Abstract
Dysphagia aortica is an uncommon condition defined by the difficulty in swallowing caused by extrinsic compression of the esophagus due to an ectatic, tortuous, or aneurysmatic atherosclerotic thoracic aorta. We report the case of a 93 year old woman with dysphagia secondary to extrinsic compression by a giant sacciform aneurysm of the descending thoracic aorta. As a consequence of small liniar dissection tracts of the aneurysm, the patient developed disseminated intravascular coagulopathy with spontaneous forearm hematoma and multiple bruising, a clinical setting with a difficult therapeutic approach. Taking into account that aneurysm of the thoracic aorta is a very rare cause of dysphagia, the case report on this rare cause should contribute to better diagnosis of dysphagia aortica and swallowing difficulties in general. Dissecting aneurysm of the thoracic aorta is a very rare cause of DIC so the combination of easy bruising or spontaneous hematoma and an aortic aneurysm demands special caution.
Keywords: dysphagia aortica, aortic aneurysm, disseminated intravascular coagulation
If you do not expect the unexpected you will not find it, for it is not to be reached by search or trail.
Heraclitus
Dysphagia aortica is a difficulty in swallowing caused by extrinsic compression of the esophagus due to an ectatic, tortuous, or aneurysmatic atherosclerotic thoracic aorta. This condition is very uncommon, and it is usually associated with old age, women with short stature, hypertension, and kyphosis.
Patients with large thoracic aortic aneurysms may develop a coagulopathy resulting from localized, intraluminal activation and consumption of clotting factors. ❑
CASE REPORT
We report the case of a 93 year old women, with no significant medical history or hypertension, presenting for the development of a spontaneous right forearm hematoma (without pre-existing trauma) a week before admission with subsequent extension to the upper arm and the appearance of multiple bruises on her chest and legs. The patient also reported marked fatigue, weight loss of about 15 kg in the last months and dysphagia for solids and semisolids.
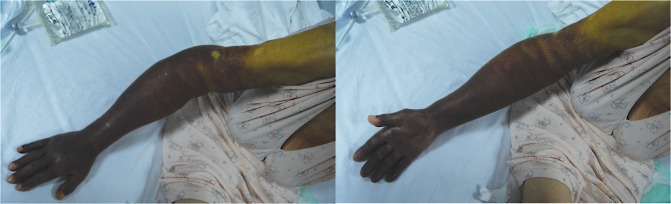
On admission, she had a chronically ill appearance. Clinical examination showed pale skin and mucosae, hematoma of the upper right limb (Figure 1) and multiple petechiae and ecchymosis on her body, kyphosis, normal lung auscultation, blood pressure was 120/90 mmHg at both upper limbs, heart rate of 100 beats per minute, with no cardiac murmurs, no lymphadenopathy or hepatosplenomegaly.
Figure 1. Haematoma of the upper right limb (arm and forearm and the distal part of the arm).
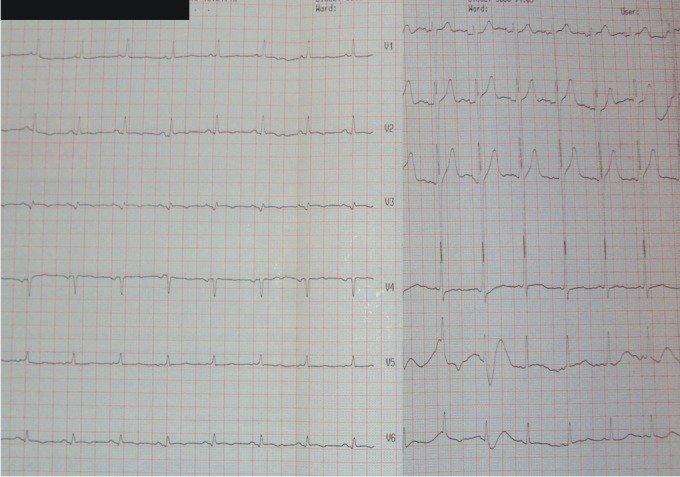
Electrocardiogram showed normal sinus rhythm, 100 beats/min, QRS axis +30°, with no criteria for left ventricular hypertrophy (Figure 2).
