures | 62(5.1) | 25(20.8) | 15(8.4) | 22(2.5) | 0(0.0) | |
| Drug use | 17(1.4) | 13(10.8) | 2(1.1) | 1(0.1) | 1(3) | |
| Other | 9(0.7) | 2(1.7) | 1(0.6) | 6(0.7) | 0(0.0) | |
|
| ||||||
| Estimated Duration of HBV (yrs) A | N=1168 | N=101 | N=154 | N=875 | N=32 | <.001 |
| 31(20,43) | 25(13,35) | 15(6,32) | 34(23,45) | 30(21,42) | ||
|
| ||||||
| Ever Received HBV TreatmentB * | N=1623 | N=184 | N=237 | N=1157 | N=38 | 0.02 |
| Yes | 235(14.5) | 24(13) | 20(8.4) | 183(15.8) | 8(21.1) | |
|
| ||||||
| Genotype B | N=1379 | N=152 | N=162 | N=1023 | N=35 | <.001 |
| A | 247(17.9) | 84(55.3) | 105(64.8) | 50(4.9) | 6(17.1) | |
| B | 531(38.5) | 7(4.6) | 7(4.3) | 510(49.9) | 6(17.1) | |
| C | 450(32.6) | 4(2.6) | 3(1.9) | 420(41.1) | 23(65.7) | |
| D | 106(7.7) | 50(32.9) | 12(7.4) | 40(3.9) | 0(0.0) | |
| E | 35(2.5) | 2(1.3) | 31(19.1) | 2(0.2) | 0(0.0) | |
| F/G/H/Multiple | 10(0.7) | 5(3.3) | 4(2.5) | 1(0.1) | 0(0.0) | |
|
| ||||||
| HBeAg B | N=1386 | N=156 | N=189 | N=1003 | N=32 | <.001 |
| Positive | 365(26.3) | 31(19.9) | 19(10.1) | 305(30.4) | 9(28.1) | |
|
| ||||||
| HBV DNA (log10 IU/mL) A | N=1592 | N=178 | N=234 | N=1136 | N=38 | <.001 |
| 3.6(2.5,5.6) | 3.0(2.0,4.2) | 3.0(2.0,3.9) | 4.0(2.7,6.1) | 3.4(2.5,4.5) | ||
|
| ||||||
| ALT (U/L), males A | N=800 | N=109 | N=127 | N=548 | N=13 | 0.06 |
| 38(27,61) | 46(30,68) | 36(26,63) | 37(26,58) | 46(33,91) | ||
|
| ||||||
| ALT (U/L), femalesA | N=764 | N=71 | N=101 | N=564 | N=24 | <.001 |
| 26(19,39) | 29(22,44) | 20(16,27) | 27(19,40) | 27(16,46) | ||
|
| ||||||
| Albumin (g/dL) A | N=1516 | N=175 | N=219 | N=1079 | N=36 | <.001 |
| 4.3(4.1,4.6) | 4.3(4.1,4.6) | 4.2(3.9,4.4) | 4.4(4.1,4.6) | 4.3(4.0,4.6) | ||
|
| ||||||
| Total Bilirubin (mg/dL) A | N=1544 | N=179 | N=225 | N=1096 | N=37 | <.001 |
| 0.6(0.4,0.8) | 0.6(0.4,0.8) | 0.5(0.4,0.7) | 0.6(0.5,0.9) | 0.5(0.4,0.7) | ||
|
| ||||||
| Platelets (103/mm3) A | N=1389 | N=167 | N=209 | N=974 | N=34 | 0.2 |
| 217(179,256) | 208(170,257) | 208(170,263) | 219(184,255) | 216(186,263) | ||
Continuous variables were summarized by N of observed data and median (interquartile range). The p-values were based on the Kruskal-Wallis test.
Categorical variables were summarized by N of observed data and percentages. The p-values were based on the χ2 test or its exact version when appropriate.
Patients currently on antiviral therapy were excluded from enrollment with the exception of pregnant women.
