Abstract
Objectives:
Body composition assessment in chronic obstructive pulmonary disease (COPD) is important, as weight loss and muscular wasting are responsible for low exercise capacity in these patients, and low body mass index (BMI) and fat free mass index (FFMI) are important prognostic factors. Our study aims were: (a) to describe body composition in COPD patients referred to a pulmonary rehabilitation center in Bucharest; (b) to examine the relationships between body composition and disease severity (bronchial obstruction, exercise capacity, quality of life); (c) to test if segmental wasting of lower limbs muscle mass (measured by segmental body composition analysis) correlates with decreased exercise capacity.
Material and methods:
We studied 36 consecutive COPD patients referred to our clinic for pulmonary rehabilitation. Patients performed pulmonary function tests, six minutes walking test (6MWT), and health status was evaluated with COPD Assessment Test (CAT). Body composition measurements were performed by direct segmental multi-frequency bioelectrical impedance analysis (BIA).
Outcomes:
This study offers the first data on body composition of Romanian COPD patients
The prevalence of nutritional depletion (defined by low BMI and/or low FFMI) among our COPD patients was 22.2%. Mean FFMI was significantly lower in normal or underweight patients versus overweight or obese patients. Patients with low FFMI had lower exercise capacity at the 6MWT and higher CAT scores than patients with normal FFMI.
Depending on the BMI and FFMI values the patients were divided in four categories: normal, semistarvation, sarcopenia and cachexia. The group of patients with sarcopenia (low FFMI and normal BMI) had the lowest mean MIP (Maximal Inspiratory Pressure), the lowest mean 6MWD (six minutes walking distance) and the higher CAT mean scores among all groups. Exercise capacity was significantly lower in muscular depleted patients (with low skeletal muscle mass index - SSMI). MIP correlated significantly with FFMI and SMMI. No correlations were found between parameters of body composition and FEV1 or CAT. Segmental body composition assessment revealed that unbalanced upper/lower skeletal muscle mass is associated with a lower exercise capacity as measured by 6WMT.
Conclusions:
This study offers the first data on body composition of Romanian COPD patients. The prevalence of nutritional depletion is similar to that found in other European studies. No significant correlations were found between FFMI and severity of the disease (bronchial obstruction, distance walked, CAT score). FFMI and SSMI correlated significantly with MIP. Sarcopenic patients had the lowest mean 6MWD, the lowest mean MIP and the highest CAT mean scores. SMMI significantly correlated with 6MWD. Segmental body composition assessment of revealed that "unbalanced" patients had lower results at 6MWT. These results show that body composition evaluation is useful for the assessment of COPD patients referred to pulmonary rehabilitation and should be routinely performed
Keywords: body composition, chronic obstructive pulmonary disease, pulmonary rehabilitation
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a disease with multiple systemic effects. Among them, weight loss and muscular wasting are responsible for low exercise capacity (1). Body composition is therefore important in the assessment of COPD patients, as well as exercise capacity and muscular strength. Body mass index (BMI) is an independent prognostic factor in COPD (2,3), but the negative effect of low body weight on survival can be reversed by appropriate therapy in some of COPD patients (2). Body mass can be divided into two types: fat mass and fat free mass (FFM) which can be directly measured by bioelectrical impedance analysis (BIA). Fat free mass index (FFMI = FFM/height2) is a strong predictor of peripheral skeletal muscle weakness (4), exercise capacity (5-7), reduced health status (8), and mortality (9).
Low BMI and involuntary weight loss are frequent in COPD patients, with a wide prevalence range depending on the selection criteria of patients, as well as on the severity of the disease. Cachexia (low BMI and low FFMI) is found in 5-15% of patients with congestive heart failure and COPD (10); due to COPD prevalence, COPD cachexia is the most frequent cachexia subtype, and mortality rates of these patients range from 10% to 15% per year (10). Although loss of FFM is more frequent in patients with severe COPD or with hypoxemia, data regarding the correlation of FFM with bronchial obstruction are controversial (11,12). FFMI of COPD patients correlates with their exercise capacity expressed as six minutes walking distance (6MWD) (12,9). Sarcopenia in COPD is defined as reduced FFM with normal or increased body weight (13). These patients are more severely disabled than those with low BMI and normal FFMI (14). Tissue depletion is an important determinant of health status independent of exercise capacity and dyspnoea (8). Assessment of FFM is important in patients referred to pulmonary rehabilitation, as exercise training results in a significant gain in FFM in normal-weight COPD (15).
