Abstract
The relationship between chronic hepatitis C virus infection (HCV) and oral lichen planus (OLP) is a current topic in the field of oral medicine. Many studies of this association have been made over time. The geographic variation of the hepatitis C prevalence proved to be an important factor influencing the statistical results of the studies analyzing the association of the oral plan lichen with the hepatitis C virus. Approaching this issue is not to be neglected. Treatment outcomes in patients with oral lichen planus associated with chronic hepatitis C virus are often unsatisfactory compared to patients suffering from idiopathic oral lichen planus. Also, the evolution of oral lesions is often fluctuating, with repeated periods of relapse according to the degree of liver function decompensation. Background therapy for liver disease itself may influence lichen planus lesions. Thus, during therapy with interferon and ribavirin oral lesions may appear or become acute.
Keywords: hepatitis C virus, oral lichen planus
Since 1989, once the virus has been discovered, infection with hepatitis C virus (HCV) has become a serious public health problem worldwide. The number of infected people gradually increased to some 130-170 million cases worldwide, affecting approximately 3% of the world population and still another 3-4 million people get infected each year (1). HCV infection is one of the major causes of chronic hepatitis but if it is untreated, 15-30% of chronic HCV infections progress to cirrhosis or hepatocellular carcinoma over the next 30 years (2). Moreover, several extrahepatic disorders may be associated with chronic HCV infection: mixed cryoglobulinemia, diabetes, porphyria cutanea, non-Hodgkin lymphoma. Among the general complications associated with HCV infection there are also dermatological disorders such as lichen planus (2).
There is certain percentage of patients with oral lichen planus who associate chronic hepatitis with C virus. The presence of hepatitis is often completely unknown to the patient, being discovered by chance during diagnostic protocol for lichen planus. Prevalence of chronic hepatitis C in patients with oral lichen planus varies between 0.5-35% as reported by different authors for different geographical areas (3). This important difference resides also in the way hepatitis C virus incidence varies in different parts of the world.
Oral lichen planus (OLP) is a muco-cutaneous dermatosis of unknown nature that may occur either as a single condition of oral mucosa or in association with characteristic lesions in the skin, genital mucosa, scalp or nails (4).
This disease is estimated to affect between 0.2 and 2.3% of the general population and represents about 0.6% of all diseases that dentists frequently meet (4). In Romania there is no study on the incidence of oral lichen planus in general population. In a hospital based-population of the Oral Medicine department in Bucharest the incidence of oral lichen planus is 12.31% (5). According to some authors it is really one of the most common diseases affecting the oral mucosa (4). Oral lichen planus is spread worldwide and equally affects all racial groups, but it is most common in middle-aged adult females (6).
Although the etiopathogenic mechanism of oral lichen planus is not fully elucidated, recent research suggests that immunological mechanisms play an important role, this disease being considered as having auto-immune nature, mediated by T CD 8 + cells, macrophages and Langerhans cells. Langerhans cells and macrophages in the basal epithelial layer provide antigenic information for T CD 8 + cells activated against basal layer (6). Cell destruction is done through immune mechanisms that trigger apoptosis, resulting in the appearance of characteristic histological changes (6).