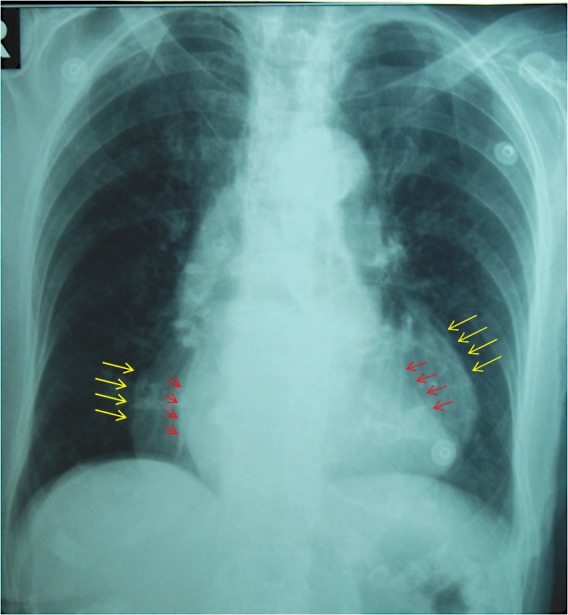
Figure 2. Chest X-ray – normal cardiothoracic index with a bizarre double contour of the cardiac silhouette.
Laboratory tests showed severe normocytic-normochromic anemia, moderate thrombocytopenia, and mildly elevated serum creatinine concentration, with no signs of liver disease. Coagulation tests were altered with spontaneous prolongation of the prothrombin time (PT) and activated partial thromboplastin time (aPTT), low plasma fibrinogen concentration and elevated D-Dimer levels.
Chest X-ray caught our attention by a normal cardiothoracic index but with a bizarre double contour of the cardiac silhouette (Figure 3).
Figure 3. Admission ECG: Sinus rhythm, 100 beats /min, QRS axis +30°, with no criteria for left ventricular hypertrophy.
Even though the patient denied a recent history of trauma, we still did forearm, elbow and wrist x-rays which excluded a possible posttraumatic bone lesion.
Presence of severe anemia in a patient with dysphagia for solids and semisolids and significant weight loss has led to endoscopic examination of the upper gastrointestinal tract to exclude an esophageal tumor. The endoscopic exploration brought interesting informations - at 35 cm from the dental arch, indeed there was a narrowing of the lumen but caused by an extrinsic compression with normal mucosa at this level.
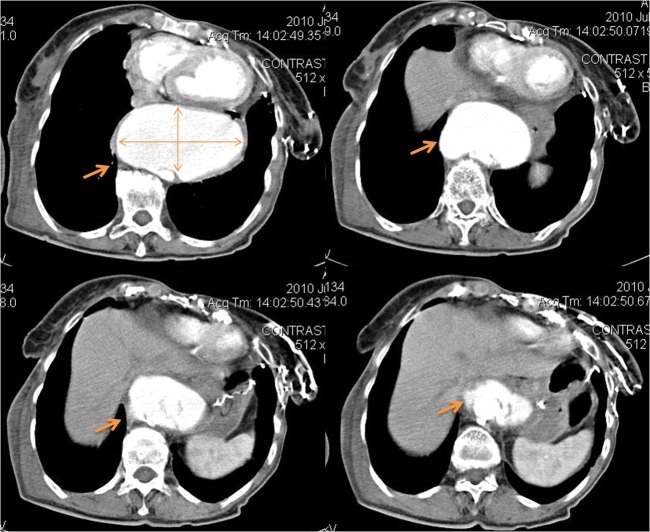
Echocardiography brought light on the diagnosis. At transthoracic examination, we found severe dilatation of the descending aorta altering the echographic anatomy of the heart by pushing-it. At this point chest tomography was mandatory to assess the thoracic aorta. This revealed the presence of a giant sacciform aneurysm of the descending thoracic aorta with a transverse diameter of 120 mm and antero-posterior diameter of 70 mm, extending over a length of about 90 mm (Figure 4). On the wall above and below the aneurysm there were small linear tracks of incomplete intimal dissection. Ascending aorta, the aortic arch and the abdominal aorta had normal dimensions with multiple intramural calcifications.
Figure 4. Chest CT - giant sacciform aneurysm of the descending thoracic aorta - transverse diameter of 120 mm and antero-posterior diameter of 70 mm, extending over a length of about 90 mm. On the wall above and below the aneurysm were revealed small linear tracks of incomplete intimal dissection.
One question remained, why did the right arm hematoma appear? Since the patient had spontaneous bleeding in her arm, with thrombocytopenia, signs of increased thrombin generation (hypofibrinogenemia) as well as increased fibrinolysis (elevated D-dimer), with PT and aPTT as a sign of reduced activity of the components of the coagulation pathways, the cause was probably the installation of disseminated intravascular coagulation secondary to the dissection of the thoracic aneurysm. ❑
DISCUSSION
Dysphagia, a term derived from the Greek words "dys" (with difficulty) and "phagia" (to eat), is the subjective awareness of difficulty in the passage of solids or liquids from the oropharynx to the stomach (1).