There are no available data regarding body composition in Romanian COPD patients.
Our study aims were:
to describe body composition in COPD patients referred to a pulmonary rehabilitation center in Bucharest
to examine the relationships between body composition and disease severity (bronchial obstruction, exercise capacity, quality of life)
to test if segmental wasting of lower limbs muscle mass (measured by segmental body composition analysis) correlates with decreased exercise capacity. ❑
METHODS
We studied 36 consecutive COPD patients referred to our clinic for pulmonary rehabilitation. All patients were in a stable state. Patients with cardiac failure class III or IV NYHA, neoplasic disease and hyperthyroidism were excluded, as these can be causes of weight loss. Cardiac comorbidities (arterial hypertension, atrial fibrillation, coronary heart disease) and history of COPD exacerbations were recorded from medical files; patients with at least two COPD exacerbations in the last year were defined as frequent exacerbators.
Weight and height were assessed with barefoot patients wearing light clothes. Pulmonary function tests were performed with Quark PFT Cosmed and included spirometry, lung volumes, diffusion tests, maximal inspiratory and expiratory pressures (MIP and MEP). Health status was evaluated with COPD Assessment Test (CAT) - Romanian version (16). Exercise capacity was assessed by the 6-minutes-walking test (6MWT); each patient performed at least two tests and the best results for the distance walked (6MWD) were expressed as absolute values (meters) and as a percentage of predicted values (17).
Body composition measurements were performed by direct segmental multi-frequency bioelectrical impedance analysis (BIA) using the Inbody 720 Body Composition Analyzer (Biospace Co. Ltd.), with patients standing (18). Parameters of body composition included: body mass index (BMI), fat free mass (FFM), fat free mass index FFMI (FFM/height2), percent of body fat (PBF), body fat mass (BFM), visceral fat area (VFA), skeletal muscle mass (SMM), skeletal muscle mass index (SMMI - SMM/height2), segmental lean mass (lean mass right arm - LMRA, lean mass left arm - LMLA, lean mass trunk, lean mass right leg – LMRL and lean mass left leg – LMLL, expressed as kilograms or as percentage of normal) and upper/lower body balance (unbalanced defined as more than 20% weighted difference in percentages of normal between upper and lower limbs lean mass; the weights are based on 80-120% and 90-110% normal ranges for upper and lower limbs respectively). Skeletal muscle mass is computed based on muscle mass of the limbs, which is mostly composed of skeletal muscle, comprising about 70% of total body skeletal muscle. The cut-offs used for low BMI (≤21 kg/m2) and low FFMI (≤16 kg/m2 in men and ≤15 kg/m2 in women) were recommended by ERS/ATS guidelines for pulmonary rehabilitation (19). Normal or altered body composition were defined in 4 categories using the criteria defined by Schols (14,20):
normal body composition: BMI >21 and FFMI >16 (men) or >15 (women)
semistarvation: BMI ≤21 and FFMI >16 (men) or >15 (women)
muscle atrophy/sarcopenia: BMI >21 and FFMI ≤16 (men) or ≤15 (women)
cachexia: BMI ≤21 and FFMI ≤16 (men) or ≤15 (women).
Patients were divided in two categories: muscular depleted or normal, based on the cut-off for SMMI used by Janssen (21) to define moderate or severe disability: SMMI lower than 6.75 in women and 10.75 in men.
The study was approved by the local Ethics Committee.
Statistics: Data were analyzed with SPSS for Windows. Comparisons between groups stratified by body composition or disease severity were evaluated by Mann-Whitney analysis. Correlations were assessed with Spearman test. A p-value lower than 0.05 was considered statistically significant. ❑
RESULTS
The characteristics of the patients are displayed in Table 1 and the results of body composition assessment in Table 2.
Table 1.
Characteristics of the patients
| Mean value | Standard deviation | Range | |
|---|---|---|---|
| Age (years) | 65.6 | 7.5 | 51 – 79 |
| FEV1 (% of predicted) | 50.7 | 14.5 | 30 – 80 |
| FEV1 (liters) | 1.4 | 0.4 | 0.8 – 2.5 |
| MIP (cm H2O) | 49.6 | 15.8 | 17 - 83 |
| MEP (cm H2O) | 74 | 24.4 | 24 - 127 |
| 6MWD (meters) | 385.2 | 102.8 | 145 – 536 |
| 6MWD (% of predicted) | 75.9 | 19.2 | 30.7 – 100.1 |
| CAT (points) | 18.3 | 8.2 | 6 – 40 |
| Number of subjects (percent) | |||
|---|---|---|---|
| Male / female | 33 / 3 | ||
| GOLD classes I / II / III / IV | 0 / 14 / 15 / 7 | ||
| Frequent exacerbators | 7 (19.4%) | ||
| Cardiac comorbidities | 22 (61.1%) | ||
| Resting hypoxemia (SaO2 <90%) | 4 (13.9%) | ||
Table 2.
