Abstract
The heart is a very complex conglomeration of organized interactions between various different cell types that all aid in facilitating myocardial function through contractility, sufficient perfusion, and cell-to-cell reception. In order to make sure all features of the heart work effectively, it is imperative to have a well-controlled communication system among the different types of cells. One of the most important ways the heart regulates itself is by the use of extracellular vesicles, more specifically, exosomes. Exosomes are types of nano-vesicles, naturally released from living cells. They are believed to play a critical role in intercellular communication through the means of certain mechanisms including direct cell-to-cell contact, long-range signals as well as electrical and extracellular chemical molecules. Exosomes contain many unique features like surface proteins/receptors, lipids, mRNAs, microRNAs, transcription factors and other proteins. Recent studies indicate that the exosomal contents are highly regulated by various stress and disease conditions, in turn reflective of the parent cell status. At present, exosomes are well appreciated to be involved in the process of tumor and infection disease. However, the research on cardiac exosomes is just emerging. In this review, we summarize recent findings on the pathologic effects of exosomes on cardiac remodeling under stress and disease conditions, including cardiac hypertrophy, peripartum cardiomyopathy, diabetic cardiomyopathy and sepsis-induced cardiovascular dysfunction. In addition, the cardio-protective effects of stress-preconditioned exosomes and stem cell-derived exosomes are also summarized. Finally, we discuss how to epigenetically reprogram exosome contents in host cells which makes them beneficial for the heart.
Keywords: Exosomes, Cardiomyopathy, Cardiac remodeling, Stem cells, miRNAs
1. Introduction
Cardiovascular disease is the number one cause of death in human beings, with approximately one in every four deaths being cardiac related [1]. The mammalian heart is thought of as a highly stressed organ which continually keeps beating and outputs blood/nutrition to the whole body. To maintain effective myocardial function, synchronized communication among different cell types within the heart is needed. It is estimated that the human heart contains two to three billion cardiomyocytes, which account for approximately one-third of the total number of cells in the heart [2]. This balance includes a wide array of additional cell types, such as smooth muscle cells, endothelial cells, fibroblasts, mast cells, immune system-related cells, other connective tissue cells and pluripotent cardiac stem cells [2-4]. It is becoming clear that a number of local and long-distance cell-cell communications contribute to the maintenance of normal cardiac homeostasis as well as responses to hypertrophic stimuli [4-12]. These include growth factor-mediated paracrine/autocrine and adiponectine-mediated endocrine signals, gap junction-mediated cell-cell contacts, cell-matrix interactions and signaling through adhesion molecules including integrins that may modulate ventricular growth in crosstalk with circulating growth factors and other humoral mediators [4-12].
Recently, a group of small membrane vesicles naturally released from mammalian cells have been implicated as a new tool for cardiac cell communication [13-15]. These extracellular vesicles have a fluid lipid bilayer and contain a specific set of proteins, genetic materials, and lipids [13-15]. The types of membrane vesicles produced from cardiac cells include apoptotic bodies, microvesicles and exosomes [15]. These different vesicles are distinguished from one to another on the basis of their subcellular origin, size, and content. Apoptotic bodies (1-5 μm in diameter) are released from the plasma membrane of cells as blebs when they undergo apoptosis [14-16]. They contain a variety of cellular organelles, intracellular fragments, histones and even fragmented DNA and can be detected by extensive amounts of phosphatidylserine. Apoptotic bodies are considered to be mobile as they have the ability to float on a sucrose substance with a density between 1.16 and 1.28 g/mL [14-16]. While the exact role of apoptotic bodies is not fully understood, it is believed that apoptotic bodies could be used in activating cell signals to promote the elimination of other damaged cells [15]. Usually, microvesicles (100-1000 μm) are generated from cells by a direct outward budding of the cell membrane when they are exposed to certain stimuli including exposure to pro-thrombotic and pro-inflammatory as well as cellular differentiation and senescence [14-16]. Unlike apoptotic bodies, microvesicles float on a different sucrose gradient of 1.04-1.06 g/mL and are usually derived from platelets, endothelial and red blood cells [15]. Microvesicles contain a variety of bioactive molecules that include a variety of adhesive structures, cytokines, chemokines, cytoplasmic proteins, membrane proteins, cellular receptors, non-coding RNA, and even mRNA and miRNAs. The various different protein markers for microvesicles include integrins, selectins, and the CD40 ligand [15]. Exosomes are types of extracellular vesicles whose sizes ranges between 30-100nm and float to a density ranging from 1.13-1.19g/ml using ultracentrifugation on linear sucrose gradient (2M-0.25M sucrose) [15-18]. They have very unique qualities that greatly set them apart from other extracellular vesicles (apoptotic bodies and microvesicles). Secretion of exosomes from viable cells was proposed to be a mechanism through which cells discard their cellular waste [19-22]. However, over the past years, exosomes have emerged as important players for inter-cellular communication that are involved both in normal physiology, e.g. cardiac development, myocardial angiogenesis, and vesicle formation during reticulocyte maturation [13, 15, 20-22], and in pathophysiological conditions, e.g. progression of cancer, liver disease, immune-defective disease, and neurodegenerative disease [16, 23-25]. In this review, we will focus on exosomes and their role in cardiac patho-physiology. Specifically, we will: 1) discuss how to regulate exosome biogenesis and secretion, 2) summarize the advances on the detrimental and beneficial role of exosomes in cardiac remodeling, and 3) discuss the therapeutic potential of exosomes in cardiovascular diseases.
2. Biogenesis and Secretion of Exosomes
2.1 Exosome Biogenesis
The term “exosomes” was coined by Johnstone et al [21] in their investigation of vesicle formation during reticulocyte maturation. Since their initial discovery, much has been learned about the biogenesis of exosomes. Unlike microvesicles that are derived from the direct outward blebs of plasma, exosomes are generated within the endosomal network, beginning with the inward budding of the cell membrane to form early endosomes, followed by second inward budding of the endosomal membrane to form the various intraluminal vesicles (ILVs) (late endosomes). The late endosomes containing ILVs are also called as multivesicular bodies (MVBs). Finally, the MVBs may fuse with the cell membrane, releasing its ILVs in an exocytotic fashion to the extracellular environment. These ILVs are then referred to as exosomes. Alternatively, the MVBs move towards the perinuclear area of the cell where they can directly fuse with lysosomes, creating hybrid organelles where endocytosed cargos are degraded. The process of exosome biogenesis is summarized in Fig. 1.
Figure 1.
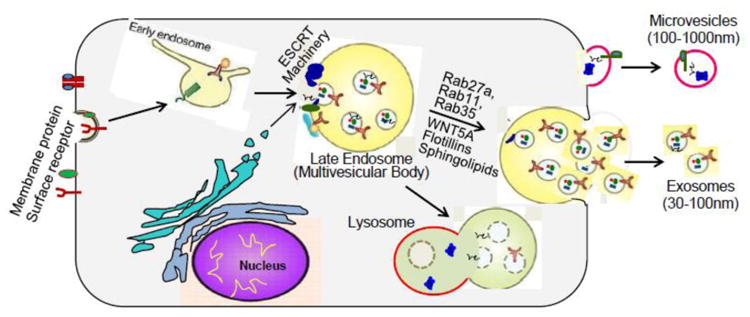
The generation of microvesicles and exosomes. Microvesicles (MVs) are formed directly by outward budding or blebbing of the plasma membrane. Specific loading of membrane proteins, lipids and RNAs to the MVs in known to occur, but the exact molecular mechanism are largely unknown. Biogenesis of exosomes is initiated with inward budding of the cell membrane, with specific membrane proteins/receptors incorporated, to form early endosomes. Subsequently, the cargo is packaged into intraluminal vesicles (ILVs) upon second inward budding of the early endosome membrane, and then transformed into multivesicle bodies (MVBs). Four different mechanisms have been described to facilitate the cargo loading: 1) ESCRT machinery and associated proteins; 2) lipid rafts; 3) higher-ordered oligomerization; and 4) segregation into microdomains by ceramide. MVBs can then fuse with the lysosomal membrane and release ILVs for degradation. Alternatively, MVBs fuse with the plasma membrane and release ILVs into the extracellular space as exosomes, a process which is regulated by Rab27a, Rab11, Rab35, WNT5A, SNAREs, glycosphingolipids and flotillins, etc.
