Abstract
Objective
There is increasing pre-clinical and clinical evidence that metformin, a commonly used diabetes medication, has a protective effect in cancer. The aim of this review is to discuss metformin's anti-cancer molecular mechanisms of action and to summarize the current literature demonstrating metformin's potential in gynecologic cancer prevention and treatment.
Methods
A PubMed search was conducted combining the keywords “metformin” with “neoplasm”, “uterine neoplasms”, “ovarian neoplasms”, and “uterine cervical neoplasms”. Studies published in English between 1994 and 2014 were included.
Results
Pre-clinical studies in endometrial, ovarian, and cervical cancer suggest that metformin inhibits the growth of cancer cells. The primary molecular mechanism mediating this effect appears to be the activation of AMP-activated protein kinase (AMPK) and the subsequent inhibition of mammalian targets of rapamycin (mTOR). The pre-clinical findings are augmented by clinical studies indicating that metformin use is associated with a reduced risk of cancer and improved survival in diabetic women with ovarian and endometrial cancer. No clinical analyses have evaluated metformin use and cervical cancer. Overall, the data showing a favorable effect of metformin is strongest for endometrial and ovarian cancer and prospective clinical testing is ongoing in these two malignancies.
Conclusions
Numerous clinical studies have reported an association between metformin use by diabetic patients and improved outcomes in gynecologic cancers. In addition, pre-clinical reports have identified plausible biological mechanisms to explain the molecular mechanism of action of metformin in cancer. However, the most important question remains unanswered: Will metformin be effective against cancer in patients without diabetes? Until this question is answered with prospective clinical testing, the role of metformin in the treatment or prevention of gynecologic malignancies remains theoretical and the clinical use of metformin as a cancer therapeutic is experimental.
Keywords: metformin, gynecologic cancer, endometrial cancer, ovarian cancer, glucose
INTRODUCTION
Despite the investment of considerable time and money, there has been minimal success in the development of new gynecologic cancer therapies. One approach to decreasing the cost and increasing the efficiency of new cancer drug development is drug repurposing [1]. This approach exploits the anti-cancer effects of medications that are FDA approved for non-cancer indications and builds on available safety and pharmacology data to develop the drug as a cancer therapeutic. Currently, one of the medications with the greatest potential for repurposing in gynecologic oncology is metformin, an oral biguanide, currently used for the treatment of diabetes and polycystic ovarian syndrome.
A group of investigators at the University of Dundee first suggested that metformin has a protective effect in cancer in 2005. In this study that included 11,867 men and women, Evans et al. found that patients with type II diabetes taking metformin had a lower risk of dying from cancer when compared to patients who did not use metformin (OR 0.76; 95% CI: 0.67-0.93)[2]. These findings were not cancer-type specific. Following this landmark report, several other observational studies suggested an association between metformin use and reduced cancer risk and/or improved cancer survival [3-5]. In 2013, a meta-analysis which included 6 observational studies reported a significant reduction in the risk of cancer death in metformin users compared to non-users (OR 0.65, 95% CI: 0.53-0.80, p<0.0001) [6], confirming the findings of a 2010 meta-analysis [7].
At first, metformin may seem an unlikely candidate for repurposing as a cancer therapeutic. However, metformin alters metabolism and it is becoming increasingly clear that cancer cells have metabolic derangements that may make them uniquely vulnerable to drugs that target metabolism. Perhaps the most well characterized metabolic derangement in cancer cells is that of glucose utilization. Non-transformed cells, in the presence of oxygen, process glucose through glycolysis, the tricarboxylic acid (TCA) cycle, and the mitochondrial respiratory transport chain to produce 38 molecules of adenosine 5’-triphosphate (ATP) per molecule of glucose. In contrast, cancer cells preferentially generate energy only through glycolysis, even when oxygen is present, which inefficiently generates lactic acid and only 2 molecules of ATP per molecule of glucose. This reliance of cancer cells on glycolysis alone is referred to as “aerobic glycolysis” and was first described in the 1920s by German Nobel laureate Otto Warburg [8]. In addition, cancer cells metabolize lipids in a unique fashion. Cancer cells are capable of de novo fatty acid synthesis, whereas non-transformed cells are dependent on fatty acids obtained from dietary intake [9]. In ovarian cancer, recent findings indicate that cancer cells also obtain lipids from adjacent normal adipocytes, using the lipids to fuel rapid growth [10, 11].
Since cancer cell-specific derangements in glucose and lipid metabolism may represent an “Achilles heel” of cancer, there has been a surge of interest in identifying agents, such as metformin, that may be directed against cancer cell metabolism. In fact, both pre-clinical research on metformin and prospective trials; testing it as a cancer therapeutic are advancing quickly. The aim of this review is to examine the anti-cancer mechanisms of metformin and to summarize the literature indicating metformin's potential in gynecologic cancer prevention and treatment.