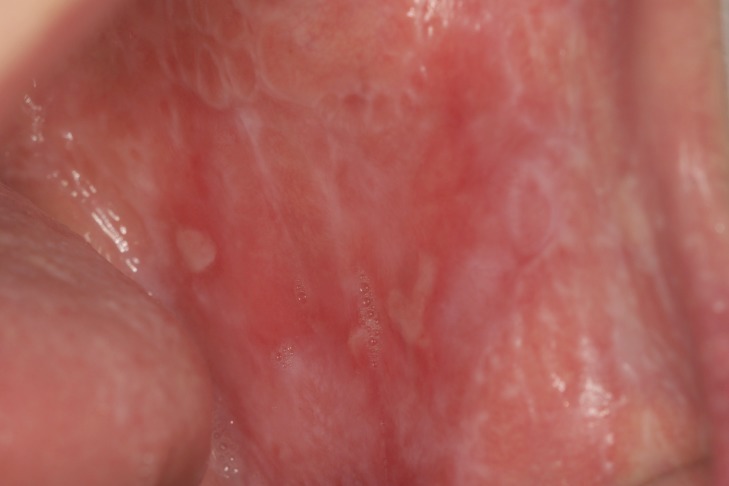
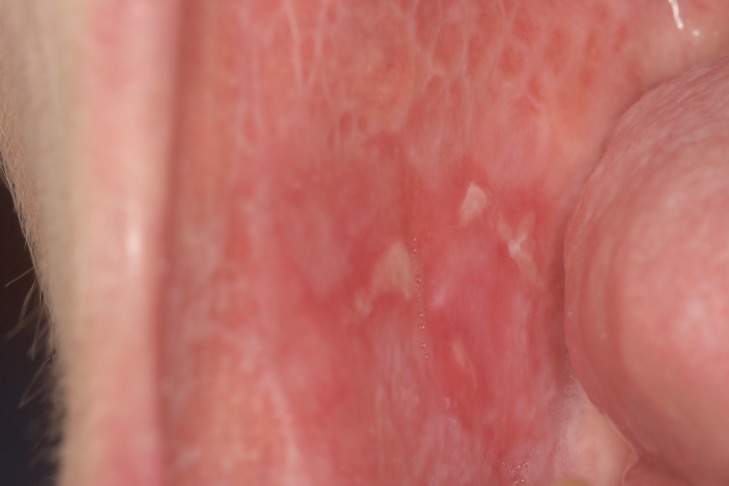
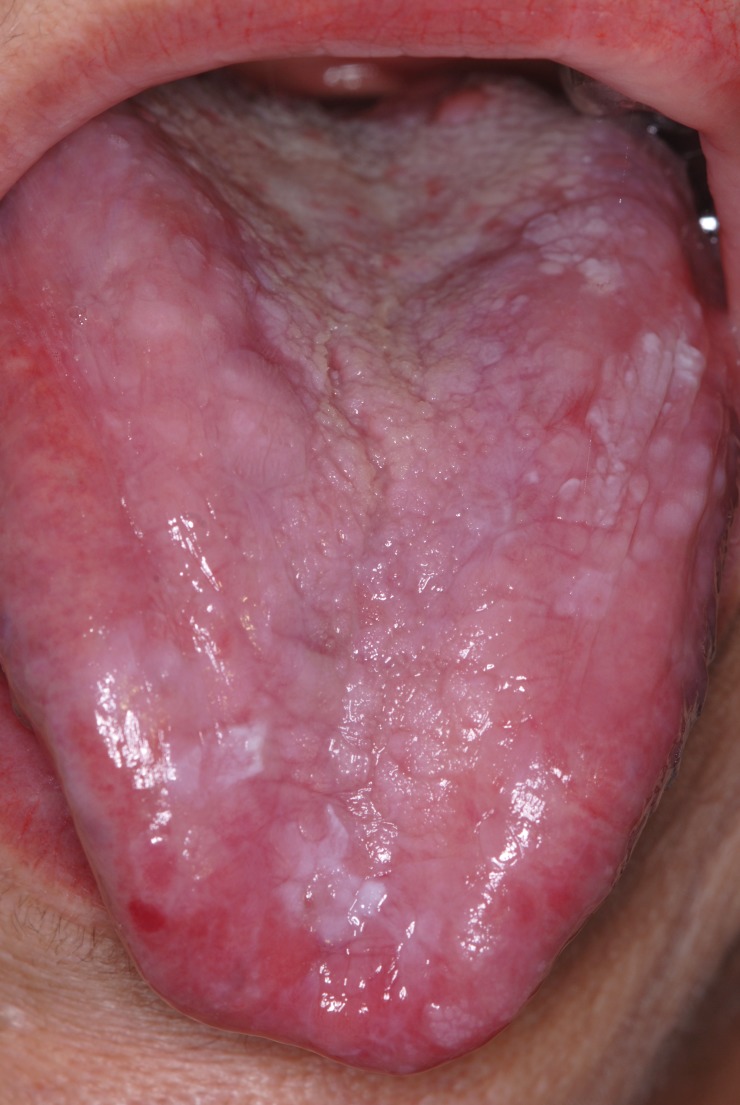
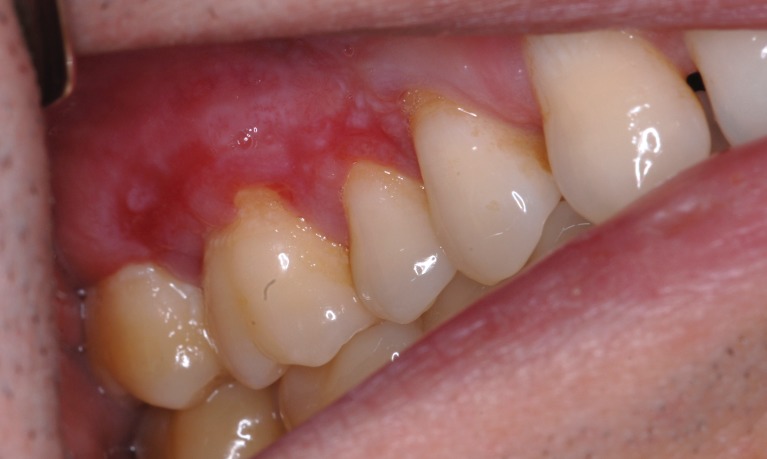
The clinical aspect of OLP is mostly polymorphic. Keratotic lesions such as papules, reticular or plaque-like lesions are associated with atrophic, erosive-ulcerative or bullous lesions (6) (Figure 1A, Figure 1B). The erosive form is accompanied by various symptoms from mild discomfort to serious function disturbances affecting the quality of life. Usually, lesions are located on the posterior buccal mucosa, bilaterally. Other sites can also be affected: the dorsal tongue (Figure 2), labial mucosa or gingiva. The involvement of gingival mucosa is regarded as a particular clinical entity named desquamative gingivitis (Figure 3) (4,6).
Figure 1. Polymorphic lesions of oral lichen planus – reticular keratosis, atrophy and ulcers involving bilateral buccal mucosa.
A.
B.
Figure 2. Dorsal tongue mucosa with atrophyc aspect.
Figure 3. Descumative gingivitis with keratosis and atrophy.
An important aspect of oral lichen planus is its association with chronic hepatitis C in certain groups of the population and in variable proportions (2).
Globally, the prevalence of HCV infection shows regional variations with an average of 3%, which is very likely to be underestimated (7). The incidence and prevalence of HCV infection varies by geographic region. It is high in Africa and the Eastern Mediterranean and low in Western Europe and North America. The highest