Nowadays, the molecular mechanisms of ILV (exosome) formation and sorting of endosomal proteins into these vesicles are partially understood. The best described machinery that drives the process of MVB formation is coordinated by the endosomal sorting complexes required for transport (ESCRT) system, which has been widely reviewed recently in the literature [26-29]. In brief, this machinery consists of four soluble multi-protein complexes, called ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III, and its associated proteins. ESCRT-0 is responsible for cargo clustering in an ubiquitin-dependent manner; ESCRT-I and ESCRT-II induce bud formation; ESCRT-III drives vesicle scission, and the accessory proteins (especially the VPS4 ATPase) allow dissociation and recycling of the ESCRT machinery. Recently, Colombo et al [30] performed an RNA interference screen targeting 23 components of ESCRT complex and associated proteins in HeLa cells. They observed that depletion of HRS, STAM1 (ESCRT-0 members), and Tsg101 (ESCRT-I component) inhibited exosome biogenesis; by contrast, silencing of VPS4b (ESCRT-III member) promoted exosome formation. Notably, Colombo et al [30] observed that members of ESCRT-II did not have any influences on exosome biogenesis/release in HeLa cells. Of interest, several recent studies show that the ESCRT-III-associated protein, ALIX, can augment intraluminal budding of vesicles in endosomes and enhance CD63-positive exosome biogenesis in primary dendritic cells (DCs) and muscle cell line C2C12 [31-33].
Although many studies use ESCRT silencing as a tool to inhibit biogenesis of exosomes, one should be aware that ESCRT- independent mechanisms are also involved in the ILV formation and exosome biogenesis [34, 35]. These mechanisms are associated with proteolipid proteins, tetraspanins or heat shock proteins (see reviews elsewhere [26]). Currently, it remains obscure whether these multiple mechanisms of biogenesis of exosomes/ILVs can occur in a single MVB, or different MVB populations can co-exist within the cell. In addition, it is not completely known how to decide different MVB subpopulations' fate for either lysosomal degradation or exosomal release. Future studies will be needed to clarify such issues.
2.2 Exosome Secretion
Exosomes can be released from the cell by two mechanisms: constitutive or inducible. The constitutive secretion pathway is regulated by certain RAB GTPases (Rab27a/b, Rab11 and Rab35), hetrotrimetric G-protein, WNT5A, glycosphingolipids and flotillins [36-41]. On the other hand, the inducible secretion is regulated by stress stimuli including aberrant intracellular calcium release, DNA damage, thrombin, heat shock, hypoxia and lipopolysaccharide (LPS) stimulation [42-51]. For example, we and others have shown that low dose of H2O2 (20 μM) can stimulate cardiomyocytes to produce exosomes [48-50]. Recent studies have also shown that ischemic preconditioning can induce exosome secretion from the heart [52]. In addition, change in membrane pH has been shown to trigger the secretion of exosomes from various cell types [49, 53].
No matter constitutive or inducible release, the final step of exosome secretion requires the fusion of MVBs with the plasma membrane. This process possibly involves a specific combination of Soluble N-ethylmaleimide-sensitive factor-attachment protein receptors (SNAREs): vesicular SNAREs (v-SNAREs, localized on MVBs) interact with target SNAREs (t-SNAREs, localized on the intracellular side of the plasma membrane), to form a membrane-bridging SNARE complex, responsible for membrane fusion [54, 55].
2.3 Molecular Composition of Exosomes
Increasing evidence shows that exosome composition contains both a common and a specific set of DNA, RNA and proteins (see database available at www.exocarta.org; www.evpedia.info) [56, 57]. The common set of exosomal proteins that have been identified are found in parent's cell membranes of endocytic compartment or the plasma membrane, also in the cytosol, the Golgi, even the nucleus, but rarely the endoplasmic reticulum or mitochondria [56-58]. The typical membrane proteins encased in exosomes include GPI-anchored proteins, Tetraspanins (CD81, CD82 CD63, and CD9) and receptors [i.e., Tumor Necrosis Factor Receptor 1 (TNFR1)]. Other typical types of molecules wrapped in exosomes include: 1) luminal proteins such as annexin 2 and cytokines; 2) antigen-presentation (MHC-I, MHC-II) an co-presentation molecules (CD86), cell adhesion (Integrins, MFGE8, etc.), cell structure and motility (actins, myosin, tubulin, etc.); 3) Heat shock proteins and chaperones (HSP90, HSP70, Hsp60, Hsp20, αB-Crystalline); 4) metabolic enzymes (β-enolase, fatty acid synthase, glyceraldehyde-3-phosphate dehydrogenase, peroxidases, pyruvate kinase); 5) proteins involved in exosome biogenesis (ESCRT complex, i.e. Tsg101, Alix); 6) signalling proteins (kinases, 14-3-3, GTPase Hras, RhoA, RAP1B, Guanine nucleotide-binding protein subunits -G proteins, etc.); 7) proteins involved in transcription and protein synthesis (transcription factors, histones, ribosomal proteins, ubiquitin, etc.), and 8) proteins involved in trafficking and membrane fusion (Annexins, Rab protein family, ARF).
In addition to a common set of proteins, it has been characterized that exosomes display a particular lipid organization and composition (See reviews elsewhere [18, 59, 60]). A recent unexpected finding showed that exosomes contain double-stranded DNA, mRNA and noncoding RNA (microRNA and lncRNA) [61-64]. Importantly, we and others have demonstrated that these exosomal mRNA and microRNA are functional in recipient cells [65-69]. This suggests that the exchange of exosome contents between cells may represent an effective and efficient inter-cellular communication. Furthermore, it is now known that protein and RNA sorting into exosomes is highly regulated by various patho-physiological stress stimuli and disease conditions [46, 64]. This allows cells to produce tailor-made exosomes with different functional characteristics, reflective of their parent cell status. In this regard, any stress or disease conditions may be mirrored in the contents of the exosomes, which can be used to develop future biomarkers for the diagnosis and prognosis of diverse cardiovascular disease.
3. Pathological Effects of Exosomes on Cardiac Remodeling
At present, exosomes have been implicated to mediate cell-to-cell communication through different mechanisms of interaction with recipient cells, including: 1) directly ligand-receptor interaction, resulting in activation of downstream signaling, 2) extracellular proteases cleave exosomal membrane proteins, releasing soluble ligands that bind to target receptors of recipient cells, 3) directly membrane fusion, leading to release exosomal contents into recipient cells, and 4) internalization of exosomes by endocytic mechanisms (phagocytosis, macropinocytosis or receptor-mediated endocytosis) [70]. Considering that the multiple properties of exosomes (reviewed above) and the secretion of exosomes from a variety of different cell types within the heart, it is reasonable to expect that exosomes are likely to be involved in many cardiovascular physiological and pathological disorders. However, the published studies on such issues are scarce. This may be ascribed to either lack of knowledge or some technical difficulties on exosomes. As a matter of fact, we only collect and summarize a small number of recent publications that investigated the roles of exosomes in cardiomyocyte hypertrophy, peripartum cardiomyopathy, diabetes-, and sepsis-induced cardiomyopathy, as reviewed below.
3.1 Exosomes in Cardiomyocyte Hypertrophy
It is now known that cardiac stress (i.e. systemic hypertension, cardiac valve disease, and myocardial infarction) can lead to cardiomyocyte hypertrophy, fibroblast proliferation, and secretion of extracellular matrix proteins and proinflammatory cytokines [71-73]. These fibroblast-derived mediators may act in either an autocrine or paracrine fashion between fibroblasts and cardiomyocytes to further stimulate cardiac remodeling [73, 74]. Notably, cardiomyocytes comprise only 56% (mouse), 30% (rat), and 28% (human) of all the cells in the healthy adult heart; whereas cardiac fibroblasts have been reported to make up 27% (mouse), 64% (rat), and 70% (human) of total cardiac cells [75]. Traditionally, cardiac fibroblasts are considered to play passive roles in the heart and to be solely responsible for maintaining homeostasis of extracellular matrix proteins, including type I and III collagens and fibronectin [76]. However, when co-cultured with cardiac fibroblasts or treated with conditioned fibroblast media, adult murine cardiomyocytes develop hypertrophy [77, 78]. This suggests that cardiac fibroblast may be active participants in cardiomyocyte remodeling, but the underlying mechanisms of fibroblast-cardiomyocyte communication are not yet fully understood.