Analysis by Race
There were marked differences in almost all of the demographic, clinical and laboratory features among the major racial groups (Table 1). Compared to Asians, Whites and Blacks were older, more likely to be male and have a shorter estimated-duration of infection. Vertical transmission was the predominant source of infection among Asians whereas horizontal transmission was more common among Whites and Blacks, with sexual transmission and medical exposure being the most common modes of horizontal transmission. A majority of all races were HBeAg negative, including 70% of Asians, 80% of Whites and 90% of Blacks. The distribution of HBV genotypes varied markedly among the 3 major racial groups. The predominant genotypes among Asians were B (50%) and C (41%), among Whites A (55%) and D (33%) and among Blacks A (65%) and E (19%). The majority of Whites in the cohort (65%) were born in North America compared to only 24% of Blacks and 8% of Asians.
ALT levels were also different across racial groups (p=0.01, Table 1 and Figure 3a). A higher percentage of Blacks (42%) than Asians (32%, p=0.01) or Whites (24%, p=0.001) had normal ALT, and proportionally more Whites (31%) had an elevated ALT level >2x ULN compared to Asians (24%, p=0.05) and Blacks (20%, p=0.02). HBV DNA levels were highest among Asians (Table 1 and Figure 3b). Physician-assigned phenotype differed by race (Figure 4). Among Asians, 13% were designated as having immune tolerant disease, 29% had HBeAg negative CHB, 15% had HBeAg positive CHB and 30% were inactive carriers. In contrast, among Whites and Blacks, immune tolerant disease was uncommon (1% and 2%) and the inactive carrier phenotype was the most common (47% and 66%, respectively) followed by HBeAg negative CHB (25% and 14%, respectively). Notably, 13% (10-15% by race) of all participants had an indeterminate phenotype. Among participants with an indeterminate phenotype for whom each characteristic was known, 57% were male, 74% Asian and 83% were HBeAg negative. There was a broad spread of HBV DNA levels with 33% <103 IU/mL, 27% 103-<104 IU/mL, 34% 104-<107 IU/mL and 5% >107 IU/ml and ALT levels, 28% had an ALT ≤ULN, 54% >1-2x ULN and 18% >2x ULN.
Figure 3a.

Distribution of serum ALT (categorized by upper limit of normal) by race. Serum ALT was categorized as ≤1 x ULN, >1 to 2 x ULN and >2 x ULN, with ULN defined as 30 U/L for men and 20 U/L for women.
Figure 3b.

Distribution of serum HBV DNA level by race. Serum HBV DNA was categorized as <103 IU/mL, 103 to 104 IU/ml, 104 to 107 IU/mL and ≥107 IU/mL.
Figure 4.

Distribution of phases of chronic HBV infection by race. Each slice of the pie represents the percent phenotype distribution.
Analysis by Continent of Birth
There were also racial and genotype differences with respect to immigration status. Nearly three-quarters of the participants in the HBRN adult cohort were Asians of whom 92% were born outside North America. In addition, 15% of the cohort was Black of whom 70% reported Africa as their birthplace. The genotype distribution differed significantly (p<.001) by Continent of birth (Figure 5). The most common genotypes were A and C among those born in the U.S. and Canada, B and C for those born in Asia, A and E in those born in Africa, A and D in those born in Europe, and A and others (F, G and H) for those born in South America and Mexico.
Figure 5.

Distribution of subjects in the HBRN Cohort Study by Continent of birth and HBV genotype. The size of each pie represents the proportion of enrollees who were born in each continent (with the exception of Europe and South America-which were enlarged slightly to facilitate viewing) and each slice of the pie represents the percent genotype distribution.
Discussion
The objectives, organizational structure, and initial findings of the NIDDK-sponsored HBRN are described in this report. The HBRN is the largest research program in North America created to study the epidemiology, natural history, and treatment of CHB. During a period of 32 months, 1,625 adults with chronic HBV infection were enrolled from 21 clinical centers in the United States and Canada. The potential importance of the HBRN is due to its wide catchment distribution, the racial and ethnic diversity of the participants, the planned long-term follow up of study participants, and the breadth of data and biosamples collected which will enable investigators to study a wide range of topics related to hepatitis B. Efforts will continue to enroll targeted patient groups for studies of specific aspects of natural history of chronic HBV infection and treatment trials. Information on pediatric participants enrolled in the HBRN cohort studies will be analyzed and published separately.7
While it is generally accepted that chronic HBV infection can be classified into four phases, 13% of subjects in the HBRN did not meet criteria for any of these phases. Intriguingly, using recent definitions of ‘normal’ ALT instead of laboratory reference ranges, 43% of our cohort displayed low level elevations in ALT (1-2x ULN) that may not be neatly categorized into commonly accepted immune active, inactive or tolerant phases of chronic HBV infection. Further follow-up will determine the natural history of these participants. Furthermore, virtually all of the immune tolerant patients were Asian and were born abroad.