Body composition measurements
| Characteristics | Mean value | Standard deviation | Range |
|---|---|---|---|
| BMI (kg/m2) | 27.5 | 6.1 | 18-34.2 |
| FFM (kg) | 53.3 | 8.4 | 37-71.7 |
| FFMI (kg/m2) | 18.5 | 2.3 | 13.8-23.7 |
| SMM (kg) | 29.1 | 5.3 | 19.5-39.7 |
| SMMI (kg/m2) | 10.1 | 1.5 | 6.3-13.2 |
| BFM (kg) | 26.0 | 12.1 | 6.0-54.9 |
| PBF (%) | 31.4 | 9.4 | 10.5-45.4 |
| LMRA (kg and % of predicted) | 3.1 (107.1%) | 0.7 | 1.8-4.8 |
| LMLA (kg and % of predicted) | 3.1 (105.2%) | 0.7 | 1.8-4.8 |
| LMRL (kg and % of predicted) | 8.2 (88.1%) | 1.3 | 5.8-11.0 |
| LMLL (kg and % of predicted) | 8.1 (89.3%) | 1.3 | 5.8-10.8 |
| Balanced / unbalanced (number of subjects) | 11 / 25 | ||
Five patients had low BMI (13.9%). Among the remaining 31 patients, 17 patients were overweight (8 patients) or obese (9 patients, 25%). Six patients had low FFMI (16.7%), 3 with low BMI and 3 with normal BMI. The prevalence of nutritional depletion among our COPD patients was 22.2%, as 8 patients had low BMI and/or low FFMI.
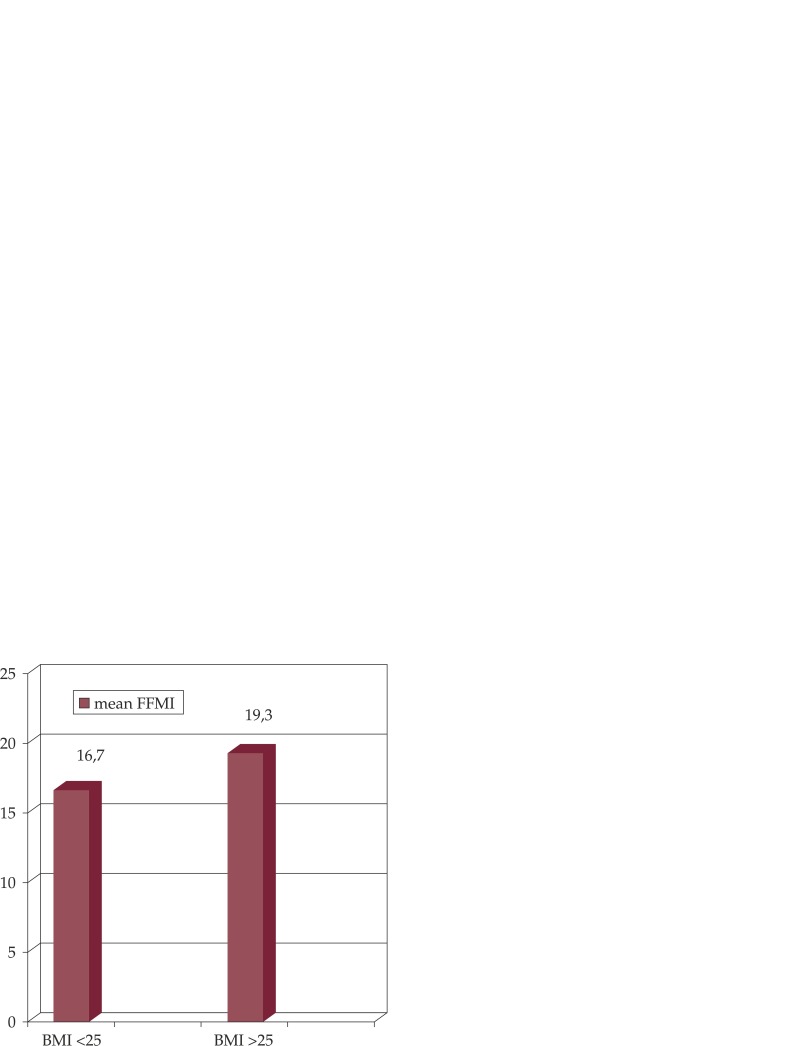
None of the patients who were overweight or obese had low FFMI; mean FFMI was significantly lower in normal or underweight patients versus overweight or obese patients (16.7 ± 1.6 versus 19.3 ± 2.2, p = 0.001) (Figure 1).