Recently, Bang and co-workers [68] reported a unique mechanism in which cardiac fibroblasts promote the hypertrophic response of cardiomyocytes through exosomal transfer of miR-21* (Fig. 2). They firstly identified that cardiac fibroblast produce and secret exosomes. Using a RNA sequencing approach, these authors analyzed 388 rat miRNAs and 50 miRNAs were found to be enriched in exosomes derived from neonatal rat cardiac fibroblasts. Interestingly, they observed that 25.5% of fibroblast exosomal miRNAs were so called “star” miRNAs, the passenger (star) strand that is often degraded within a cell. In particular, the levels of miR-21* were significantly higher in exosomes, whereas miR-21 expression was higher in their donor fibroblasts, when compared to each other. This data indicates that miR-21* is selectively packaged into exosomes. Nonetheless, it is too early to conclude that miRNAs remain inside of donor cells, whereas miRNAs* may be kicked out of cells through exosomes. The most important finding by Bang and colleagues [68] is that, fibroblast-derived exosomal miR- 21* can be taken up by cardiomyocytes, which is dependent on temperature and actin. Of significance, such exosmal miR-21*, once released within cardiomyocytes, is functional and down-regulates the expression of SORBS2 (sarcoplasmic protein sorbin and SH3 domain-containing protein 2) and PDLIM5 (PDZ and LIM domain 5), leading to substantial increase in cardiomyocyte cell size. Moreover, the study by Bang et al., [68] may support previous findings showing that miR-21* was detected in pericardial fluid of mice with transverse aortic constriction-induced cardiac hypertrophy [79], thus confirming in-vivo that miR-21* plays a critical role in regulation of the cardiac fibroblast secretome and in determining a hypertrophic response.
Figure 2.
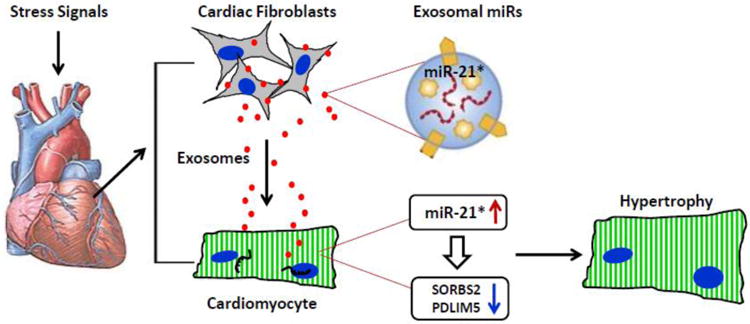
Under stress conditions, cardiac fibroblasts secret miR-21*-enriched exosomes, which are taken up by cardiomyocytes, leading to elevation of miR-21*. Consequently, the expression levels of SORBS2 and PDLIM5 are down-regulated in cardiomyocytes, resulting in cardiomyocyte hypertrophy.
Overall, under stress conditions, cardiac fibroblasts can promote an undesirable pathologic hypertrophy of cardiomyocytes through exosomes and their miRNA cargo. However, many questions remain unclear. For example, the development of fibroblast during cardiomyopathy involves multiple types of pro-fibrotic cells which may be derived from epithelial-mesenchymal transition (EMT), endothelial-mesenchymal transition (EndMT), perivascular cells, circulating monocytes/fibrocytes, or bone marrow originated progenitor cells. Therefore, it will need to clarify whether and how exosomes contribute to such a process of cardiac fibrosis. Given that cardiac fibroblast not only insulate myocyte bundles but also integrate to myocytes through connexin proteins, it will be an urgent need to address whether cardiac exosomes play a role in cardiac electrophysiology. In addition, it will need to investigate whether exosomes involve in both synthesis and degradation of extracellular matrix to form fibrosis in the heart.
3.2 Exosomes in Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a very dangerous pregnancy-associated cardiomyopathy that resides in a large population of women [80, 81]. It is characterized by sudden heart failure during the last month of pregnancy and/or in the first few months of postpartum. A 16-kDa N-terminal prolactin fragment (16K PRL), cleaved from the full-length nursing hormone prolactin (PRL) by cathepsin D, is believed to be a potential factor in initiating PPCM [82]. The underlying molecular mechanisms, however, remain obscure. Recently, Halkein et al. [69] reported that 16K PRL not only induced the expression of miR-146a in endothelial cells (ECs), leading to inhibition of angiogenesis, but also enhanced the release of miR-146a-enriched exosomes from ECs (Fig. 3). These endothelial exosomes can be taken up by cardiomyocytes, resulting in the elevation of miR-146a levels. Consequently, the expression of Erbb4, Notch1, and Irak1 (targets of miR-146a) was decreased in cardiomyocytes, leading to impaired metabolic activity and contractile function. These findings provide evidence that shows a miRNA-based intercellular communication system between ECs and cardiomyocytes via exosomes. Moreover, in situ hybridization in postpartum Stat3-knockout mouse hearts mainly detected miR-146a in ECs and other nonmyocyte cardiac cells, such as cardiac fibroblasts. This suggests that in addition to ECs, fibroblasts may also be a source for exosomal miR-146a in hearts exposed to 16K PRL.
Figure 3.
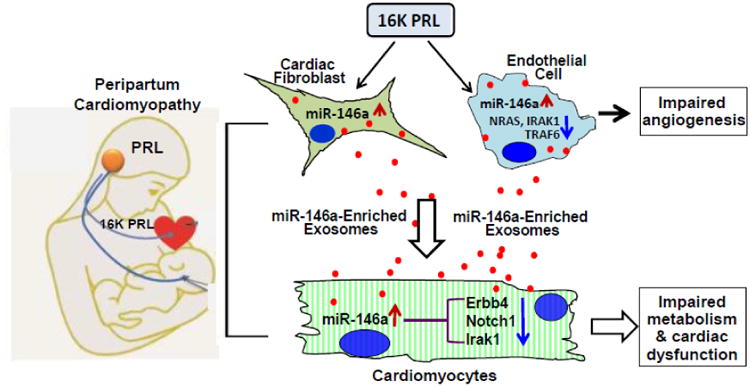
In peripartum cardiomyopathy (PPCM) patients, Cathepsin D cleaves nursing hormone prolactin (PRL) to generate an antiangiogenic 16-kDa fragment, 16K PRL. 16K PRL stimulates both cardiac fibroblasts and endothelial cells to release miR-146a-enriched exosomes, which transport miR-146a to cardiomyocytes. The exosome-mediated elevation of miR-146a in cardiomyocytes can down-regulate the expression of Erbb4, Notch1, and Irak1, leading to slowed metabolism and impaired contractile function in cardiomyocytes. In addition, 16K PRL stimulates endothelial cells to activate NF-κB, which up-regulates miR-146a expression, leading to decreased levels of NRAS, IRAK1 and TRAF6 and consequently, inhibiting angiogenesis.
Of interest, Halkein et al. [69] further observed that levels of exosomal miR-146a were significantly higher in plasma from patients with acute PPCM (n=38) than healthy postpartum controls (n=18) and patients with dilated cardiomyopathy (n=30). It is important to note here that in 12 PPCM patients who recovered after being treated with standard therapy for heart failure (beta blocker and ACE inhibitor) and 2.5-5.0 mg/d bromocriptine for 6–8 weeks, had the levels of circulating exosomal miR-146a restored to normal range. Thus, exosomal miR-146a may serve as a highly specific blood biomarker useful for diagnosis and risk stratification of patients with peripartum heart failure.