Dysphagia is considered an alarm symptom, indicating the need for an immediate evaluation to define the exact cause and initiate appropriate therapy. Dysphagia in elderly subjects should not be attributed to normal aging. Aging alone causes mild esophageal motility abnormalities, which are rarely symptomatic (2). The true prevalence of dysphagia is unknown but epidemiological studies estimate a prevalence rate of 16% to 22% among individuals over 50 years of age (3).
Dysphagia can be classified into an oropharyngeal or an esophageal location, and it is caused by neuromuscular motility disorders and intrinsic or extrinsic mechanical lesions (4). Extrinsic compression of the esophagus is a rare cause of dysphagia and may occur through mediastinal masses (lymphadenopathy, lung cancer, etc.), postoperative fibrotic changes and cardiovascular causes such as dysphagia aortica, dysphagia lusoria, enlarged left atrium (eg. from mitral stenosis) and recently reported, massive pericardial effusion (5,6).
The term dysphagia aortica was first used by Pape in 1932 to describe difficulty in swallowing caused by external compression from an ectatic, tortuous, or aneurysmal aorta as a result of age related degeneration. Typical patients are elderly, hypertensive females, often of short stature with kyphosis (7). Low sternal dysphagia is caused by the diseased aorta pushing the esophagus anterolaterally against the crural slings of the diaphragm. Compression at the arch of the aorta is less common (1).
There is no gold standard diagnostic procedure for dysphagia aortica. The association of suggestive symptoms, such as progressive intolerance to solids with concomitant weight loss along with the results of imaging and other diagnostic studies, provide a high index of suspicion. The diagnostic work-up includes radiologic, endoscopic, and manometric studies. On a standard chest radiography and CT scan the usual findings are the enlargement of the aortic arch and the tortuous dilated aorta. A barium swallow test may show partial esophageal obstruction and pulsatile movement of the barium synchronous with aortic pulsation (1). The endoscopic findings may be stenosis, band like pulsatile extrinsic compression, or kinking of the esophagus. Esophageal manometry may demonstrate a localized high-pressure band with superimposed pounding that is synchronous with the cardiac pulsation (8). Although we did not perform esophageal manometry, the patient's symptoms and imaging studies were consistent with the classic findings of dysphagia aortica.
The treatment for dysphagia aortica depends on the severity of the symptoms. Mild cases may be treated conservatively, such as avoiding sticky solids and feeding on a liquid diet. Patients with more severe symptoms may respond to surgery. The surgical procedures include transposition of the distal esophagus, separation of the distal esophagus from the aorta, esophagomyotomy, division of the right crus of the diaphragm, aortic resection, and repair of an aneurysm. For patients who are not candidates for surgery, insertion of a feeding tube via percutaneous endoscopic gastrostomy (PEG) is an option (1,8).
Patients with large thoracic aortic aneurysms may develop a coagulopathy resulting from localized, intraluminal, activation and consumption of clotting factors (9). The first case report associating dissecting aneurysm with coagulation defects was described in 1967 in a 51-year-old woman (10).
Disseminated intravascular coagulation (DIC) is a complication of an underlying illness occurring in approximately 1 percent of hospital admissions but DIC with clinical symptoms reveals aortic arterial aneurysm in less than 5% (11,12). In a series of patients with aortic aneurysm, 40 % had elevated levels of fibrin (ogen) split products, but only 4% had significant bleeding and laboratory evidence of DIC. Several factors predispose patients with aortic aneurysms to the development of DIC: a large surface area, dissection, and expansion of the aneurysm (13).
The pathogenesis is believed to involve the local release of thromboplastin, as well as contact activation of clotting factors by subendothelial procoagulant substances, locally deposited red cell fragments, and platelet aggregates (14).
Treatment of DIC is controversial but there is unanimous agreement that correction of the underlying disease and initiating factors is of central importance and probably the only effective treatment (13). In our patient, association of old age, biological state (severe anemia, acute renal failure) and an unfavorable anatomy of the giant sacciform aneurysm of the descending thoracic aorta-transverse diameter of 120 mm and antero-posterior diameter of 70 mm, extending over a length of about 90 mm - made surgical correction impossible in our clinic. Consequently, supportive measures were chosen.