One of the phases, HBeAg-negative CHB, was formerly considered relatively rare in North America and has been observed more commonly in the Mediterranean, Eastern Europe and Asia where it may constitute as much as 90% of all patients with chronic HBV infection.8,9 Our study showed that in North America, HBeAg-negative CHB is far more common than HBeAg-positive CHB among all racial groups. The largest group of HBeAg negative participants was classified as inactive carriers; excluding patients who were receiving antiviral therapy may have skewed the HBRN cohort towards this group.
Determining HBV genotypes is an important goal of the HBRN as it has been postulated that certain genotypes are associated with a higher incidence of long term complications including HCC.10 A broad range of genotypes (A to H) are represented in the HBRN cohort with genotypes A-D constituting more than 90% of cases. Compared to a previous study done a decade ago, the most striking difference of the current analysis is the markedly lower prevalence of genotype A (a decline from 35% to 18%) and increase in genotype B (from 22% to 39%).11 This could be due to the study being conducted at different sites than previous or participant selection, but could also be explained by changes in immigration patterns over the last decade.
The adult cohort study provides a unique opportunity to examine the natural history of hepatitis B across many races and ethnicity. The data on country of birth confirms that the burden of chronic HBV infection is disproportionately large among immigrant populations residing in North America, the size of which differs by race. These findings from the HBRN are well aligned with a recent report from the Centers for Disease Control and Prevention highlighting the impact that changing immigration patterns has had on the epidemiology of chronic HBV infection in the U.S. It is estimated that more than 90% of people with chronic HBV infection in the U.S. today immigrated from other countries.12 During the period from 1974-2008, nearly 30 million immigrants have entered the U.S. of whom 63% were born in countries of intermediate or high HBV prevalence (range 2-31%).12 Thus, it is estimated that 1.3 million new HBsAg carriers entered the U.S. during this period. The true burden of HBV infection in the U.S. has been under-appreciated because immigrant minorities are inadequately sampled in population-based studies such as the National Health and Nutrition Examination Survey IV study.13 The high frequency of imported chronic hepatitis B has important implications for developing public health strategies to educate and control spread of HBV infection in the community.
There are several limitations of the HBRN worth noting. Subjects enrolled in the HBRN may not be representative of those in the community. Patients who are currently on antiviral therapy (>40% of those screened for enrollment), those who do not have access to specialized liver centers and those who do not speak a language for which data can be collected by the HBRN were not included. Furthermore, over half of the potentially eligible participants were not enrolled, primarily because they refused to consent or were considered to be unable to comply with follow-up. Subjects with HIV were excluded from the main cohort, but are being studied as part of a parallel NIDDK R01 funded ancillary study.
In summary, the NIDDK-sponsored HBRN is the largest prospective cohort study in North America, and includes a diverse racial distribution and the full spectrum of disease phenotypes.5,14 Longitudinal assessments of participants should provide meaningful insights into the natural history and disease burden of chronic HBV infection in North America. With the large size of the population under study, the HBRN should provide reliable estimates of transitions from one phase of chronic HBV infection to another and the virologic and immunologic basis behind those transitions. Collectively, immigrants with chronic HBV infection constitute the largest group of patients with hepatitis B in North America. A better understanding of the epidemiology and natural history could improve management, delay disease progression and save lives.