METHODS
This article reviews the English language literature for studies on metformin and gynecologic cancers. We searched the MEDLINE (PubMed) database for literature published between January 01, 1994- September 30, 2014, combining the MESH keywords “metformin” with “neoplasm”, “uterine neoplasms”, “ovarian neoplasms”, and “uterine cervical neoplasms”. Articles published in peer-reviewed journals and published abstracts from national meetings were included. Pre-clinical studies were selected if they assessed the effects of metformin on carcinogenesis or treatment response in gynecologic cancers. Clinical studies were selected if the study population was described, metformin use was reported and if the measures of association (hazard ratio (HR) or odd ratio (OR)) were provided. Additional reports were collected by systematically reviewing all references from retrieved papers. The website clinicaltrials.gov was searched to identify ongoing prospective clinical trials of metformin in gynecologic cancers.
RESULTS
Metformin's Proposed Mechanism of Action in Cancer
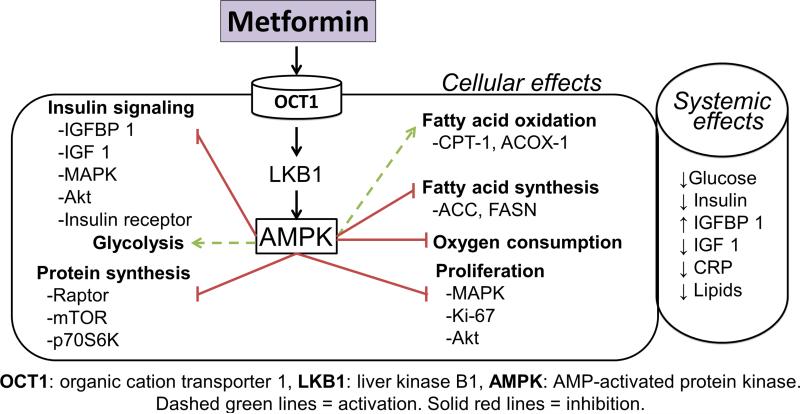
Studies investigating metformin's anti-cancer mechanisms of action focus on both the systemic effects of the drug and the direct effects on cancer cells (Fig 1). Systemically, insulin and insulin-like growth factors (IGF) stimulate cancer cell proliferation through activation of PI3K-AKT signaling, leading to tumor growth [12]. One hypothesis is that by improving peripheral insulin sensitivity and increasing insulin growth factor binding proteins (IGFBP), metformin treatment results in a net reduction in systemic insulin and IGFs, ultimately leading to inhibition of tumor growth [13]. The current hypothesis for metformin's direct action in cancer cells points to AMPK activation as primarily mediating metformin's effects [14]. Metformin enters the cell through the organic cation transporter (Oct 1/2) and inhibits complex I of the respiratory transport chain which reduces ATP production [15]. The reduction in ATP levels triggers the tumor suppressor, LKB1 (Liver Kinase B1), to phosphorylate (activate) AMPK [16]. AMPK is charged with maintaining energy hemostasis and when activated AMPK decreases ATP consumption by inhibiting fatty acid and protein synthesis. Since cancer cells have baseline metabolic derangements, the additional metabolic stress induced by unchecked AMPK activation ultimately results in cell death.
Fig. 1.
Possible mechanisms of action of metformin in cancer.
Recent research has produced a more detailed characterization of metformin's action in cancer cells. For example, through inhibition of endogenous reactive oxygen species produced by complex 1 of the electron transport chain, metformin has been shown to reduce DNA damage, oxidative stress and reduce mutation rate [17]. In addition, reports indicate that metformin inhibits mitochondrial glycerophosphate dehydrogenase, a redox shuttle enzyme that reduces the conversion of lactate and glycerol to glucose and impairs hepatic gluconeogenesis [18].
Metformin also has novel anti-cancer effects that are independent of its impact on metabolism. Metformin may protect against cancer through modulation of small non-coding RNA segments (miRNA) that inhibit gene expression at the posttranslational level. A report by Blandino et al. in breast cancer, showed that metformin's protective effect was mediated by up regulation of a key enzyme in miRNA biogenesis (DICER) and increased expression of miRNA33a [19]. Another study in breast cancer demonstrated that metformin up-regulates tumor suppressive miRNAs, including let-7a (Lethal 7a)[20]. Another interesting hypothesis proposes that metformin targets cancer stem cells. Selective targeting of cancer stem cells by metformin has been reported in breast [21], pancreatic [22], and ovarian [23] cancer. Through the molecular mechanisms outlined above, it is biologically plausible that metformin may have anti-cancer effects in gynecologic malignancies.
Ovarian cancer
Ovarian cancer is the 4th leading cause of cancer death among women (estimated 21,890 new cases and 4,020 deaths from ovarian cancer in the US,2014) [24]. Platinum-based treatment for ovarian cancer was introduced 30 years ago, and taxanes were added in 1996. This combination treatment is the standard first line chemotherapy regimen currently used to treat epithelial ovarian cancer. Unfortunately, since the introduction of these agents, there has been little change in the therapeutic approach and little improvement in overall survival [25]. This lack of progress has motivated ongoing investigations into novel therapeutics including approaches, like metformin, aimed at tumor metabolism.