prevalence was reported in Egypt, where approximately 20% of blood donors had HCV- Antibodies (World Health Organization). Across Europe there is a North-South gradient (prevalence of 0.5% in the north compared to 2% in the south) and an east-west gradient, with a lower prevalence among Western countries. Although the prevalence of infection in the general population is not well known, the following endemic areas can be identified (7):
Low (below 1.5%) North America, Northern Europe;
Medium (1.5-3.5%): South Asia, sub-Saharan Africa, Central and Latin America, the Caribbean, Oceania, Australia, Central and Eastern Europe, Western Europe;
High (over 3.5%): Central and Eastern Asia, Middle East, North Africa.
In 1994, Molnar and collaborators assessed the prevalence of infections with hepatitis viruses in Romania, reporting a 4.9% prevalence of the infection with C virus. He also reported variations between urban and rural areas (8). These figures place our country in the group of countries with an average prevalence of chronic HCV hepatitis.
Another study, more recently conducted in our country, reported a prevalence rate of hepatitis C virus infection in the adult Romanian population of 3.23%, with relevant differences between major geographic regions. The results of this nationwide survey showed a maximum prevalence in Moldova (4.25%), while the lowest values were found in Transylvania - Banat area (2.63%). In Muntenia and Dobrogea regions, prevalence was 3.35%, very close to that found nationwide (9).