3.3 Exosomes in Diabetic Cardiomyopathy
Diabetes affects approximately 8% of the United States population and it is predicted that, over 400 million people by 2030 will have cases of diabetes [83]. Diabetes mellitus is a group of metabolic diseases characterized by high blood glucose either due to lack of (type 1) or resistance to (type 2) insulin [84]. Early in the course of diabetes, high glucose levels in the bloodstream can lead to endothelial dysfunction and microvascular rarefaction [85, 86]. Interestingly, insufficient myocardial angiogenesis is the major manifestation of diabetes-caused ischemic cardiovascular disease [87]. Within the mammalian heart, it is well recognized that endothelial cells play a critical role in cardiomyocyte survival and myocardial contraction [88]. However, in response to stress conditions (i.e. hyperglycemia), whether cardiomyocytes have an ability to affect endothelial cell function remains largely unknown. Recently, we and others have observed that exosomes derived from cardiomyocytes harbor a variety of mRNAs, miRNAs and proteins, which may be transferred to the adjacent endothelial cells and modulate their function[48, 49, 67, 89]. In particular, we showed that when cardiac endothelial cells (EC) were co-cultured with cardiomyocytes derived from type 2 diabetic Goto-Kakizaki (GK) rats, EC proliferation and migration were significantly inhibited, compared with EC culture alone [67]. By contrast, co-culture of ECs with cardiomyocytes isolated from healthy rats promoted EC proliferation and migration [67]. Thus, these data provide evidence that normal conditions of cardiomyocytes may benefit ECs, whereas diabetic myocytes may impair EC function (Fig. 4). Of interest, either beneficial or detrimental effects of cardiomyocytes on ECs were eliminated when treated with exosome inhibitor GW4869. Our further investigations revealed that exosomes derived from diabetic GK cardiomyocytes contain higher levels of miR-320, lower levels of miR-126 and Hsp20 proteins, compare with exosomes collected from healthy cardiomyocytes [67]. Importantly, the cardiomyocyte exosomal miR-320 can be transferred to endothelial cells and consequently, down-regulates the expression of IGF-1, Hsp20, and Ets-2, leading to inhibition of EC proliferation, migration and tube formation [67]. Therefore, our study may provide a novel mechanism underlying the impairment of myocardial angiogenesis in diabetes which may be caused by secretion of anti-angiogenic exosomes from cardiomyocytes (Fig. 4).
Figure 4.
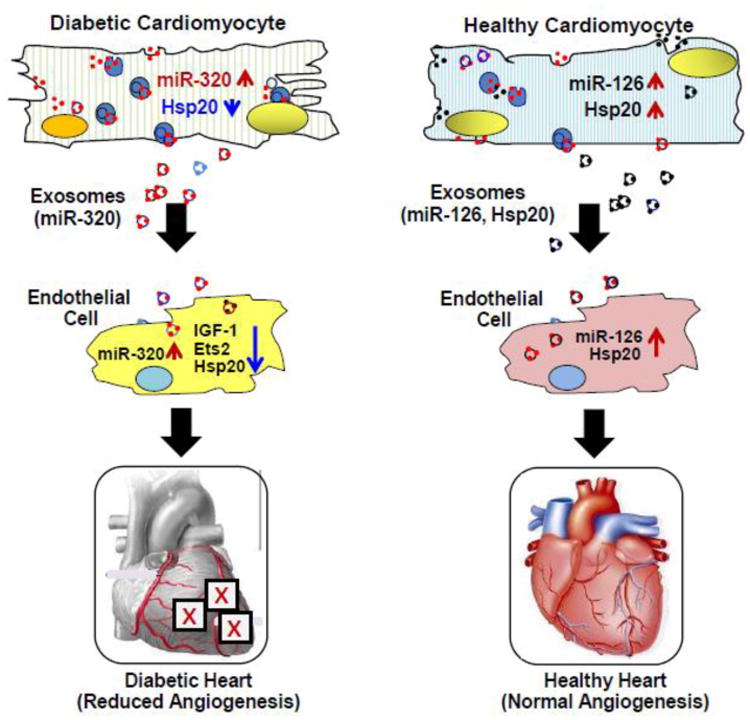
In type-2 diabetic rat hearts, miR-320 is up-regulated, whereas Hsp20 is down-regulated in cardiomyocytes. Accordingly, exosomes released from diabetic cardiomyocytes contain higher levels of miR-320, compared with those from healthy cardiomyocytes. The exosomal miR-320 is then transported to endothelial cells, resulting in decreased levels of IGF-1, Ets2 and Hsp20 and thereby, inhibiting angiogenesis in diabetic hearts. By contrast, healthy cardiomyocytes can secret miR-126- and Hsp20-enriched exosomes, which transfer miR-126 and Hsp20 to endothelial cells, leading to angiogenesis.
3.4 Exosomes in Septic Cardiomyopathy
Cardiovascular dysfunction is a major player contributing to mortality associated with sepsis, a disease initiated by local severe infection [90]. There is compelling evidence that myocardial depression is present in >40% of sepsis patients [91], and that exosomes may be involved [92]. Sepsis is triggered by a reactive immune response to an infection that can potentially result in mass deterioration of multiple organs [90]. Inflammation induced by sepsis results in blood clotting that restricts blood flow, thus causing a lack of supply of nutrients and oxygen to vital organs, like the heart. As a matter of fact, cardiovascular dysfunction is a major factor contributing to mortality associated with sepsis [91]. Numerous studies have shown that biventricular dilation and decrease in ejection fraction are associated with septic patients [91]. Much of prior work has implicated cytokines (IL-6 and TNFα) and reactive oxygen/nitrogen species (nitric oxide, superoxide, and peroxynitrite) as myocardial depressors [90]. However, several recent studies indicate that the presence of exosomes in the plasma of sepsis patients may cause vascular and cardiac dysfunction as well as coagulation in sepsis [51, 92, 93]. In particular, Gambim et al.[51] reported that in sepsis, both increased generation of nitric oxide (NO) and the presence of LPS can trigger the release of exosomes from platelets. These septic exosomes contain higher levels of NADPH oxidase, nitric oxide synthases (NOS) and protein disulfide isomerase (PDI) than healthy exosomes, as evidenced by western-blotting. Importantly, these authors observed that NO-induced and human septic platelet-derived exosomes induced caspase-3 activation and apoptosis of target endothelial cells through generation of active ROS/RNS by NADPH oxidase and NO synthase type II (Fig. 5). Azevedo et al. [92] further provided evidence that circulating platelet-derived exosomes from septic patients induced a decrease in myocardial contractility in isolated rabbit heart and rat papillary muscle preparations. These detrimental effects of exosomes were enhanced by pre-exposure to LPS. They confirmed that septic platelet-derived exosomes contain NOS, which can generate NO in exosomes and induce NO production in the heart. More recently, numerous studies have profiled circulating exosomal miRNAs in septic patients [63]. Specifically, the levels of exosomal miR-223 are significantly lower in the blood of non-survival septic patients, compared with survivors [94]. Of interest, reduced miR-223 may increase the expression of miR-223 targets (e.g. ICAM-1, IL-6, and IL-1β) [95-97]. Consequently, these increased target proteins may be enriched in septic exosomes and contribute to myocardial depression (Fig. 5). Put together, sepsis-induced cardiomyopathy may be mostly ascribed to both an increased number of exosomes and an intrinsic property of exosome (e.g. enriched NOS, reduced miR-223).
Figure 5.
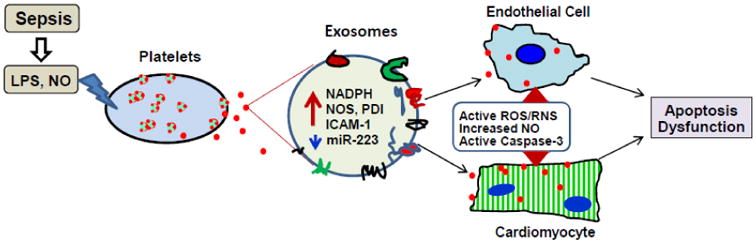
Sepsis can cause higher levels of lipopolysaccharide (LPS) and nitric oxide (NO) in the blood, which stimulate platelets to secret inflammatory exosomes. Such exosomes are enriched with NADPH, NOS, PGI, ICAM-1 and less miR-223, which activate ROS/RNS signaling pathways, increase NO production and activate Caspase-3 in both endothelial cells and cardiomyocytes, resulting in cell apoptosis and dysfunction and consequently, cardiomyopathy.