Pre-clinical studies in ovarian cancer
In 2008, Gotlieb et al. (McGill University, Quebec) published the first report indicating that metformin was cytotoxic to ovarian cancer cells in vitro [26], a finding that has now been supported by numerous other publications [27-30]. The initial molecular studies of metformin in ovarian cancer, like those in other cancers, focused on activation of AMPK and AMPK's downstream targets, including decreased protein synthesis via mTOR inhibition, increased fatty acid oxidation, and decreased fatty acid synthesis. A number of studies demonstrated that, in ovarian cancer cells, metformin activated AMPK in a time and dose dependent manner and altered known downstream targets of AMPK [26, 27, 31, 30]. The in vitro molecular effects of metformin in ovarian cancer are outlined in Table 1.
Table 1.
Molecular effects of metformin in gynecologic cancers in vitro
| Molecular effect | Ovarian CA | Endometrial CA | Cervical CA |
|---|---|---|---|
| Inhibits proliferation of cell lines | Gotlieb, 2008 [26] Rattan, 2011 [27] Chan, 2012 [28] Wu, 2012 [29] Lengyel, 2014 [30] |
Cantrell, 2010 [46] Zhang, 2011 [47] Hanna, 2012 [48] Iglesias, 2013 [49] Sarfstein, 2013 [50] |
Xiao, 2012 [76] Kwan, 2013 [77] Yung, 2013 [78] |
| Activates AMPK and alters targets downstream of AMPK | Gotlieb, 2008 [26] Rattan, 2011 [27] Liao, 2012 [31] Lengyel, 2014 [30] |
Cantrell, 2010 [46] Xie, 2011 [59] Zhang, 2011 [47] Iglesias, 2013 [49] Sarfstein, 2013 [50] |
Xiao, 2012 [76] Yung, 2013 [78] |
| Induces cell cycle arrest | Rattan, 2011[27] Yasmeen, 2011 [32] Lengyel, 2014 [30] |
Cantrell, 2010 [46] Hanna, 2012 [48] Iglesias, 2013 [49] Sarfstein, 2013 [50] Takahashi, 2014 [51] |
None |
| Induces apoptosis and/or autophagy | Yasmeen, 2011 [32] Li, 2012 [33] |
Hanna, 2012 [48] Iglesias, 2013 [49] Sarfstein, 2013 [50] Takahashi, 2014 [51] |
Xiao, 2012 [76] |
| Reduces invasion of cancer cells | Wu, 2012 [29] | Tan, 2011 [52] | None |
| Synergistic with chemotherapy or hormone therapy | Gotlieb, 2008 [26] Yasmeen, 2011 [32] Shank, 2012 [23] Erices, 2013 [37] Xie, 2014 [38] Lengyel, 2014 [30] |
Xie, 2011 [59] Zhang, 2011 [47] Hanna, 2012 [48] |
Do, 2013 [80] |
Several investigators have shifted the focus from AMPK and attempted to categorize the molecular effects of metformin in the framework of the hallmarks of cancer. They have found that metformin's anti-ovarian cancer action is multifaceted. When evaluating the mechanisms mediating metformin's anti-proliferative effect in ovarian cancer, investigators have reported that metformin modulates the cell cycle [27, 32, 30]. Two studies have reported that metformin induces apoptosis in ovarian cancer cells [32, 33], although another study found no induction of apoptosis with metformin treatment [29]. In addition, metformin has been shown to inhibit ovarian cancer cell adhesion, invasion, and migration [29], highlighting a potential role combating metastasis. Inhibition of angiogenesis was suggested in a study that noted a reduction in CD31 staining [34]. Moreover, a recent report indicates that, in ovarian cancer, metformin inhibits adipocyte-induced proliferation and migration of cancer cells [35]. This is the first report in a gynecologic cancer indicating that metformin's effect extends beyond cancer cells to the adjacent stromal cells in the tumor microenvironment.
Novel effects of metformin in ovarian cancer have also been investigated. Reports have indicated that combining metformin with either a PI3K inhibitor (LY294002) [33] or an inhibitor of complex III of the electron transport chain (phenethyl isothiocyanate) [28] achieves greater ovarian cancer cell death than either compound alone. Ronald J. Buckanovich and his group at the University of Michigan demonstrated, in vitro and in mouse models, that metformin therapy alone and in combination with cisplatin inhibits the growth of ovarian cancer stem cells [23]. These findings have been augmented by a recent report indicating that metformin down regulates expression of ovarian cancer stem cell markers through activation of FOXO3 [36].