Chronic hepatitis with HCV affects not only the liver, but is a metabolic disorder that has effects throughout the whole body, general findings including the following (10):
Mixed Cryoglobulinemia associated with lymphoproliferative diseases.
Membrano-proliferative glomerulonephritis.
Porphyria cutanea tarda.
Oral or cutaneous lichen planus.
Although the association of oral lichen planus with chronic hepatitis C virus is well documented, pathogenesis remains unclear. There are currently several hypotheses that may explain this association:
Hepatitis C virus triggers an auto-immune process, and the association of a lichen planus with other auto-immune diseases such as vitiligo, myasthenia gravis or diabetes supports this hypothesis (10).
HCV interferes directly on cellular replication and produces immunological changes having a role in the occurrence of muco-cutaneous lesions of lichen planus (11).
In chronic hepatitis C virus, immunological changes and circulating autoantibodies can appear: anti-nuclear autoantibodies (ANA), anti-cardiolipine antibodies (ACL), anti-smooth muscle antibodies (ASMA), anti-mitochondria antibodies (AMA), anti-thyroperoxydase antibodies (ATPO) and rheumatoid factor (RF). Serological auto-immune manifestations were explained by the lymphotropism of hepatitis C virus (12).
Arguments in favor of lichen planus association with chronic liver disease are:
Presence of chronic hepatitis and specific markers in a much higher proportion in patients with lichen planus than in the general population (3,11,13).
The occurrence of antibodies directed against basal keratinocytes was found in chronic hepatitis (11).
Several cases were reported when lichenoid eruptions or oral lichen planus lesions occurred after hepatitis B vaccination (14).
The association of the lichen planus with the chronic liver disease has been known since 1980. The prevalence of the chronic hepatitis in the patients with lichen planus has been found to be between 0.5-35% in studies conducted in various geographic areas (2,3). In 1991, Monki and collaborators reported the first case of histologically confirmed lichen planus in a patient with chronic active CV hepatitis, suggesting an association between the two. Since then, more research has been conducted that supported this connection. The reported data showed a prevalence of chronic active hepatitis with C virus between 16 and 55% for the study groups and between 0.17 and 4.7% for controls (3).
A study conducted by Lodi and collaborators (2004) investigated the presence of HCV- Ab in a group of 581 patients, of whom 303 were diagnosed with oral lichen planus based on clinical and histopathological criteria. The remaining 278 subjects, who did not have suggestive clinical oral lesions, were included in the control group. The results of the study showed that 19.1% of the patients diagnosed with lichen planus had HCV Ab versus only 3.2% of the controls (13).
Imhof and collaborators, in a study conducted in 1996 in a region of Germany, found HCV Antibodies in 16% of patients diagnosed with oral lichen planus compared to 1.1% in the control group (15). This percentage is lower than the results from the research in the Mediterranean areas, due to the reduced incidence of chronic HCV in this country.
In a study by Bellman and collaborators from Miami, on a group of patients with oral lichen planus, HCV Antibodies were found in 23% of the study group compared to only 4.8% of the control group (16).
Mignona, in a large study on 263 patients with oral lichen planus, found anti-HCV Antibodies in 29% of the subjects, compared to the control group where the proportion was only 3% (17).
In a meta-analysis of retrospective studies conducted since 1990 till now, we can find a prevalence of chronic hepatitis C virus 3-9 times higher in patients with oral lichen planus compared to controls (3). Depending on the geographic areas in which research was conducted, the prevalence of hepatitis C virus on patients diagnosed with oral lichen planus varies quite widely - 25.7% (Lodi, Campisi, Carozzo, Mignon) in Italy (13,17-19), 19% (Sancez-Perez Gimenez-Garcia) in Spain (20-22), 5.8% (Denli, Harman, Karavelioglu, KirtaK) in Turkey (23-27). These values are higher than the reported prevalence of chronic hepatitis C virus in the general population of these countries.