4. Protective Effects of Exosomes in the Myocardium
As reviewed above, exosomes derived from disease conditions elicit detrimental effects on cardiac remodeling. Recent studies have also demonstrated that exosomes collected from ischemic preconditioning or various stem cells provide cardioprotection, which may have therapeutic potential in the treatment of cardiovascular diseases. In the following sections, we will summarize recent findings on the role of stem cell-derived, stress-preconditioned and gene-modified cell-derived exosomes in cardiac protection including enhanced myocardial angiogenesis, reduced oxidative stress, limited inflammatory response, decreased cardiomyocyte death and myocardial infarction size (Fig. 6).
Figure 6.
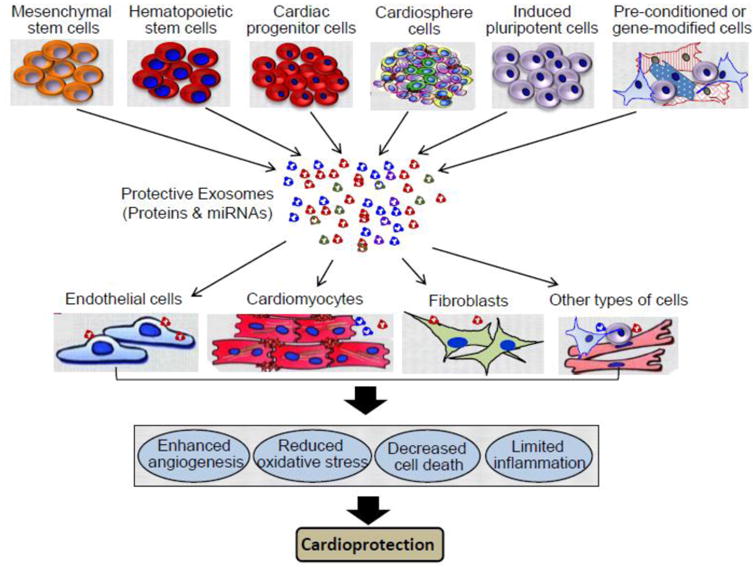
Multiple sources of stem cells, stress-preconditioned cells and gene-modified cells can yield cardio-protective exosomes. These protective exosomes, when injected into hearts, can interact with endothelial cells, cardiomyocytes, fibroblasts and other types of cells within hearts by endocytosis, receptor/ligand-mediated action, or membrane fusion, resulting in enhanced angiogenesis, reduced oxidative stress, decreased cell apoptosis/necrosis, and limited inflammatory response.
4.1 Mesenchymal Stem Cell-Derived Exosomes Confer Protection in Cardiovascular Diseases
Various kinds of stem cells (embryonic stem cells, adult stem cells, and induced pluripotent stem cells) have been extensively investigated for the therapeutic potential in cardiovascular disease [98-100]. It was previously thought that stem cell therapy acts to regenerate tissue through replication and then differentiation, but recent studies indicate that the beneficial effects of adult stem cells in the repair of cardiac tissue is through the release of paracrine and autocrine factors [101]. Indeed, stem cells can secret numerous types of factors/molecules, including proteins, microRNAs, growth factors, antioxidants, proteasomes, microvesicles and exosomes. In particular, exosomes has garnered a specific attention in cell-free-based stem cell therapy for cardiovascular disease.
Lai et al. [102] first showed that human ESC-derived mesenchymal stem cells (MSCs) secreted 50- to 100-nm membrane vesicles. Using an ex-vivo Langendorff model of ischemia/reperfusion injury, they observed that these purified exosomes were able to reduce infarct size in mouse hearts. Arslan et al. [103] further demonstrated that a single intravenous bolus of exosomes 5 min prior to reperfusion reduced infarct size by 45% in mice. Importantly, they found that exosome treatment restored energy depletion and redox state in mouse hearts within 30 min after I/R, evidenced by elevation of ATP and NADH levels, and reduction of oxidative stress. This can be interpreted that MSC-derived exosomes contain all five enzymes [namely glyceraldehyde 3-phosphate dehydrogenase (GAPDH), phosphoglycerate kinase (PGK), phosphoglucomutase (PGM), enolase (ENO) and pyruvate kinase m2 isoform (PKm2), see database available at www.exocarta.org] required for ATP generation. In addition, active CD73 (the major enzyme responsible for the formation of extracellular adenosine from released adenine nucleotides) and the phosphorylated PFKFB3 (which upregulates phosphofructose kinase) are also wrapped in MSC-derived exosomes [103]. Moreover, exosome treatment could reduce systemic inflammation in mice after myocardial I/R [103]. Therefore, MSC-derived exosomes may have powerful therapeutic potential for patients suffering from acute myocardial infarction.
Similarly, Lee et al.[104] reported that the anti-inflammatory activity in mouse bone marrow-derived mesenchymal stem cells (MSCs) is associated with exosomes. Using a murine model of hypoxia-induced pulmonary hypertension (HPH), these authors demonstrated that MCS-derived exosomes (MEX) delivery in-vivo suppressed HPH and vascular remodeling. Moreover, hyper-proliferative signals were also blocked by MEX treatment, as evidenced by the suppression of signal transducer and activator of transcription-3 (STAT3) phosphorylation, resulting in increased lung levels of miR-204, a microRNA enriched in distal pulmonary arterioles that is downregulated in both human PH and experimental models of disease. The data provided by Lee et al.[104] point to exosomes as the key effectors of MSC paracrine function. However, their work did not identify the critical components encased in these exosomes.
4.2 Hematopoietic Stem Cell-Derived Exosomes Promotes Myocardial Angiogenesis
It is previously hypothesized that hematopoietic stem cells (HSC) could repair cardiac tissue by differentiating into cardiomyocytes [105]. However, numerous studies have showed that few of the transplanted HSC survive and even fewer of the cells become cardiomyocytes [106]. Nonetheless, it has been observed that the transplantation of these HSCs improved cardiac function in both animal models and human patients [107-110]. This may be the result of a variety of paracrine factors including vascular endothelial growth factor (VEGF), FGF, HGF, and IGF, released from implanted HSCs. Recently, a study by Sahoo et al.[111] showed that human CD34+ stem cells had the ability to secret cup-shaped exosomes which expressed CD63, phosphatidylserine and TSG101. By the in vitro method, it was found that CD34+- exosomes replicated the angiogenic activity of the CD34+ cells by enhancing endothelial cell viability, proliferation and tube-like formation on Matrigel. In the in vivo studies, these authors observed that both CD34+-cells and CD34+-exosomes induced the formation of vessel-like endothelial structures, accompanied with significantly increased proportion of endothelial cells in the Matrigel plug. In the corneal angiogenesis assay, pellets containing CD34+-exosomes, but not CD34--exosomes, were associated with significantly greater vessel growth. Hence, these findings indicate that the CD34+ -exosomes are the key paracrine component of CD34+-cell– induced vessel growth. Nonetheless, the mechanisms underlying CD34+- exosome-mediated angiogenesis are not clearly understood. It could be ascribed to exosomal receptor-induced activation of angiogenic signaling cascades or the overall transfer of exosomal contents (proteins/RNAs) into the cytosol of endothelial cells. Indeed, Sahoo et al.[111] has presented data demonstrating that CD34+-exosomes are highly enriched with pro-angiogenic miR-126 and miR-130a, compared with CD34--exosomes. However, the extent to which these exosomal miRs are transferred and induce any molecular changes in the recipient cells remains to be clarified.