Figure 1. Fat-free mass index (FFMI, kg/m2) in normal or underweight patients (BMI ≤25 kg/m2) versus overweight or obese patients (BMI >25 kg/m2), p=0.001.
a. GOLD classes
Patients were divided in GOLD categories of severity using FEV1 values, as follows: GOLD 2 - 14 patients (38.9%), GOLD 3 - 15 patients (41.7%) and GOLD 4 - 7 patients (19.4%). There were no patients in GOLD I category. The characteristics of the patients of the three GOLD categories are illustrated in Table 3.
Table 3.
The characteristics of the patients divided by GOLD categories.
| GOLD categories |
GOLD 2 (14 patients) |
GOLD 3 (15 patients) |
GOLD 4 (7 patients) |
|---|---|---|---|
| BMI (kg/m2) | 27.3 | 28.1 | 26.3 |
| FFM (kg) | 51.9 | 56.0 | 50.0 |
| FFMI (kg/m2) | 18.2 | 18.9 | 17.9 |
| SMM (kg) | 28.5 | 30.5 | 27.2 |
| SMMI (kg/m2) | 10.0 | 10.3 | 9.7 |
| 6MWD (meters) | 406.2 | 418.8 | 301.7 |
| 6MWD (% of predicted) | 81.6 | 79.3 | 58.6 |
| CAT (points) | 14.1 | 19.9 | 23 |
| MIP (cm H2O) | 51.8 | 49.1 | 46.1 |
| MEP (cm H2O) | 75.8 | 76.3 | 65.9 |
GOLD 4 patients had the lowest mean BMI, FFM, FFMI, SMM and SMMI; their exercise capacity and maximal respiratory pressures were reduced compared with GOLD 2 and 3 patients, but all these differences reached no statistical significance. As expected, CAT score was significantly higher in GOLD 4 patients versus GOLD 2 patients (p=0.036).
b. Low FFMI patients
The characteristics of the patients with low FFMI versus those with normal FFMI are shown in Table 4.
Table 4.
The characteristics of the patients with low FFMI versus those with normal FFMI.
|
Low FFMI (6 patients) |
Normal FFMI (30 patients) |
p value | |
|---|---|---|---|
| FEV1 (% of predicted) | 53.2 (11.5) | 50.2 (15.1) | 0.395 |
| FEV 1 (liters) | 1.4 (0.5) | 1.4 (0.5) | 0.949 |
| 6MWD (meters) | 363.5 (138.3) | 389.5 (96.7) | 0.579 |
| 6MWD (% of predicted) | 72.9 (23.6) | 76.5 (18.7) | 0.682 |
| CAT (points) | 20.8 (11.1) | 17.7 (7.7) | 0.702 |
| MIP (cm H2O) | 36 (12.8) | 52.3 (15) | 0.029 |
| MEP (cm H2O) | 52.8 (17.6) | 78.2 (23.5) | 0.020 |
Patients with low FFMI had lower exercise capacity at the 6MWT (either in absolute distance and in percent of predicted) and higher CAT scores than patients with normal FFMI, but without statistical significance. Their MIP and MEP were significant lower than in patients with normal FFMI.
There were more patients with low FFMI in the "frequent exacerbators" group (two or more exacerbation during the previous year) – 28.6%, than in COPD patients with less than 2 exacerbation/year – 13.8%, but no statistical significance was found (p=0.346). No significant correlation could be found between FEV1 and FFMI (p=0.482).
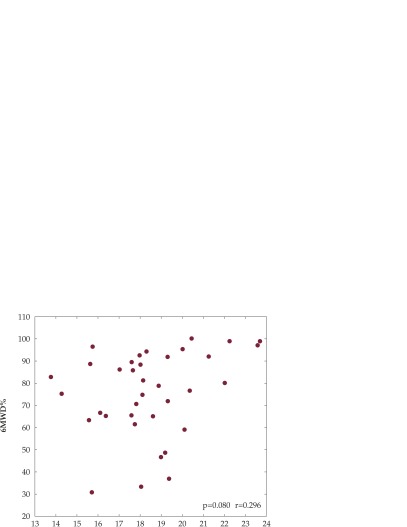
The positive correlation with r = 0.296 (p = 0.080) between FFMI and exercise capacity evaluated by 6MWD (% predicted), is illustrated in Figure 2.