In xenograft mouse models of ovarian cancer, metformin has been shown to decrease tumor burden, with up to 60% lower mean tumor weights in mice treated with metformin compared to mice treated with placebo [34, 23, 29, 30]. Many of the in vitro molecular mechanisms of action of metformin in ovarian cancer were also found to be present in mouse models, including activation of AMPK signaling pathways [34]. In a recent study using a both xenograft and genetic mouse models of ovarian cancer, our group at the University of Chicago show that metformin prevents tumor growth through induction of cell cycle arrest and sensitizes tumors to paclitaxel [30]. These findings support earlier reports in xenograft mouse models and in vitro indicating that metformin improves response to cisplatin [34, 23, 37, 38]. The findings regarding metformin and ovarian cancer in mouse models are outlined in Table 2.
Table 2.
Molecular effects of metformin in gynecologic cancers in vivo
| Molecular effect | Ovarian CA | Endometrial CA | Cervical CA |
|---|---|---|---|
| Inhibits tumor growth in animal models | Rattan, 2011 [34] Shank, 2012 [23] Wu, 2012 [29] Lengyel, 2014 [30] |
Iglesias, 2013 [49] | None |
| Attenuates endometrial proliferation induced by estrogen. | NA | Zhang, 2013 [60] | NA |
| Activates AMPK and alters targets downstream of AMPK | Rattan, 2011 [34] | None | |
| Synergistic with chemotherapy or hormone therapy | Rattan, 2011 [34] Shank, 2012 [23] Lengyel, 2014 [30] |
Zhang, 2011 [47] Hanna, 2012 [48] |
None |
| Restricts growth of cancer stem cells | Shank, 2012 [23] Hu, 2014 [36] |
None |
Clinical studies in ovarian cancer
Metformin's potential role in the prevention of ovarian cancer was evaluated in one case-control analysis of 1,611 ovarian cancer cases, which reported that long-term users of metformin (>30 prescriptions) had a trend toward a reduced risk of ovarian cancer (OR 0.61, 95% CI 0.30-1.25) [39]. Two published studies have evaluated the relationship between metformin use and ovarian cancer survival, both reporting promising findings. In a retrospective cohort analysis of ovarian cancer patients at University of Chicago, we found that progression-free survival at 5 years was 51% for diabetic patients who used metformin compared with 23% for non-diabetic patients and 8% for diabetic patients who did not use metformin (p=0.03). Overall survival at five years was 63% for diabetic patients who used metformin compared with 37% for non-diabetic patients and 23% for diabetic patients who did not use metformin (p=0.03) [40]. In an independent study at Mayo Clinic Rochester, the Shridhar group found a nearly identical magnitude of improved survival among ovarian cancer patients who used metformin [41]. Both of these studies used death from ovarian cancer, not all-cause mortality, as the primary outcome. The findings that diabetics on metformin had an improved outcomes when compared to nondiabetics was unexpected in light of prior published evidence indicating that patients with diabetes have worse ovarian cancer outcomes [42]. The clinical studies of metformin and ovarian cancer are outlined in Table 3.
Table 3.
Summary of clinical studies of metformin and gynecologic cancers
| Cancer Type | Outcome | Study Design | Finding | Reference |
|---|---|---|---|---|
| Ovary | Prevention | Case control Cases: n=1,611 Controls: n=9,170 |
Long-term use of metformin is associated with a trend toward decreasing ovarian cancer risk (OR 0.61, CI 0.30-1.35). | Bodmer, 2011 [39] |
| Ovary | Survival | Cohort: n=341 Diabetics: n=44 Metformin users: n=16 |
In uni-variable analysis, metformin use was associated with improved progression-free and overall survival. In multi-variable analysis, metformin users had decreased hazard for disease recurrence (HR 0.38, 95% CI 0.16-0.90). | Romero, 2012 [40] |
| Ovary | Survival | Case control Cases: n=72 (ovarian cancer patients using metformin) Controls: n=143 (ovarian cancer patients without diabetes) |
In uni-variable analysis, metformin use was associated with improved overall survival. In multi-variable analysis, patients that did not use metformin had increased hazard for death (HR 2.7, 95% CI 1.4-5.4). | Kumar, 2013 [41] |
| Endometrial | Prevention | Case control Cases: n=2,554 Controls: n=15,324 Cohort: n=1,241 |
No association between metformin use and risk of endometrial cancer. | Becker, 2013 [61] Luo 2014 [62] |
| Endometrial | Survival | Cohort: n=1,495 Diabetics: n=363 Metformin users: n=200 |
Non-metformin users had 1.8 times worse RFS (95%CI: 1.1-2.9 p-0.02) and 2.3 times worse OS (95%CI CI: 1.3-4.2 p=0.005) after adjusting for age, grade, stage, histology and adjuvant treatment. | Ko, 2013 [63] |
| Endometrial | Survival | Cohort: n=985 Diabetics: n=250 Metformin users: n=114 |
Metformin use associated with improved OS (HR=0.57; 95% CI: 0.31-0.97, p=0.04) only in patients with non-endometroid subtypes. | Nevadunsky, 2013 [64] |
| Endometrial | Window of Opportunity | Cohort: n= 11-15 | Non-diabetic patients prospectively treated with metformin before surgery for endometrial cancer. | Schuler, 2013 [65] Soliman, 2013 [66] Laskov, 2014 [67] Mitsuhashi, 2014 [68] |
OR: odds ratio, CI: confidence interval, HR: hazards ration, RFS: recurrence-free survival, OS: overall survival
Endometrial Cancer
Endometrial cancer is the fourth most common cancer in women in the United States (estimated 52,630 new cases/8,590 deaths in the US in 2014) [24]. Risk factors for endometrial cancer include unopposed estrogen [43], obesity [44], and hyperinsulinemia [45]. It is biologically plausible that metformin could favorably influence these risk factors because of the drug's beneficial effects on diabetes and metabolic syndrome.