Acknowledgments
In addition to the authors, the HBRN would like to acknowledge the contributions of the following: Harvard Consortium: Nezam Afdhal, MD, Asad Javaid, MBBS, Jianghe Niu, Johanna Han, Imad Nasser, MD (Beth Israel Deaconess Medical Center, Boston, MA). Minnesota Alliance for Research in Chronic Hepatitis B Alisha C. Stahler, Linda Stadheim, RN (Mayo Clinic Rochester, Rochester, MN), Mohamed Hassan, MD (University of Minnesota, Minneapolis, MN). Saint Louis Midwest Hep B Consortium: Debra L. King, RN, Rosemary A. Nagy, MBA, RD, LD (Saint Louis University School of Medicine, St Louis, MO) (Washington University, St. Louis, MO). University of Toronto Consortium: Danie La, RN (Toronto Western & General Hospitals, Toronto, Ontario), Lucie Liu (Toronto Western & General Hospitals, Toronto, Ontario). HBV CRN North Texas Consortium: Stacey Minshall, RN, BSN (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas), Sheila Bass (University of Texas Southwestern, Dallas, TX). Los Angeles Hepatitis B Consortium: Samuel French, MD, Velma Peacock, RN (David Geffen School of Med, UCLA, Los Angeles, CA). San Francisco Hepatitis B Research Group Consortium: Ashley Ungermann, MS, Claudia Ayala, MS, Emma Olson, BS, Ivy Lau, BS (University of California-San Francisco), Veronika Podolskaya, BS, NCPT, Nata DeVole, RN (California Pacific Medical Center, Research Institute). Michigan Hawaii Consortium: Barbara McKenna, MD, Kelly Oberhelman, PAC, Sravanthi Kaza, Bpharm, Cassandra Rodd, BS (University of Michigan, Ann Arbor, MI), Leslie Huddleston, NP, Peter Poerzgen, PhD (University of Hawaii/Hawaii Medical Center East, Honolulu, HI). Chapel Hill, NC Consortium: Jama M. Darling, M.D., A. Sidney Barritt, M.D., Tiffany Marsh, BA, Vikki Metheny, ANP, Danielle Cardona, PA-C (University of North Carolina at Chapel Hill, Chapel Hill, NC). Virginia Commonwealth University Medical Center Velimir A. Luketic, MD, Paula G Smith, RN, BSN, Charlotte Hofmann, RN (Virginia Commonwealth University Health System, Richmond, VA). PNW/Alaska Clinical Center Consortium: Terri Mathisen, RN, BSN, Susan Strom, MPH (University of Washington Medical Center, Seattle WA) Jody Mooney, Lupita Cardona-Gonzalez (Virginia Mason Medical Center, Seattle WA). Liver Diseases Branch, NIDDK, NIH: Nancy Fryzek, RN, BSN, Elenita Rivera, BSN, Nevitt Morris, Vanessa Haynes-Williams. Immunology Center: Mary E. Valiga, RN, Keith Torrey, BS, Danielle Levine, BS, James Keith, BS, Michael Betts, PhD (University of Pennsylvania, Philadelphia, PA), Luis J. Montaner, DVM, DPhil (Wistar Institute, Philadelphia, PA). Data Coordinating Center: Yona Cloonan, PhD, Michelle Danielson, PhD, Tamara Haller, Geoffrey Johnson, MS, Stephanie Kelley, MS, Sharon Lawlor, MBA, Ruosha Li, PhD, Manuel Lombardero, MS, Joan M. MacGregor, MS, Andrew Pelesko, BS, Donna Stoliker, Barbara Walters, Ella Zadorozny, MS (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA).
Funding: The HBRN was funded by a U01 grant from the National Institute of Diabetes and Digestive and Kidney Diseases to the following investigators William Lee (U01 DK082872), Steven Belle PhD (DK082864), Harry Janssen, MD, PhD (DK082874), Norah Terrault, MD, MPH (U01 DK082944), Robert C Carithers, MD (DK082943), Daryl T-Y Lau, MD, MPH (DK082919), W. Ray Kim, MD (DK 082843), Michael W. Fried, MD (DK082867), Richard K. Sterling, MD, MSc (DK082923), Adrian Di Bisceglie, MD (DK082871), Steven-Huy B. Han, MD (DK082927), Kyong-Mi Chang, MD (DK082866), Anna SF Lok MD (DK082863), an interagency agreement with NIDDK: Lilia Milkova Ganova-Raeva, PhD (A-DK-3002-001) and support from the intramural program, NIDDK, NIH: Marc G Ghany. Additional funding to support this study was provided to Kyong-Mi Chang, the Immunology Center, (NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306, NIH Public Health Service Research Grant M01-RR00040), Richard K. Sterling (UL1TR000058, NCATS (National Center for Advancing Translational Sciences, NIH), Norah A. Terrault (CTSA Grant Number UL1TR000004), Michael W. Fried (CTSA Grant Number UL1TR001111), and Anna Suk-Fong Lok (CTSA Grant Number UL1RR024986.) Additional support was provided by Gilead Sciences, Inc. and Roche Molecular Systems via a CRADA through the NIDDK.