Figure 2. Six minutes walking distance (6MWD, % predicted) versus fat-free mass index (FFMI, kg/m2) showing positive correlation, r = 0.296, p = 0.080.
c. Body composition categories
Depending on the BMI and FFMI values the patients were divided in the four categories described above:
normal: 28 patients (77.8%)
semistarvation: 2 patients (5.6%)
sarcopenia / muscular atrophy: 3 patients (8.3%)
cachexia: 3 patients (8.3%).
Their characteristics are illustrated in Table 5.
Table 5.
Characteristics of the patients in the four body composition categories.
| Parameters (mean values) | Categories depending on the BMI and FFMI values | |||
|---|---|---|---|---|
|
Normal (28 patients) |
Semistarvation (2 patients) |
Sarcopenic (3 patients) |
Cachexia (3 patients) |
|
| FEV1 (liters) | 1.5 | 1.2 | 1.1 | 1.7 |
| FEV1 (% of predicted) | 51.3 | 35.5 | 46 | 60.3 |
| 6MWD (meters) | 388.6 | 401.5 | 274 | 453 |
| 6MWD (% of predicted) | 77.2 | 66 | 62.9 | 82.8 |
| CAT (points) | 18.2 | 11.5 | 23.3 | 18.3 |
| MIP (cm H2O) | 52.3 | 51.7 | 27.7 | 44.3 |
| MEP (cm H2O) | 80.5 | 47 | 41.7 | 64 |
Patients in the sarcopenic group (low FFMI and normal BMI) had the lowest mean MIP, the lowest mean results at 6MWT (expressed either in meters or in percentage of predicted values), and the highest CAT mean scores, which defines low health status. Low numbers in each category did not allow statistical analysis.
Using the cut-points defined by Janssen (21) to define moderate or severe disability (SMMI lower than 6.75 in women and 10.75 in men), patients were divided in two categories: muscular depleted (22 patients) and normal (no muscular depletion) (14 patients). Exercise capacity was significantly lower in muscular depleted patients: mean 6MWD 69% ± 19.9 versus 86.6 % ± 12.3 in patients with no muscular depletion, p=0.006; (mean distance 358.8 m ± 115.1, versus 426.6 m ± 63.6 p=0.05). Comparison of mean CAT in the two categories showed no significant differences: 18.6 points ± 10 in muscular depleted patients versus 17.7 ± 4.6 points in normal patients, p 0.897. Mean MIP in muscular depleted patients was 48.36 ± 15.27 cmH2O versus 51.43 ±16.9 in non-depleted patients (p=0.399). Mean MEP had similar values in the two categories (74 cmH2O).
d. Segmental body composition assessment
The segmental body composition assessment revealed that upper limbs lean mass was greater than lower limb lean mass: mean lean mass percent of predicted was 107.1% and 105.2% for right and left arm and 88.1% and 89.3 % for right and left leg. Thus, an important part of the patients had unbalanced upper/lower skeletal muscle mass. This category of patients, named "unbalanced " had lower results at the 6MWT: mean walking distance was 73% ± 20.9 in unbalanced patients and 82.4% ± 13.5 in patients with balanced muscular mass (p 0.170). Among the 30 patients with normal FFMI, 23 were muscular unbalanced, compared with only 2 from the 6 patients with low FFMI. ❑
DISCUSSIONS
The results of the present study are the first on body composition in Romanian COPD patients and are in general similar with other studies on European populations. Regarding the correlations found, parameters of exercise tolerance correlated better with skeletal muscle mass than with fat free mass; no correlations were found between parameters of body composition and FEV1 or CAT; MIP correlated significantly with FFMI and SMMI. Segmental body composition assessment revealed that unbalanced upper/lower skeletal muscle mass is correlated with exercise capacity assessed by 6WMT.
Bioelectrical impedance analysis is an easy, safe and noninvasive method of measuring body composition, which has been validated extensively in COPD (20). DSM-BIA (direct segmental multi-frequency BIA) used in this study offers additional advantages, like direct segmental measurement (trunk and four limbs) and reproducibility due to the same 8 points tactile electrode system. DSM-BIA is a valid tool for the assessments of total body and segmental body composition, particularly for the quantification of body lean mass (22).