Although commonly quoted as fact, the hypothesis that hemostatic replacement therapy in DIC "fuels the fire" has never been proved. Platelet concentrates, cryoprecipitate, and fresh-frozen plasma contain the hemostatic factors and inhibitors of blood coagulation commonly depleted in patients with DIC. In general, patients should be transfused with blood components only when they have bleeding and depleted hemostatic factors. Also eligible are patients being prepared for emergency surgery and patients with gunshot-induced brain injury (13). Patients with marked (<20,000/microL) or moderate thrombocytopenia (<50,000/microL) and serious bleeding should be given platelet transfusions (1 to 2 units per 10 kg per day). With respect to replacement therapy, actively bleeding patients with a significantly elevated prothrombin time (INR) and/or a fibrinogen concentration <50 mg/dL, should receive fresh frozen plasma or cryoprecipitate, the latter for fibrinogen replacement. It is preferable to keep the fibrinogen level >100 mg/dL (11). The administration of heparin is generally limited to the subset of patients with chronic, compensated DIC who have predominantly thrombotic manifestations. Also, in patients unsuitable for surgery, chronic DIC associated with aortic aneurysm could be controlled by heparin. There are case reports of inoperable aortic aneurysm with chronic DIC that were managed with once daily subcutaneous injection of low molecular weight heparin (dalteparin) on long term (15,16).
Our patient received red blood cell transfusion to correct the severe anemia (hemoglobin of 6 g/dL and hematocrit of 22.8%). The coagulation profile was not severe enough to justify administration of fresh frozen plasma or platelet transfusion.
We were able to discharge the patient in stable conditions but acknowledging the poor prognosis on the short term.
Taking into account that aneurysm of the thoracic aorta is a very rare cause of dysphagia, the case report on this rare cause should contribute to better diagnosis of dysphagia aortica and swallowing difficulties in general. Dissecting aneurysm of the thoracic aorta is a very rare cause of DIC as such, the combination of easy bruising or spontaneous hematoma and an aortic aneurysm demands special caution.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Kim JH, Jang SW, Kim DB, et al. A Patient With Dysphagia due to an Aortic Aneurysm. Korean Circ J. 2009;39:258–60. doi: 10.4070/kcj.2009.39.6.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamburek RD, Farrar JT. Disorders of the digestive system in the elderly. N Engl J Med. 1990;322:438–438. doi: 10.1056/NEJM199002153220705. [DOI] [PubMed] [Google Scholar]
- 3.Lind CD. Dysphagia: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:553–575. doi: 10.1016/s0889-8553(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Spieker MR. Evaluating dysphagia. Am Fam Physician. 2000;61:3639–48. [PubMed] [Google Scholar]
- 5.Hemender SV. Dysphagia from Extrinsic Compression of Esophagus by Pericardial Effusion. Clin Med Res. 2008;6:78–79. doi: 10.3121/cmr.2008.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fass R, Feldman M, Travis AC. Evaluation of dysphagia in adults, UpToDate 2011. www.uptodate.com [Google Scholar]
- 7.Keates PG, Magidson O. Dysphagia associated with sclerosis of the aorta. Br J Radiol. 1955;28:184–90. doi: 10.1259/0007-1285-28-328-184. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson JM, Euinton HA, Smith LF, et al. Diagnostic dilemmas in dysphagia aortica. Eur J Cardiothorac Surg. 1997;11:222–7. doi: 10.1016/s1010-7940(96)01053-6. [DOI] [PubMed] [Google Scholar]
- 9.Micallef-Eynaud PD, Ludlam CA. Aortic aneurysms and consumption coagulopathy. Blood Coag Fibrinol. 1991;2:477–481. doi: 10.1097/00001721-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Fine NL, Applebaum J, Elguezabal A, et al. Multiple Coagulation Defects in Association With Dissecting Aneurysm. Arch Intern Med. 1967;119:522–526. [PubMed] [Google Scholar]
- 11.Leung L. Clinical features, diagnosis, and treatment of disseminated intravascular coagulation in adults, UpToDate 2010. www.uptodate.com [Google Scholar]
- 12.Deleplanque G, Kraimps JL, Rouffineau J, et al. Abdominal aortic aneurysm detected by a consumption coagulopathy. J Chir (Paris). 1990;127:325–9. [PubMed] [Google Scholar]
- 13.Beutler E, Lichtman MA, Coller BS, et al. Disseminated intravascular coagulation. In Williams Hematology 6th Edition. 2000:1191–1202. [Google Scholar]
- 14.Scola M, Brophy M, Fiore L. Localized Consumptive Coagulopathy. Circulation. 2001;104:120–121. doi: 10.1161/hc2601.093181. [DOI] [PubMed] [Google Scholar]
- 15.Garcia Fernandez JR, Lopez Berenguel F, Ais C. Long-term treatment with low molecular weight heparin, of chronic disseminated intravascular coagulation. Ann Med Interna. 2003;20:191–194. [PubMed] [Google Scholar]
- 16.Majumdar G. Long-term management of chronic DIC associated with inoperable aortic aneurysm with low molecular weight heparin. Hematol J. 2004;5:447–448. doi: 10.1038/sj.thj.6200411. [DOI] [PubMed] [Google Scholar]