Pre-clinical studies in endometrial cancer
In endometrial cancer, in vitro studies have demonstrated that metformin inhibits the growth of cancer cells in a dose-dependent fashion [46-50]. The in vitro molecular effects of metformin in endometrial cancer are outlined in Table 1. Several studies have evaluated the molecular mechanisms by which metformin may inhibit endometrial cancer cell growth. The findings of those studies indicate that metformin induces cell cycle arrest and apoptosis [46, 48-51]. Metformin has also been shown to decrease migration and invasion of endometrial cancer cells. In an elegant study, Tan et al. showed that the ability of endometrial cancer cells to invade was impaired by treatment with sera from PCOS patients on metformin as compared to treatment with sera from PCOS patients not using metformin [52].
Endometrial cancer harbors genetic aberrations, including loss of PTEN and activation of mTOR [53, 54], which may make metformin particularly effective for this malignancy. Drugs that inhibit mTOR, such as rapamycin, are already approved for use in the treatment of renal cell carcinoma [55, 56] and have undergone prospective testing in endometrial cancer [57, 58]. Functionally, metformin acts as an mTOR inhibitor by activating AMPK, which negatively regulates mTOR. Consistent with this signaling cascade, endometrial cancer cells treated with metformin show a dose dependent increase in AMPK activation and a decrease in mTOR signaling [46, 59, 47, 49, 50]. In vivo studies have confirmed several of these molecular effects in endometrial cancer (Table 2). In a study led by Karen H. Lu at the University of Texas MD Anderson Cancer Center, investigators found that, in two different mouse models, metformin treatment reduced mean tumor weight by almost 50% and lowered mTOR signaling (measured by phosphorylated S6 ribosomal protein) in tumors [49]. In a separate study, this group also evaluated metformin in the context of endometrial cancer prevention. Here the investigators analyzed the endometrium of Zucker rats without cancer and reported that metformin attenuates proliferative pathways induced by estrogen [60].
Studies in endometrial cancer have also evaluated the possibility that metformin could improve response to standard therapy. For example, investigators have demonstrated synergism between metformin and paclitaxel in vitro and in animal models [48]. When metformin's effect on hormonal therapy was evaluated, it was found to be synergistic with medroxyprogesterone acetate and to overcome progestin resistance [59, 47, 49]. There have been no published reports evaluating if metformin modulates radiation therapy.
Clinical and window of opportunity trials in endometrial cancer
Metformin's potential role in the prevention of endometrial cancer has been evaluated in two studies, both indicating that metformin use was not associated with a reduced risk of endometrial cancer [61, 62]. The clinical studies of metformin and endometrial cancer are outlined in Table 3. Two studies, however, have reported that metformin use is associated with improved endometrial cancer survival. In a retrospective analysis of 1,495 endometrial cancer cases, the Bae-Jump group (University of North Carolina) found that metformin use was associated with significantly improved overall survival and recurrence free survival, but was not significantly associated with time to recurrence. Nonmetformin users were 2.0 times as likely to die as metformin users (95% CI:1.3-3.2, p = 0.003) [63]. This improvement in overall survival was echoed by Nevadunsky et al, (Albert Einstein College of Medicine) but only among patients with non-endometroid histology. In this study the authors report that, among endometrial cancer patients with non-endometroid histologic subtypes, diabetics who used metformin had a greater overall survival (HR=0.57; 95% CI: 0.31-0.97, p = 0.04) when compared to non-diabetics [64]. Of note, the primary outcome in both of these studies was all-cause mortality, not death from endometrial cancer. Therefore, one must use caution in inferring that metformin improves survival from endometrial cancer specifically, since it is possible that metformin is decreasing other causes of mortality (e.g. diabetes complications or obesity).