The results of body composition assessment in our study group revealed that only 13.9% of patients had low BMI, which is lower than in other patients groups (32-64%) (18, 19). This difference could be explained by the outpatient type of our rehabilitation program which excluded from the beginning more severe patients with potentially low BMI. Indeed, the mean BMI of 27.5 kg in our study was a little higher than in other European studies: 22.8-26.1 kg (11,15,19,23,24).
The analysis of BMI in our patients revealed a 54.8% prevalence of patients who were overweight or obese. This is similar with a recent EUROSTAT survey (25), which reported 57.5% overweight or obese in men and 43.9% in women for the Romanian population (healthy subjects over 18 years). Still, our group of patients had a much greater prevalence of obesity (25% versus 7.6% in men and 8% in women in the above-mentioned study). Due to the small number of patients in our study this cannot be extrapolated to the global population of Romanian COPD patients, even if their exercise intolerance could determine a low physical activity and thus explain the higher obesity prevalence. A recent meta-analysis showed that for patients with COPD being overweight or obese had a protective effect against mortality (26).
We used for BMI and FFMI the cut-offs recommended by ATS/ERS guidelines for pulmonary rehabilitation, as these cut-offs are discriminative for exercise capacity and health status (8,20,27). Mean FFM in our group was 53.3 kg, similar with other studies who found mean FFM 49.8 kg (4) and 52.4 kg (15). The mean FFMI in our study group was 18 kg/m2 which is also similar with the results of other studies 18.1 kg/m2 (15), 18.7 kg/m2 (2), 16.4 kg/m2 (20), 17.2 kg/m2 (11).
In our group of patients 16.7% had low FFMI (3 sarcopenic and 3 cachectic patients). Thus, only 9.7% of our patients with normal BMI had a reduced FFMI, which is less than in other studies (2,11). Fat free mass index (FFMI) is still more suitable in detecting muscular wasting than BMI, as an unapparent and possibly asymptomatic loss of skeletal muscle mass (27).
Mean FFMI was significantly greater in overweight or obese patients versus normal or underweight patients, which is concordant with another study which showed that severe COPD patients who were overweight or obese had greater FFM, exercise capacity and inspiratory muscle strength than patients with the same degree of airflow obstruction who were of normal weight or underweight (7).
The fact that none of the overweight or obese patients had low FFMI can be speculated in the sense of the "obesity paradox"; the protection against hyperinflation could reduce the cost of breathing and furthermore reduce energy expenditure, with a better mechanical efficiency of respiratory muscles. It seems that being overweight protects against the depletion of fat free mass, as no patient overweight or obese had low FFM.
The prevalence of nutritional depletion (low BMI and/or low FFMI) was 22.2% in our study group, which is similar with the prevalance of 27% reported by Vermereen (11) and with 21% reported by Engelen (28).
The distribution of the patients in the four categories of body composition defined by Schols (14,20): normal, semistarvation, sarcopenia and cachexia was 77.8%, 5.6%, 8.3% and 8.3% respectively which is very similar with data from Vermereen: 73%, 1%, 15% and 11% respectively (11). We note that Schols reported a distribution on the four categories of 56 %, 6%, 10% and 29% in men and 59%, 5%, 10% and 27% in women, in a group of patients referred to rehabilitation (20).
Patients in the sarcopenic group (low FFMI and normal BMI) had the lowest mean MIP, the lowest mean results at 6MWT and the highest CAT mean scores. It was shown in previous studies (27) that in patients with low FFM and preserved BMI the decrease in exercise performance is more important than in patients with low FFM and low BMI (26), and that a higher lean-to-fat ratio was associated with a greater distance walked in 6 minutes (29). One might expect that the cachectic group would be the most impaired, and emphasize the need of assessing FFM in COPD patients, for an adequate tailoring of an individualized pulmonary rehabilitation program.
Data regarding the relation between FFMI and FEV1 are controversial; some authors showed a significant correlation between FFMI and COPD stages (12), while other studies failed to find a correlation between FFMI and FEV1, dyspnoea scores or health status (11). In our study, FFMI did not correlate with the severity of bronchial obstruction expressed as FEV1; indeed, the mean FFMI was lower in GOLD group II than in GOLD group III, which emphasizes that loss of fat-free mass can occur even in the less severe forms of the disease.