A follow-up study conducted in Japan on a group of patients diagnosed with hepatitis C showed an incidence of oral lichen planus of 12.5%, while other studies have not shown any connection between the two conditions. Opposing results were reported by Nagao, who found HCV antibodies in 68% of patients with lichen planus (28).
Comparing oral lichen planus patients who associate various liver diseases and those without significant general pathology, several differences and observations were noted. Thus regarding clinical form at the time of presentation, the evolution during follow-up and therapeutic it was observed that patients who associate a form of chronic liver disease generally show extensive forms of oral lichen planus, with frequent periods of exacerbation of clinical lesions and symptoms refractory to treatment, which is in line with the severity of liver disease (29-32).
Inconsistencies between the results of these observational studies can be explained by the different prevalences of hepatitis C virus in the general population. Specifically, the prevalence of lichen planus tends to reach a peak in adulthood. In addition, hepatitis C prevalence varies by age in endemic areas (3). Therefore, the association between the two disorders may be due to the fact that patients with lichen planus are usually middle-aged adults, generally older than subjects in control groups, thus more likely to be infected with Hepatitis C virus (3). Another explanation of the very different results reported in literature may be the small size of the study groups, resulting in a low statistical value (1). Some authors remarked that research conducted in countries where the prevalence of hepatitis C virus is high, such as Southern Europe and subtropical areas, showed a significant association with oral lichen planus, while studies conducted in regions where the prevalence of hepatitis C is low, such as Northern Europe , had insignificant or even negative results (33). However, these regional variations suggest that other factors than patient's age and prevalence of hepatitis C virus are involved in the etiology of the lichen planus and could be responsible for the inconsistent results of the observational studies. Studies conducted in countries with different lifestyles and behavioral habits could help research on this hypothesis.
Thus, in a study that took place in Iran, a country with a totally different lifestyle from Western countries and in which the prevalence of hepatitis with C virus is very low, only 1%, Ghaderi and Makhamalbaf found an association of only 1.4% of hepatitis C in patients diagnosed with oral lichen planus (34).
In another study conducted by Henerson and collaborators in 2001 which examined the patients infected with hepatitis C virus during regular dental check-ups found evident clinical signs suggestive for the oral lichen planus diagnosis in 20% of these patients (3). Since the incidence of hepatitis C in the UK population is only 1%, the authors of this study concluded that there is a direct proportion between the prevalence of hepatitis C and oral lichen planus (3).
The topic of this article is interesting and useful as it is connected to the regular follow-up of the patients for both the oral disease and the liver involvement. Often the acute, erosive form of lichen planus is associated with chronic hepatitis C virus in an active stage which is biochemically releaved by elevated levels of serum transaminases and accelerated viral replication. In this situation it is necessary to investigate the hepatitis and an interdisciplinary collaboration with specialists of infectious diseases for balancing the underlying and the appropriate therapy. Another important aspect is the need to test all patients diagnosed with oral lichen planus for the presence of hepatitis C virus as this liver disease has often a long time asymptomatic evolution and goes undiagnosed. ❑
CONCLUSIONS
Chronic HCV is a disease with multiple social implications and response throughout the whole body, having many extrahepatic manifestations.
Association of oral lichen planus with chronic hepatitis C may result in a longer evolution of oral lesions of lichen planus, with repeated exacerbations according to the degree of liver function decompensation.
The type of lichen planus frequently associated with chronic hepatitis C is the erosive-ulcerative type, accompanied by acute symptoms with significant functional disorders and affecting the patient's quality of life.
Results of studies in literature have shown that the prevalence of hepatitis C in patients with oral lichen planus is higher compared to the general population; these values vary to a great extent because chronic HCV has a worldwide distribution which is different from one geographical region to another.
ACKNOWLEDGMENTS
This paper was supported by the Sectorial Operational Program Human Resources Development (SOP HRD) 2007-2013, financed from the European Social Fund and the Romanian Government under the contact number POSDRU/107/1.5/S/82839
CONFLICT OF INTEREST
none declared.