While the benefit of CD34+-cell therapy on functional recovery after ischemic injury could be induced primarily through the exosome-mediated transfer of angiogenic factors to surrounding cells, both viability and angiogenic quality of autologous CD34+-cells decline with advanced age [112, 113]. In this regard, Mackie et al. [114] recently attempted to genetically modify CD34+-cells with the sonic hedgehog (Shh) gene (CD34Shh) for enhancing their angiogenic quality. They observed that treatment with CD34Shh induced robust increases in capillary development within the infarct border zone, accompanied by reduced infarct sizes. Importantly, they found that CD34Shh deposit a greater amount of Shh in exosomes as compared with other Shh-modified cell types. Shh-containing exosomes derived from CD34Shh are capable of transferring Shh to other cell types (endothelial cells and fibroblasts) and thereby, activating the Shh signaling pathway. Thus, exosome-mediated delivery of Shh to ischemic myocardium may represent a major mechanism explaining the observed preservation of cardiac function in mice treated with CD34Shh cells.
4.3 Cardiac Progenitor Cells-Derived Exosomes Provide Cardioprotection
Cardiac progenitor cells (CPC) can also release exosomes into their environment and those exosomes contain matrix metalloproteinases (MMP) and extracellular matrix metalloproteinase inducer (EMMPRIN) that all aid in the breakdown of the extracellular matrix as well as activate MMP [115]. In addition, GTAT4-responsive miR-451 is highly enriched in these exosomes [116, 117]. As a matter of fact, Vrijen et al.[115] observed that CPC-derived exosomes can stimulate the migration of endothelial cells in an in vitro scratch assay. Chen et al. [116] found that in vivo delivery of CPC-exosomes in an acute mouse myocardial ischemia/reperfusion model inhibited cardiomyocyte apoptosis by about 53% in comparison with PBS control. Together, these studies implicate exosomes as the important cardioprotective component in CPC-mediated paracrine effects.
Currently, it is recognized that cardiac progenitor cells can be clonally expanded from murine and human myocardial biopsy specimens and form “spheres” in vitro which is referred to as cardiospheres (CSs) [118-121]. Because CSs contain both primitive cells and committed progenitors for the three major cell types present in the heart (cardiomyocytes, endothelial cells, and smooth muscle cells), they represent an attractive cell source for cardiac regeneration. Indeed, numerous studies have demonstrated that cardiosphere-derived cells (CDCs) can stimulate regeneration, angiogenesis, and functional improvement in the infarcted human heart [122-124]. Most recently, Ibrahim et al. [125] showed that CDC-derived exosomes can replicate CDC-induced therapeutic effects, and blockade of exosome production negates the beneficial effects of CDCs on the infarcted mouse hearts. They further identified miR-146a as being particularly enriched in CDC exosomes. Of interest, miR-146a may largely contribute to the protective effects of CDC-exosomes, but does not suffice to confer comprehensive therapeutic benefit alone. Hence, other miRNAs (i.e. miR-22, miR-24) in the repertoire may exert synonymous or perhaps synergistic effects with miR-146a. Overall, the data provided by Ibrahim et al [125] indicate that CDC-exosomes contain rich signaling information, and specifically the miRNAs transferred by these exosomes, that is capable of producing regeneration in a murine model of myocardial infarction (MI), and confer the same benefits as CDCs without transplantation of living cells.
4.4 Ischemic-Preconditioned (IPC) Exosomes Mediate Cardioprotection
It is well appreciated that ischemic preconditioning (IPC) elicits strong protective effects against cardiac ischemia/reperfusion (I/R)-induced injury [126. 127]. However, the underlying mechanism is still not completely understood. Recently, Giricz et al.[52] showed that coronary effluent collected from IPC rat hearts contained more extracellular vesicles (EVs, microvesicles and exosomes) than effluent from control hearts, as evidenced by Western blotting. Furthermore, they observed that infarct size was significantly smaller in hearts perfused with coronary effluent collected from IPC hearts than those hearts perfused with coronary effluent from control hearts. Of interest, infarct size in hearts perfused with EV-depleted IPC coronary effluent was similar to controls. Therefore, the study by Giricz et al. [52] indicates that microvesicles/exosomes released from IPC hearts are responsible for the transmission of protective signals in the heart. It is important to note here, a recent study by Li et al. [128] showed that cardio-protection elicited by remote ischemic preconditioning (rIPC) was associated with the elevation of circulating miR-144, which was not included in plasma micropaticles/microvesicles (50-400 nm), but incorporated with Argonaute 2. Nonetheless, they observed that the hairpin precursor miR-144 was 4-fold increase in the exosome pellet derived from rIPC plasma [128]. Similarly, Jeanneteau et al. [129] recently observed that microvesicles derived from the blood of animals underwent hind limb ischemia/reperfusion failed to decrease myocardial infarct size in rats. Put together, these studies suggest that exosomes rather than microvesicles (microparticles) contribute to the propagation of cardioprotective signals. Further studies will definitely be needed to elucidate the mechanisms underlying the IPC-exosome-mediated cardioprotection, using a clinically relevant in vivo model.
Recently, Feng et al. [130] performed a microRNA array for profiling the miRNAs in exosomes released from ischemic-preconditioned mesenchymal stem cells (MSCs). These MSCs were isolated from mouse bone marrow and subjected to two cycles of anoxia (30 min)-reoxygenation (10 min) as ischemic preconditioning (IPC). Feng et al. [130] observed that the levels of miR-22 were 4.5-fold higher, accompanied with miR-21 (3.3-fold), miR-210 (3.1-fold), miR-199a-3p (2.8-fold), and miR-24(2.5-fold) in exosomes collected from IPC-MSCs (ExoIPC) than control exosomes (Exonon-IPC). Importantly, in vivo treatment of mice with ExoIPC by directly injection into infarcted hearts resulted in a significant reduction of cardiac fibrosis and apoptosis, compared with Exonon-IPC-treated hearts. Furthermore, these authors identified that the ExoIPC-mediated protective effects are largely associated with transfer of exosomal miR-22 to surrounding cells. However, whether these preconditioned exosomes contain other beneficial proteins or ligands/receptors remains unclear.
5. Conclusions and Future Studies
Over the past decade, exosomes have been extensively studied as miRNA-carriers, as biomarkers for diagnosis of disease, and as a critical tool for cell-to-cell communication. However, their physiological and pathological roles in cardiovascular diseases are still vague. As reviewed above, exosomes derived from different conditions may contain different functional factors, which decide their properties; either harmful or beneficial (Tables 1 & 2). Most studies consistently implicate that disease messages may be spread around the body through exosomes (pathological role). But, we do not know whether specifically blockade of exosome production during disease is able to have therapeutic effects. Additionally, while it is well appreciated that stem cell-derived exosomes have therapeutic potential in cardiovascular disease, the in vivo mechanisms underlying the exosome-elicited action are not clear yet. Of interest, Yuan et al. [131] recently observed that only certain miRNAs wrapped in exosomes/microvesicles were efficiently transferred to recipient cells, which raises the possibilities that the transfer of miRNAs is selective and may be regulated by proteins located at either exosomes or recipient cells. Future studies will be needed to test this hypothesis. Moreover, the ability of exosomes to transfer lipid, RNA, and proteins raises very exciting possibilities for future therapeutic uses. For example, based on the regulatory effects of miR on therapeutic proteins [132, 133], we could engineer host cells to overexpress a specific miR that is capable to be delivered via exosomes. These engineered cells can be either encapsulated to provide sustained local delivery or utilized as “factory” to produce therapeutic exosomes. The latter may be used as viral-free or cell-free agents in replacement of viral vectors or intact cells, which offers a safer approach for clinical application. It is also possible that the pathologic exosomes can be re-uploaded and be able to replace the harmful factors with beneficial ones. Overall, further understanding of pathways involved in exosome biogenesis, exosomal cargo loading, exosome secretion and exosome uptake will be of fundamental importance to address how to alter the quality and quantity of exosomes, as well as therapeutic efficacy for the treatment of cardiovascular disease.