Four independent preoperative window of opportunity trials have been completed in endometrial cancer patients [65-68]. All of the studies had a similar design; non-diabetic patients with endometrial cancer (n=12-30) were treated with metformin (1500-2250 mg/day) for 4 to 6 weeks. Serum and endometrial samples were collected before and after metformin use (at hysterectomy). All four studies reported reduced levels of a cell proliferation marker (Ki67) in endometrial tumor samples after metformin treatment and decreased expression of metformin targets, including phospho-AMPK, phospho-AKT and Ras-Mitogen activated protein kinase (Ras-MAPK). Two of the studies analyzed serum and reported a reduction in insulin and IGF-1 levels with metformin treatment [67, 68]. One study, conducted at Chiba University in Japan, reported that the concentration of metformin in the cancer tissue was approximately 20% of the plasma metformin concentration. This was an important step forward in the field, since it was the first evidence that metformin actually reaches measurable concentrations in tumors [68].
Cervical cancer
Worldwide, cervical cancer is among the most common female cancers with over 528,000 new cases and 266, 000 deaths in 2012 [69]. In the United States, the incidence of cervical cancer is decreasing [70]. However, patients with advanced stage or relapsed cervical cancer continue to have a five-year survival of only 10-20% [71]. mTOR overexpression has been associated with a poor prognosis in cervical cancer [72-74]. Recently, a phase ll trial of the mTOR inhibitor, Temsirolimus, in patients with advanced cervical cancer resulted in 9 patients (57.6%) with stable disease after treatment [75]. Since metformin also inhibits mTOR there is strong rationale for evaluating metformin as a protective agent in cervical cancer.
Pre-clinical studies in cervical cancer
There have been few studies of the effects of metformin in cervical cancer as compared to those in ovarian and endometrial cancers. The in vitro molecular effects of metformin in cervical cancer are outlined in Table 1. Three studies using cell lines have reported inhibition of cervical cancer growth with metformin treatment [76-78]. Metformin's anti-proliferative effect in cervical cancer appears to be due to induction of apoptosis and autophagy [76], rather than the induction of cell cycle arrest found in other gynecologic cancers. In cervical cancer cells, metformin activates AMPK and alters its downstream targets [76, 78]. Of note, Xiao et al. found that metformin's effect in cervical cancer cells was reliant on intact LKB1 [76]. This may be clinically relevant, since 20% of cervical cancers have LKB1 mutations [79]. The novel molecular mechanisms of action of metformin that have been identified in in vitro studies of cervical cancer include targeting Wnt/β-catenin signaling [77], inhibition of FOXM1 signaling [78], and the inhibition of heme oxygenase-1 expression, which sensitizes cervical cancer cells to paclitaxel [80]. Metformin has not been tested in animal models of cervical cancer.
Clinical studies in cervical cancer
To the best of our knowledge there have been no epidemiologic studies evaluating the association between metformin use and cervical cancer risk or survival.
Ongoing clinical trials in gynecologic cancers
Based on the abundance and consistency of the findings in epidemiologic and pre-clinical studies, the evaluation of metformin as a treatment for endometrial and ovarian cancer appears to be warranted. There are currently a number of registered trials testing metformin as a treatment for endometrial or ovarian cancer [81] (Table 4). The University of Chicago (PI S.D Yamada) is conducting a phase II trial in ovarian cancer in which newly diagnosed patients are treated upfront with either metformin or placebo in addition to standard chemotherapy. After completion of chemotherapy, the patients continue metformin or placebo for 2 years as maintenance therapy (NCT02122185). The University of North Carolina and the Gynecologic Oncology Group (PI V. Bae-Jump) is conducting a phase II/III trial in endometrial cancer in which patients with advanced or recurrent disease are treated with either metformin or placebo in addition to paclitaxel and carboplatin (NCT02065687). Both of these trials are enrolling non-diabetic patients and are using doses of metformin prescribed for diabetes treatment (850 mg bid). Translational studies embedded in the trials are aimed at determining whether metformin's anticancer effect is a result of systemic alterations in insulin and glucose, a direct effect on the cancer cell, or both. There are no ongoing trials of metformin as primary prevention of gynecologic malignancy or as adjuvant treatment for cervical cancer. Certainly, if the treatment trials have a positive result then it would be a natural progression to initiate primary prevention trials of metformin in endometrial and ovarian cancer.
Table 4.