References
- 1.Antonelli A, Ferri C, Gianotti C, et al. HCV infection pathogenesis, clinical, manifestations and therapy. Clin Exp Rheumatol. 2008;26:S39–47. [PubMed] [Google Scholar]
- 2.Lodi G, Pellicano R, Carrozzo M. Hepatitis C virus infection and lichen planus: a systematic review with meta-analysis. Oral Dis. 2010;16:601–12. doi: 10.1111/j.1601-0825.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 3.Petti S, Rabiei M, De Luca M et al. The magnitude of the association between hepatitis C virus infection and oral lichen planus: meta-analysis and case control study. Odontology. 2011;99:168–78. doi: 10.1007/s10266-011-0008-3. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Carozzo M. Oral mucosa disease-lichen planus. Br J Oral Maxilofac Surg. 2008;46:15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]
- 5.Tovaru S. Incidence of oral lesions in a selected hospital populations Poster 11 th Biennal Congress of EAOM Athens 2012 [Google Scholar]
- 6.Sugerman PB, Savage NW, Walsh LJ, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 7.Mohd Hanafiah K, Groeger J, Flaxman AD. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 8.Molnar GB, Cocean S, Jebeleanu L, et al. Evaluarea epidemiologica a infectiei anamnestice cu virus hepatitic C la populatia din 11 judete ale Romaniei. Rom J Infect Dis. 2005;8:3–7. [Google Scholar]
- 9.Gheorghe L, Csiki IE, Iacob S, et al. The prevalence and risk factors of hepatitis C virus infection in adult population in Romania: a nationwide survey 2006-2008. J Gastrointestin Liver Dis. 2010;19:373–9. [PubMed] [Google Scholar]
- 10.Ibrahim HA, Baddour MM, Morsi MG, et al. Should we routinely check for hepatitis B and C in patients with lichen planus or cutaneous vasculitis? East Mediterr Health J. 1999;5:71–8. [PubMed] [Google Scholar]
- 11.Ko HM1, Hernandez-Prera JC, Zhu H, et al. Morphologic Features of Extrahepatic Manifestations of Hepatitis C Virus Infection Clinical and Developmental Immunology. 2012;2012:740138–740138. doi: 10.1155/2012/740138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouelhi L, Debbeche R, Sfar I, et al. Auto-immune serological disorders in chronic viral C hepatitis: prevalence and clinical significance. Tunis Med. 2008;86:777–81. [PubMed] [Google Scholar]
- 13.Lodi G, Giuliani M, Majorana A, et al. Lichen planusand hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br. J Dermatol. 2004;151:1172–81. doi: 10.1111/j.1365-2133.2004.06257.x. [DOI] [PubMed] [Google Scholar]
- 14.Ciaccio M, Rebora A. Lichen planus following HVB vaccination: a coincidence? Br J Dermatol. 1990;122:424–424. doi: 10.1111/j.1365-2133.1990.tb08294.x. [DOI] [PubMed] [Google Scholar]
- 15.Imhof M, Popal H, Lee JL. Prevalence of hepatitis C virus antibodies and evaluations of hepatitis C virus genotypes in patients with lichen planus. Dermatology. 1997;195:1–5. doi: 10.1159/000245675. [DOI] [PubMed] [Google Scholar]
- 16.Bellman B, Reddy R, Falanga V. Lichen planus associated with hepatitis C. Lancet. 1995;346:1234–1234. doi: 10.1016/s0140-6736(95)92945-2. [DOI] [PubMed] [Google Scholar]
- 17.Mignona MD, Lo M, Favia G, et al. Oral lichen planus and HCV infection: a clinical evaluation of 263 cases. Int J Dermatol. 1998;37:575–8. doi: 10.1046/j.1365-4362.1998.00510.x. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani M, Lajolo C, Miani CM, et al. Hepatitis C virus chronic infection and oral lichen planus: an italian case control study. Eur. J Gastroenterol Hepatol. 2007;19:647–52. doi: 10.1097/MEG.0b013e32821f6134. [DOI] [PubMed] [Google Scholar]