Table 1. Pathologic Effects of Exosomes in the Heart.
| Exosome Sources | Key Findings (Function of Exosomes) | Cargo Responsible For Effect | Refs |
|---|---|---|---|
| Neonatal rat cardiac fibroblasts | Promote cardiomyocyte hypertrophy | miR-21* | [68] [133] |
| Goto-Kakizaki (GK) rat cardiomyocytes | Inhibit myocardial endothelial cell promotion, migration and tube formation | miR-320 | [67] |
| Platelets from patients with sepsis | Induce endothelial cell apoptosis and cardiac dysfunction | NADPH, NOS, PDI | [92] [93] |
| 16K PRL-treated HUVECs | Reduce metabolic activity in cardiomyocytes | miR-146a | [69] |
| Heart and plasma from patients with acute peripartum cardiomyopathy | miR-146a-enriched exosomes are associated with dilated with cardiomyopathy | miR-146a | [69] |
Table 2. Protective Effects of Exosomes in the Heart.
| Exosome Sources | Key Findings (Function of Exosomes) | Cargo Responsible For Effect | Refs |
|---|---|---|---|
| Human CD34+ cells; Shh-modified CD34+ cells | Promote angiogenesis and reduce infarct size in mice after acute myocardial infarction | miR-126, miR-130a, Shh protein | [111] [114] |
| Human ES9.E1-derived MSCs | In myocardial ishcmic-reperfused mice, exosome-treated animals exhibited significant preservation of left ventricular geometry and contractile performance during 28 days follow-up. | GAPDH, PGK, PGM, ENO, PKm2, phosphorylated PFKFB3 | [102] [103] |
| Human cardiac progenitor cells (CPCs) | Inhibit cardiomyocyte apoptosis and improve cardiac function after injection of exosomes into rat infarcted hearts. | miR-210, miR-132, miR-146a-3p | [134] |
| Human foetal heart – derived progenitor cells | Enhance the migration of human microvascular endothelial cells (HMECs), using in vitro scratch wound assay. | MMPs and EMMPRIN | [115] |
| Human cardiosphere-derived cells (CDCs) | Injection of exosomes into injured mouse hearts recapitulates the regenerative and functional effects produced by CDC transplantation. | miR-146a, miR-22, miR-24, miR-210 | [125] |
| Mouse mesenchymal stem cells subjected to two cycles of anoxia (30 min) and re-oxygenation (10min) (IPC). | In vivo delivery of IPC exosomes ameliorates fibrosis and reduces infarction sizes in mouse myocardial infarction model. | miR-21, miR-22, miR-24, miR-199a-3p, miR-210 | [130] |
| Mouse mesenchymal stromal cells | In the murine model of hypoxic pulmonary hypertension, intravenous delivery of exosomes inhibits vascular remodeling and hypoxic pulmonary hypertension. | miR-17 superfamily, miR-204 | [104] |
| Rat hearts upon three cycles of global ischemia (5 min) and reperfusion (5 min, IPC) in Langendorff mode. | Exosomes collected from perfusates of IPC hearts attenuate infarction size in non-preconditioned recipient hearts. | N/A (not available) | [52] |
Highlights.
Secretion of exosomes from living cells occurs in normal physiological conditions.
Secretion of exosomes is increased in response to stress or pathological conditions.
Pathological exosomes may be harmful for cardiac remodeling through the transfer of exosomal contents.
Exosomes released from stem cells are protective in the heart.
Exosomes collected ischemic-preconditioned cells/tissues are protective in the heart.
Acknowledgments
The research in Dr. Guo-Chang Fan's lab is supported by NIH grant 2R01-087861.
Footnotes
Conflict of interest: The authors confirm that this article content has no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, et al. Executive summary: heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Ieda M. Heart development and regeneration via cellular interaction and reprogramming. Keio J Med. 2013;62:99–106. doi: 10.2302/kjm.2012-0020-re. [DOI] [PubMed] [Google Scholar]
- 3.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–37. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc Med. 2009;19:182–90. doi: 10.1016/j.tcm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lionetti V, Bianchi G, Recchia FA, Ventura C. Control of autocrine and paracrine myocardial signals: an emerging therapeutic strategy in heart failure. Heart Fail Rev. 2010;15:531–42. doi: 10.1007/s10741-010-9165-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Su J, Mende U. Cross talk between cardiac myocytes and fibroblasts:from multiscale investigative approaches to mechanisms and functional consequences. Am J Physiol Heart Circ Physiol. 2012;303:H1385–96. doi: 10.1152/ajpheart.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab. 2012;303:E937–49. doi: 10.1152/ajpendo.00061.2012. [DOI] [PubMed] [Google Scholar]
- 10.Tian Y, Morrisey EE. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ Res. 2012;110:1023–34. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doroudgar S, Glembotski CC. The cardiokine story unfolds: ischemic stress-induced protein secretion in the heart. Trends Mol Med. 2011;17:207–14. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pentassuglia L, Sawyer DB. ErbB/integrin signaling interactions in regulation of myocardial cell-cell and cell-matrix interactions. Biochim Biophys Acta. 2013;1833:909–16. doi: 10.1016/j.bbamcr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Current Angiogenesis. 2013;2:54–59. doi: 10.2174/22115528113020020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis. 2011;1:138–149. [PMC free article] [PubMed] [Google Scholar]
- 15.Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res. 2014;102:302–11. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 16.Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–64. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 17.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles – Endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;7:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 20.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for xternalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;7:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;7:9412–9420. [PubMed] [Google Scholar]
- 22.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;7:1844–1851. [PubMed] [Google Scholar]
- 23.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108–20. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012;1826:103–11. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol. 2014;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29C:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JM, Gould SJ. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans. 2013;41:277–82. doi: 10.1042/BST20120275. [DOI] [PubMed] [Google Scholar]
- 29.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–94. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–65. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 31.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F, Powis SJ. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136:192–7. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guescini M, et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316:1977–84. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Romancino DP, Paterniti G, Campos Y, De Luca A, Di Felice V, d'Azzo A, Bongiovanni A. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013;587:1379–84. doi: 10.1016/j.febslet.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 35.Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 36.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 37.Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505–15. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 38.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–32. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal MJ, Stahl PD. The small GTP-binding proteins Rab4 and ARF are associated with released exosomes during reticulocyte maturation. Eur J Cell Biol. 1993;60:261–7. [PubMed] [Google Scholar]
- 40.Ekström EJ, Bergenfelz C, von Bülow V, Serifler F, Carlemalm E, Jönsson G, Andersson T, Leandersson K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer. 2014;13:88. doi: 10.1186/1476-4598-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214–27. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 42.Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–90. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 43.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–33. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 44.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–9. [PubMed] [Google Scholar]
- 45.Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011;186:2219–28. doi: 10.4049/jimmunol.1002991. [DOI] [PubMed] [Google Scholar]
- 46.de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1:18396. doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–99. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan GC. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One. 2012;7:e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304:H954–65. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–6. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 51.Gambim MH, do Carmo Ade O, Marti L, Veríssimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á, Buzás EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–8. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Higashijima Y, Sonoda H, Takahashi S, Kondo H, Shigemura K, Ikeda M. Excretion of urinary exosomal AQP2 in rats is regulated by vasopressin and urinary pH. Am J Physiol Renal Physiol. 2013;305:F1412–21. doi: 10.1152/ajprenal.00249.2013. [DOI] [PubMed] [Google Scholar]
- 54.Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583:3817–3826. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 57.Kim DK, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20384. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–71. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 59.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–99. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 60.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 61.Thakur BK, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–9. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gezer U, Ozgür E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38:1076–9. doi: 10.1002/cbin.10301. [DOI] [PubMed] [Google Scholar]