Our study revealed that frequent exacerbators have lower FFMI, which can be explained by the fact that FFM depletion occurs frequently in hospitalised COPD patients (30).
Patients with low FFMI had lower exercise capacity expressed as the distance walked at 6MWT, without reaching statistical significance compared with those with normal FFMI, given the low number of patients. Indeed, it was shown that patients with depletion of FFM irrespective of body weight showed greater impairment in 12MWD (8).
In our study, patients with low FFMI had greater CAT scores (without reaching statistical significance). The sarcopenic group had the highest CAT scores, even greater than in the cachectic group. This tendency suggests the impact of body composition on health status, as tissue depletion is an important determinant of health status independent of exercise capacity and dyspnea (8). It was shown that health status is more affected in underweight patients with COPD and in those with reduced FFM even more so (19).
The only parameter which in our study was significantly correlated with impaired FFMI was MIP, a finding which is similar with other studies (20,31). It was showed that in COPD patients inspiratory muscle force is affected more than peripheral muscle force (32). This can be explained by a reduced mass of the dyaphragm muscle (33), or by the reduced mechanical efficiency of the respiratory muscles in hyperinflated COPD patients.
Skeletal muscle mass is even more suitable in detecting muscular atrophy than FFM; in our study group the number of muscular depleted patients was more than double the one of patients with low fat free mass, suggesting that this method is more useful in finding patients suitable for pulmonary rehabilitation. We have to note that the cut-off for normal or depleted muscular mass is not clearly defined. Still, we used the values described in a study based on self-declared level of disability; we consider that these values can be used in selecting the right candidates for pulmonary rehabilitation.
In fact, even though due to low numbers we could not reproduce the results of other studies (8,27) regarding the significant correlation between FFMI and exercise tolerance assessed by walking tests, we found that patients with low SMMI had significantly lower exercise capacity assessed by 6MWD percent predicted than patients with normal SMMI. CAT scores were not significantly different in these two groups, despite the fact that these categories were defined on the basis of a questionnaire regarding disability.
Segmental assessment of FFM could be interesting, as mechanical efficiency and exercise performance of upper extremities are relatively preserved in COPD (34); arm and leg muscle strength are equally affected in COPD, but arm muscle endurance is not impaired (35). Muscle wasting of the extremities is frequent in COPD, independent of airflow obstruction (4). It was noted that in our study group the majority of patients with normal FFMI had unbalanced upper and lower muscle mass. We can speculate from these results that muscular atrophy begins with the lower limbs and can be detected with segmental body composition analysis in an early stage, before the impairment of the total SMM.
The results of the present study on Romanian COPD patients emphasize the utility of body composition assessment in patients referred to pulmonary rehabilitation, not only for defining the individualized exercise training program for each of them, but also for the evaluation of the benefits of the intervention (36). Future research should focus on the usefulness of segmental body composition assessment for the individualization of a rehabilitation program in order to ameliorate its outcome.
Limits of the study: BIA is not a gold standard method for assessing body composition, but is safe and relatively available. Another limit could be the fact that patients referred to pulmonary rehabilitation are the most depleted, as seen in different studies, with larger proportion of depleted patients in this category than in general outpatient population with COPD (19). Small number of patients is another important limit of this study which did not allow statistical analysis in some groups. ❑
CONCLUSIONS
This study offers the first data on body composition of Romanian COPD patients. The prevalence of nutritional depletion is similar to that found in other European studies. No significant correlations were found between FFMI and severity of the disease (bronchial obstruction, distance walked, CAT score). FFMI and SSMI were significantly correlated with MIP. Sarcopenic patients had the lowest mean 6MWD, the lowest mean MIP and the highest CAT mean scores. SMMI is more accurate in detecting exercise intolerance, as SMMI significantly correlated with 6MWD. Segmental body composition assessment revealed that lower limbs are more depleted than upper limbs, and that "unbalanced" patients had lower results at 6MWT. These results are extremely useful in patients referred to pulmonary rehabilitation, and body composition evaluation should become a routine for COPD patients.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease. Available from http://www.goldcopd.org. Updated: 2013. Date last accessed: May 15, 2013