Ongoing clinical trials of metformin in gynecologic cancers
| Cancer | Study Design | Outcome | Reference |
|---|---|---|---|
| Ovary | Phase II RCT Upfront treatment and maintenance therapy |
Progression-free survival in patients treated with metformin or placebo plus standard chemotherapy with metformin or placebo continued for 2 years. University of Chicago |
NCT02122185 PI: S.D Yamada |
| Ovary | Phase II Historic controls |
Recurrence free survival at 18 month in patients treated with metformin plus standard therapy. Molecular analysis focused on evaluation of metformin's effect on cancer stem cells. University of Michigan |
NCT01579812 PI: R. Buckanovich |
| Ovary | Phase I Recurrent OvCa |
Progression free survival following metformin and chemotherapy compared to the length of progression free survival resulting from the patient's immediate prior line of treatment. Fox Chase Cancer Center |
NCT02050009 PI: A. Jain |
| Endometrial | Phase II/III RCT Advanced or recurrent cancer |
Progression-free and overall survival in patients treated with metformin or placebo plus carboplatin and paclitaxel for 6 courses. University of North Carolina |
NCT02065687 GOG-0286B PI: V. Bae-Jump |
| Endometrial | Phase II single arm Advanced or recurrent cancer |
Clinical benefit rate from the combination of metformin, RAD001 (Everolimus), and Letrozole University of Texas MD Anderson Cancer Center |
NCT01797523 PI: P. Soliman |
| Endometrial | Phase III RCT Normal endometrium |
Effect of metformin on biomarkers in patients treated with metformin or placebo. University of Texas MD Anderson Cancer Center |
NCT01697566 PI: K. Lu |
| Endometrial | Phase II RCT Early stage cancer |
Metformin combined with levonorgestrel IUD. |
NCT01686126 PI: A. Obermair NCT01968317 PI: X. Chen NCT02035787 PI: K. Doll |
| Endometrial | Phase IIa Metformin before surgery. |
Molecular changes in endometrial samples before and after metformin treatment |
NCT01205672 PI: P. Soliman NCT02042495 PI: S. Salvador NCT01877564 PI: University of Arkansas |
Limitations
While reports attributing a potential protective effect to metformin in gynecologic malignancies have rapidly increased, there are at least two significant limitations in the published studies. First, the in vitro studies use supra-physiologic doses of metformin, ranging from 165 to 6,600 mg/l (human therapeutic plasma levels = 0.465 to 2.5 mg/l) [13]. Proving efficacy using physiologic doses is critical if metformin is to be validated as a cancer therapeutic. Second, the clinical studies have analyzed patients who were using metformin for diabetes, leaving the question of metformin's ability to protect against cancer in patients without diabetes unanswered. The ongoing clinical trials of metformin as a cancer therapeutic in non-diabetic patients (Table 4) will overcome this limitation. If these clinical trials fail to show that metformin has beneficial effects, the biological rationale for targeting tumor metabolism is sufficiently compelling that testing new molecularly targeted therapies or a more potent biguanide, such as phenformin, could be justified.
Future Directions
When the molecular mechanisms of metformin in gynecologic malignancies are understood, the process of predictive biomarker identification can begin. It is unlikely that metformin will have a protective effect in all cancer patients and may even be harmful to some. Identifying a molecular or metabolic signature that identifies patients most likely to benefit from metformin will optimize its use as a cancer treatment. For example, it has been shown that p53 is involved in mediating the energy-conserving response to AMPK activation and that loss of p53 heightens the metformin-induced energy stress on cancer cells [82], raising the interesting possibility that metformin may have increased efficacy in p53-deficient tumors, like ovarian cancer. In addition, recent studies showed that cancer cell lines with mutations in mitochondrial DNA genes have an increased response to metformin [83]. Perhaps these mitochondrial mutations might identify a subset of patients who would have a particularly positive response to metformin.
If metformin advances as a cancer therapeutic, it will also be important to identify the clinical scenarios where metformin will have the greatest impact. One can envision metformin being used as either prevention or adjuvant treatment. In the context of primary prevention, patients at high risk for cancer (i.e. BRCA or HNPCC mutation carriers) might use metformin as a chemopreventive agent until the time of risk reducing surgery. For secondary prevention, patients might take metformin as maintenance therapy to delay recurrence. In the setting of adjuvant treatment, metformin might be added to Carboplatin/Taxol for chemo sensitization. For endometrial cancer treatment, metformin might be used as a fertility-sparing approach for grade 1 disease confined to the endometrium.
Conclusion
There is increasing evidence that metabolic reprogramming in malignancy promotes a tumor's ability to rapidly proliferate, even under conditions of stress [84]. This has resulted in a surge of interest identifying novel agents that target the metabolic abnormalities in cancer. Metformin is at the forefront of this effort. The drug's long record of safety and low cost ($40/year) make it an attractive candidate for repurposing as a cancer therapeutic. In gynecologic malignancies, clinical studies suggest that metformin use is associated with improved survival and preclinical studies support anti-cancer effects of metformin in endometrial, ovarian, and cervical cancer. However, it remains to be determined whether metformin at standard therapeutic doses will improve survival from gynecologic malignancies in patients without diabetes. Ongoing prospective clinical trials in endometrial, ovarian and breast cancer [85] aim to answer this question. If the results of clinical trials are positive, the drug may ultimately be included in our armamentarium of treatment and prevention. Until that time, the data on metformin in gynecologic malignancies should be regarded as both provocative and promising, but does not justify clinical use.
Highlights.
We summarize the molecular mechanisms of action mediating metformin's protective effect in cancer.
Review the preclinical and epidemiological evidence for metformin's potential role in gynecological cancers.
Description of ongoing prospective testing of metformin in gynecologic cancers and future directions.