- 19.Carozzo M, Gandolfo S, Carbone M, et al. Hepatitis C virus infection in italian patients with oral lichen planus: a prospective case control study. J Oral Pathol med. 1996;25:527–33. doi: 10.1111/j.1600-0714.1996.tb01726.x. [DOI] [PubMed] [Google Scholar]
- 20.Gimenez-Garcia R, Perez-Castrillon JL. Lichen planus and hepatitis C virus infection. J Eur Acad Dermatol Venerol. 2003;17:291–5. doi: 10.1046/j.1468-3083.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez Perez J, De Castro M, Buezo GF, et al. Lichen planus and hepatitis C virus: prevalence and clinical presentation of patients with lichen planus and hepatitis C virus infection. Br J Dermatol. 1996;134:715–9. doi: 10.1111/j.1365-2133.1996.tb06977.x. [DOI] [PubMed] [Google Scholar]
- 22.Gimenez Arnau A, Alayon Lopez C, Camarasa JG. Lichen planus and hepatitis C. J Eur Acad Dermatol Venerol. 1995;5:S84–5. [Google Scholar]
- 23.Denli YG, Durdu M, Karakas M. Diabetes and hepatitis frequency in 140 lichen planus cases in Cukurova region. J Dermatol. 2004;31:293–8. doi: 10.1111/j.1346-8138.2004.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 24.Harman M, Akdeniz S, Dursun M, et al. Lichen planus and hepatitis C virus infection: an epidemiologic study. Int J clin Pract. 2004;58:1118–9. doi: 10.1111/j.1742-1241.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 25.Karaverioglu D, Koytak ES, Bazkaya H, et al. Lichen planus and HCV infection in Turkish patients. Turk J Gastroenterol. 2004;15:133–6. [PubMed] [Google Scholar]
- 26.Kirtak N, Inaloz HS, Ozgoztasi O, et al. The prevalence of hepatitis Cvirus infectionin patients with lichen planus in Gazinateq region of Turkey. Eur J Epidemiol. 2000;16:1159–61. doi: 10.1023/a:1010968309956. [DOI] [PubMed] [Google Scholar]
- 27.Ilter N, Senol E, Gurer MA, et al. Oral Lichen planus and hepatitis C virus in Turkish patients. Dermatol venerol. 1998;10:192–3. doi: 10.1111/j.1468-3083.1998.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 28.Nagao Y, Myoken Y, Katayama K, et al. Epidemiological survey of oral lichen planus among HCV infected inhabitantsin a town in Hiroshima Prefecturrein Japan from 2000 to 2003. Oncol Rep. 2007;18:1177–81. [PubMed] [Google Scholar]
- 29.Tovaru S, Tovaru M, Costache M, et al. Medicina si patologie Orala volumul I Q Med Publishing 2008 [Google Scholar]
- 30.Gandolfo S, Carbone M, Zulian P, et al. Oral lichen planus and liver pathology. II the clinic-statistical correlations between oral manifestations and liver damage. Minerva Stomatol. 1992;41:209–13. [PubMed] [Google Scholar]
- 31.el Kabir M, Scully C, Porter K, et al. Liver function in UK patients with oral lichen planus. Clin Exp Dermatol. 1993;18:12–6. doi: 10.1111/j.1365-2230.1993.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 32.Beaird LM, Kahloon N, Franco J, et al. Incidence of hepatitis c in lichen planus. J Am Acad Dermatol. 2001;44:311–1. doi: 10.1067/mjd.2001.111624. [DOI] [PubMed] [Google Scholar]
- 33.Bagan JV, Ramon C, Gonzalez L, et al. Preliminary investigation of the association of oral lichen planus and hepatitis C. Oral surg Oral Med Oral Pathol Oral radiol Endod. 1998;85:532–6. doi: 10.1016/s1079-2104(98)90286-4. [DOI] [PubMed] [Google Scholar]
- 34.Ghaderi R, Makhmalbaf Z. The relationship between lichen planus and hepatitis C in Birjand, Iran. Shiraz E-med J. 2007;8:72–9. [Google Scholar]