- 2.Schols AMWJ, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–7. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 4.Engelen MP, Schols AM, Does JD, et al. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–8. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 5.Schols AM, Mostert R, Soeters PB, et al. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax. 1991;46:695–9. doi: 10.1136/thx.46.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baarends EM, Schols AM, Mostert R, et al. Peak exercise response in relation to tissue depletion in patients with chronic obstructive pulmonary disease. Eur Respir J. 1997;10:2807–13. doi: 10.1183/09031936.97.10122807. [DOI] [PubMed] [Google Scholar]
- 7.Sabino PG, Silva BM, Brunetto AF. Nutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patients. Clinics (Sao Paulo) 2010;65:599–605. doi: 10.1590/S1807-59322010000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostert R, Goris A, Weling-Scheepers C, et al. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:859–867. doi: 10.1053/rmed.2000.0829. [DOI] [PubMed] [Google Scholar]
- 9.Budweiser S, Meyer K, Jörres RA, et al. Nutritional depletion and its relationship to respiratory impairment in patients with chronic respiratory failure due to COPD or restrictive thoracic diseases. Eur J Clin Nutr. 2008;62:436–43. doi: 10.1038/sj.ejcn.1602708. [DOI] [PubMed] [Google Scholar]
- 10.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeeren MAP, Creutzberg EC, Schols AMWJ, et al. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med. 2006;100:1349–55. doi: 10.1016/j.rmed.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Ischaki E, Papatheodorou G, Gaki E, et al. Bodymass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132:164–9. doi: 10.1378/chest.06-2789. [DOI] [PubMed] [Google Scholar]
- 13.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol. 2003;58:1012–7. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 14.Schols AM. Pulmonary cachexia. Int J Cardiol. 2002;85:101–110. doi: 10.1016/s0167-5273(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 15.Franssen FM, Broekhuizen R, Janssen PP, et al. Effects of Whole-Body Exercise Training on Body Composition and Functional Capacity in Normal-Weight Patients With COPD. Chest. 2004;125:2021–8. doi: 10.1378/chest.125.6.2021. [DOI] [PubMed] [Google Scholar]
- 16.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 17.Enright PL, Sherill DL. Reference equations for the six minute walk in healthy adults. Am J Resp Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 18.Nordstrand N, Gjevestad E, Dinh KN, et al. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord. 2011;11:7–7. doi: 10.1186/1471-2261-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 20.Schols AMWJ, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–9. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Baumgartner RN, Ross R, et al. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am J Epidemiol. 2004;159:413–21. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 22.Ling CH, de Craen AJ, Slagboom PE, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30:610–5. doi: 10.1016/j.clnu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 24.Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory Response and Body Composition in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2001;164:1414–8. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 25."Overweight and obesity - BMI statistics" - Statistics Explained (2013/7/1) http://epp.eurostat.ec.europa.eu/statistics_explained/index.php/Overweight_and_obesity_-_BMI_statistics.
- 26.Cao C, Wang R, Wang J, et al. Body Mass Index and Mortality in Chronic Obstructive Pulmonary Disease: A Meta-Analysis. PLoS ONE. 2012;7(8):e43892. doi: 10.1371/journal.pone.0043892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schols AMWJ, Soeters PB, Dingemans MC, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–6. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 28.Engelen MPKJ, Schols AMWJ, Baken WC, et al. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in an out-patient population with chronic obstructive pulmonary disease. Eur Respir J. 1994;7:1793–7. doi: 10.1183/09031936.94.07101793. [DOI] [PubMed] [Google Scholar]
- 29.Eisner MD, Blanc PD, Sidney S, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7–7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J. 1997;10:2264–9. doi: 10.1183/09031936.97.10102264. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura Y, Tsutsumi M, Nakata H, et al. Relationship between respiratory muscle strength and lean body mass in men with COPD. Chest. 1995;107:1232–6. doi: 10.1378/chest.107.5.1232. [DOI] [PubMed] [Google Scholar]
- 32.Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2000;20:353–60. doi: 10.1097/00008483-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi: 10.1002/14651858.CD000998.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franssen FM, Wouters EF, Schols AM. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clin Nutr. 2002;21:1–14. doi: 10.1054/clnu.2001.0485. [DOI] [PubMed] [Google Scholar]
- 35.Franssen FM, Broekhuizen R, Janssen PP, et al. Limb muscle dysfunction in COPD: effects of muscle wasting and exercise training. Med Sci Sports Exerc. 2005;37:2–9. doi: 10.1249/01.mss.0000150082.59155.4f. [DOI] [PubMed] [Google Scholar]
- 36.Mineo D, Ambrogi V, Lauriola V, et al. Recovery of body composition improves long-term outcomes after lung volume reduction surgery for emphysema. Eur Respir J. 2010;36:408–16. doi: 10.1183/09031936.00142309. [DOI] [PubMed] [Google Scholar]