Conflict of Interest: Dr Perrillo reports receiving lecture fees from Bristol Myers Squibb, Gilead Sciences and consultant fees from Novartis; Dr. Janssen reports receiving consultant fees and grant support from Bristol Myers Squibb, Gilead Sciences, Novartis, Roche, & Merck; Dr Shuhart reports receiving grant funding from Gilead Sciences; Dr. Kim reports receiving consultant fees from Gilead Sciences; Dr. Fried reports receiving consultant fees from Genentech, Merck, Vertex, AbbVie, Janssen, BMS, Gilead Sciences; Dr. Sterling reposts receiving consultant fees from BMS, Gilead Sciences, Roche, and Genentech and research support from BMS, Gilead Sciences, Roche, Genentech, and Roche Molecular Diagnostics.
Dr. Di Bisceglie reports receiving consultant fees and grant support from Gilead Sciences and Bristol Myers Squibb, Dr. Han reports receiving lecture fees from Gilead Sciences and grant support from Gilead Sciences, Bristol Myers Squibb, Idenix Pharmaceuticals; Dr. Chang reports her spouse is an employee of Bristol Myers Squibb and receiving consultant fees from Bristol Myers Squibb; Dr. Lok reports receiving consultant fees from Gilead Sciences, GlaxoSmithKline, Merck, Roche and grant support from Bristol-Myers Squibb, Gilead Sciences, and Merck.
Abbreviations
- HBV
hepatitis B virus
- U.S.
United States
- HCC
hepatocellular carcinoma
- HBRN
Hepatitis B Research Network
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- DCC
Data Coordinating Center
- DNA
deoxyribonucleic acid
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis B e antigen
- DSMB
Data and Safety Monitoring Board
- ALT
alanine aminotransferase
- anti-HBe
antibody to HBeAg
- anti-HIV
antibody to human immunodeficiency virus
- anti-HCV
antibody to hepatitis C virus
- anti-HDV
antibody to hepatitis D virus
- PCR
polymerase chain reaction
- AST
aspartate aminotransferase
- anti-HBs
antibody to HBsAg
- CHB
chronic hepatitis B
- NIH
National Institutes of Health
Footnotes
Authors' contribution: Study concept & design: MGG, RP, RL, ASFL; Acquisition of data: MGG, RP, HLAJ, NT, MS, DL, RK, MF, RS, AD, SHBH, LMGR, ASFL, Analysis/interpretation: MGG, RP, RL, ASFL; Drafting: MGG, RP, RL, ASFL; Critical revision: MGG, RP, RL, SHB, HLAJ, NT, MS, DL, RK, MF, RS, AD, SHBH, LMGR, ASFL; Statistical analysis: RL.
Drs. Ghany, Li, Belle, Terrault, Lau, and Ganova-Raeva report no potential conflict of interest relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34(Suppl 1):S1–3. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 4.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–8. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–33. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 6.Ganova-Raeva L, Ramachandran S, Honisch C, Forbi JC, Zhai X, Khudyakov Y. Robust hepatitis B virus genotyping by mass spectrometry. J Clin Microbiol. 2010;48:4161–8. doi: 10.1128/JCM.00813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz KBLS, Rosenth LP, Murray KF, Rodriguez-Baez, et al. Phenotypes of North American Children with Hepatitis B Virus (HBV) Infection. Hepatology. 2012;56:635A. [Google Scholar]
- 8.Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617–24. doi: 10.1053/jhep.2001.27834. [DOI] [PubMed] [Google Scholar]
- 9.Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9:52–61. doi: 10.1046/j.1365-2893.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu CJ, Kao JH. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Semin Liver Dis. 2013;33:97–102. doi: 10.1055/s-0033-1345716. [DOI] [PubMed] [Google Scholar]
- 11.Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology. 2003;125:444–51. doi: 10.1016/s0016-5085(03)00895-3. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One. 2011;6:e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SH, Pong P, Pollack H. Hepatitis B virus in the United States. Ann Intern Med. 2011;155:204–5. doi: 10.7326/0003-4819-155-3-201108020-00020. author reply 5-6. [DOI] [PubMed] [Google Scholar]
- 14.Rotermann M, Langlois K, Andonov A, Trubnikov M. Seroprevalence of hepatitis B and C virus infections: Results from the 2007 to 2009 and 2009 to 2011 Canadian Health Measures Survey. Health Rep. 2013;24:3–13. [PubMed] [Google Scholar]