- 63.Essandoh K, Fan GC. Role of extracellular and intracellular microRNAs in sepsis. Biochim Biophys Acta. 2014;1842:2155–2162. doi: 10.1016/j.bbadis.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Sorting it out: Regulation of exosome loading. Semin Cancer Biol. 2014 doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 66.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bang C, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–46. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halkein J, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123:2143–54. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306:C621–33. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 71.Oka T, Komuro I. Molecular mechanisms underlying the transition of cardiac hypertrophy to heart failure. Circ J. 2008;72(suppl A):A13–A16. doi: 10.1253/circj.cj-08-0481. [DOI] [PubMed] [Google Scholar]
- 72.Fan GC, Kranias EG. Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J Mol Cell Cardiol. 2011;51:574–7. doi: 10.1016/j.yjmcc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abramochkin DV, Lozinsky IT, Kamkin A. Influence of mechanical stress on fibroblast-myocyte interactions in mammalian heart. J Mol Cell Cardiol. 2014;70:27–36. doi: 10.1016/j.yjmcc.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–53. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Amerongen MJ, Engel FB. Features of cardiomyocyte proliferation and its potential for cardiac regeneration. J Cell Mol Med. 2008;12:2233–44. doi: 10.1111/j.1582-4934.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107:1304–12. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol. 2005;202:891–899. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 78.LaFramboise WA, et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol. 2007;292:C1799–C1808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 79.Queirós AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, Sanchez Ruderisch H, Regitz-Zagrosek V. Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int J Cardiol. 2013;169:331–8. doi: 10.1016/j.ijcard.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Hilfiker-Kleiner D, Sliwa K, Drexler H. Peripartum cardiomyopathy: recent insights in its pathophysiology. Trends Cardiovasc Med. 2008;18:173–179. doi: 10.1016/j.tcm.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Regitz-Zagrosek V, Eghbali M. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2:192–207. [PMC free article] [PubMed] [Google Scholar]
- 82.Hilfiker-Kleiner D, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 83.Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to Calculate the Impact of Obesity and Diabetes on Cost and Prevalence of Urolithiasis in 2030. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raslova K. An update on the treatment of type 1 and type 2 diabetes mellitus: focus on insulin detemir, a long-acting human insulin analog. Vasc Health Risk Manag. 2010;6:399–410. doi: 10.2147/vhrm.s10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005;1:59–63. doi: 10.2174/1573399052952550. [DOI] [PubMed] [Google Scholar]
- 86.Cohen G, Riahi Y, Alpert E, Gruzman A, Sasson S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch Physiol Biochem. 2007;113:259–67. doi: 10.1080/13813450701783513. [DOI] [PubMed] [Google Scholar]
- 87.Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbadis.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247–88. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7:163–83. doi: 10.2174/157340311798220494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azevedo LC, Janiszewski M, Pontieri V, Pedro Mde A, Bassi E, Tucci PJ, Laurindo FR. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care. 2007;11:R120. doi: 10.1186/cc6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit Care Med. 2004;32:818–25. doi: 10.1097/01.ccm.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L. Serum microRNA signatures identified by Solexa sequencing predict sepsis patients' mortality: a prospective observational study. PLoS One. 2012;7:e38885. doi: 10.1371/journal.pone.0038885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Huang W, Yang Y, Wang Y, Peng T, Chang J, Caldwell CC, Zingarelli B, Fan GC. Loss of duplex miR-223 (5p and 3p) aggravates myocardial depression and mortality in polymicrobial sepsis. Biochim Biophys Acta. 2014;1842:701–11. doi: 10.1016/j.bbadis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taïbi F, Metzinger-Le Meuth V, Massy ZA, Metzinger L. miR-223: An inflammatory oncomiR enters the cardiovascular field. Biochim Biophys Acta. 2014;1842:1001–9. doi: 10.1016/j.bbadis.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 97.Tabet F, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van den Akker F, Deddens JC, Doevendans PA, Sluijter JP. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2013;1830:2449–58. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 99.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011;51:619–25. doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iglesias-García O, Pelacho B, Prósper F. Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling. J Mol Cell Cardiol. 2013;62:43–50. doi: 10.1016/j.yjmcc.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 101.Makridakis M, Roubelakis MG, Vlahou A. Stem cells: insights into the secretome. Biochim Biophys Acta. 2013;1834:2380–4. doi: 10.1016/j.bbapap.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 102.Lai RC, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Arslan F, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–11. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burt R, Pearce W, Luo K, Oyama Y, Davidson C, Beohar N, Gheorghiade M. Hematopoietic stem cell transplantation for cardiac and peripheral vascular disease. Bone Marrow Transplant. 2003;32(Suppl 1):S29–31. doi: 10.1038/sj.bmt.1704177. [DOI] [PubMed] [Google Scholar]
- 106.Fukuda K, Fujita J. Mesenchymal, but not hematopoietic, stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction in mice. Kidney Int. 2005;68:1940–3. doi: 10.1111/j.1523-1755.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 107.Balsam LB, Robbins RC. Haematopoietic stem cells and repair of the ischaemic heart. Clin Sci (Lond) 2005;109:483–92. doi: 10.1042/CS20050087. [DOI] [PubMed] [Google Scholar]
- 108.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–85. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Kajstura J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 110.Balmer GM, Riley PR. Harnessing the potential of adult cardiac stem cells: lessons from haematopoiesis, the embryo and the niche. J Cardiovasc Transl Res. 2012;5:631–40. doi: 10.1007/s12265-012-9386-3. [DOI] [PubMed] [Google Scholar]
- 111.Sahoo S, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–8. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li TS, Kubo M, Ueda K, Murakami M, Mikamo A, Hamano K. Impaired angiogenic potency of bone marrow cells from patients with advanced age, anemia, and renal failure. J Thorac Cardiovasc Surg. 2010;139:459–65. doi: 10.1016/j.jtcvs.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 113.Madonna R, Renna FV, Cellini C, Cotellese R, Picardi N, Francomano F, Innocenti P, De Caterina R. Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur J Clin Invest. 2011;41:126–33. doi: 10.1111/j.1365-2362.2010.02384.x. [DOI] [PubMed] [Google Scholar]
- 114.Mackie AR, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–21. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–70. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–71. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, Jegga AG, Fan GC. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol. 2010;49:841–50. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 119.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R, Marbán E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Machida M, Takagaki Y, Matsuoka R, Kawaguchi N. Proteomic comparison of spherical aggregates and adherent cells of cardiac stem cells. Int J Cardiol. 2011;153:296–305. doi: 10.1016/j.ijcard.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 121.Chimenti I, Gaetani R, Barile L, Forte E, Ionta V, Angelini F, Frati G, Messina E, Giacomello A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol Biol. 2012;879:327–38. doi: 10.1007/978-1-61779-815-3_19. [DOI] [PubMed] [Google Scholar]
- 122.Cheng K, et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail. 2014;2:49–61. doi: 10.1016/j.jchf.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sousonis V, Nanas J, Terrovitis J. Cardiosphere-derived progenitor cells for myocardial repair following myocardial infarction. Curr Pharm Des. 2014;20:2003–11. doi: 10.2174/13816128113199990445. [DOI] [PubMed] [Google Scholar]
- 124.Kreke M, Smith RR, Marbán L, Marbán E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev Cardiovasc Ther. 2012;10:1185–94. doi: 10.1586/erc.12.102. [DOI] [PubMed] [Google Scholar]
- 125.Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301:H1723–41. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Zhu H, Zhang X, Liu Y, Chen J, Medvedovic M, Li H, Weiss MJ, Ren X, Fan GC. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovasc Res. 2012;94:379–90. doi: 10.1093/cvr/cvs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 129.Jeanneteau J, Hibert P, Martinez MC, Tual-Chalot S, Tamareille S, Furber A, Andriantsitohaina R, Prunier F. Microparticle release in remote ischemic conditioning mechanism. Am J Physiol Heart Circ Physiol. 2012;303:H871–7. doi: 10.1152/ajpheart.00102.2012. [DOI] [PubMed] [Google Scholar]
- 130.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Curcio A, et al. MicroRNA-1 downregulation increases connexin 43 displacement and induces ventricular tachyarrhythmias in rodent hypertrophic hearts. PLoS One. 2013;8:e70158. doi: 10.1371/journal.pone.0070158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Indolfi C, Curcio A. Stargazing microRNA maps a new miR-21 star for cardiac hypertrophy. J Clin Invest. 2014;124:1896–8. doi: 10.1172/JCI75801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–41. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]