Acknowledgements
We thank Gail Isenberg for critically reviewing the manuscript. Dr. Febbraro is supported by Bears Care, the charitable beneficiary of the Chicago Bears Football Club. Dr. Lengyel is supported by grants from the National Cancer Institute (5R01CA111882-07 and 1R01CA169604-01A1). Dr. Romero is supported by grants from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (2K12HD000849-26) and The American Board of Obstetrics and Gynecology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflict of interest to report.
References
- 1.Chong CR, Sullivan DJ. New uses for old drugs. Nature. 2007;448:645–6. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 2.Evans JMM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Br Med J. 2005;530:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 4.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JMM. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landman GW, Kleefstra N, van Hateren KJJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC -16. Diabetes Care. 2010;33:322–6. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prevention Research. 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Reviews Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 10.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt M, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipocyte tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–41. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 13.Dowling R, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: Translational challenges. J Mol Endocrinol. 2012;48:31–43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 14.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5:536–43. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 18.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–6. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nature communications. 2012;3 doi: 10.1038/ncomms1859. doi: 10.1038/ncomms859. [DOI] [PubMed] [Google Scholar]
- 20.Oliveras-Ferraros C, Cufi S, Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B, et al. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: Induction of the tumor suppressor miRNA let-7a and suppression of the TGFbeta-induced oncomiR miRNA-181a. Cell cycle. 2011;10:1144–51. doi: 10.4161/cc.10.7.15210. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together wtih chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila) 2012;5:355–64. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, et al. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol. 2012;127:390–7. doi: 10.1016/j.ygyno.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan S, Coward JI, Bast RC, Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nature Reviews. 2011;11:719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, et al. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–50. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15:166–78. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan DK, Miskimins WK. Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures. Journal of Ovarian Research. 2012;5:19. doi: 10.1186/1757-2215-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu B, Li S, Sheng L, Zhu J, Gu L, Shen H, et al. Metformin inhibits the development and metastasis of ovarian cancer. Oncol Rep. 2012;28:903–8. doi: 10.3892/or.2012.1890. [DOI] [PubMed] [Google Scholar]
- 30.Lengyel E, Litchfield L, Mitra AK, Nieman KM, Mukherjee A, Zhang Y, et al. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.10.026. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao H, Zhou Q, Gu Y, Duan T, Feng Y. Luteinizing hormone facilitates angiogenesis in ovarian epithelial tumor cells and metformin inhibits the effect through the mTOR signaling pathway. Oncol Rep. 2012;27:1873–8. doi: 10.3892/or.2012.1745. [DOI] [PubMed] [Google Scholar]
- 32.Yasmeen A, Beauchamp MC, Piura E, Segal E, Pollak MN, Gotlieb WH. Induction of apoptosis by metformin in epithelial ovarian cancer: Involvement of the Bcl-2 family proteins. Gynecol Oncol. 2011;121:492–8. doi: 10.1016/j.ygyno.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Liu VW, Chan DW, Yao KM, Ngan HY. LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells. Int J Gynecol Cancer. 2012;22:15–22. doi: 10.1097/IGC.0b013e3182322834. [DOI] [PubMed] [Google Scholar]
- 34.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483–91. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tebbe C, Chhina J, Dar SA, Sarigiannis K, Giri S, Munkarah AR, et al. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget. 2014;5:4123–41. doi: 10.18632/oncotarget.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu T, Chung YM, Guan M, Ma M, Ma J, Berek JS, et al. Reprogramming ovarian and breast cancer cells into non-cancerous cells by low-dose metformin or SN-38 through FOXO3 activation. Sci Rep. 2014;4:5810. doi: 10.1038/srep05810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erices R, Bravo ML, Gonzalez P, Olivia B, Racordon D, Garrido M, et al. Metformin, at concentrations corresponding to the treatment of diabetes, potentiates the cytotoxic effects of carboplatin in cultures of ovarian cancer cells. Reprod Sci. 2013:1–14. doi: 10.1177/1933719113488441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y, Peng Z, Shi M, Ji M, Guo H, Shi H. Metformin combined with p38 MAPK inhibitor improves cisplatin sensitivity in cisplatin resistant ovarian cancer. Mol Med Rep. 2014;10:2346–50. doi: 10.3892/mmr.2014.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: A case-control analysis. Gynecol Oncol. 2011;123:200–4. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 40.Romero IL, McCormick A, McEwen KA, Park S, Karrison T, Yamada SD, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol. 2012;119:61–7. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, et al. Metformin intake is associated with better survival in ovarian cancer: A case-control study. Cancer. 2013;119:555–62. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakhru A, Buckanovich RJ, Griggs JJ. The impact of diabetes on survival in women with ovarian cancer. Gynecol Oncol. 2011;121:106–11. doi: 10.1016/j.ygyno.2010.12.329. [DOI] [PubMed] [Google Scholar]
- 43.Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: Review of the evidence and research perspectives. Ann N Y Acad Sci. 2001;943:296–315. doi: 10.1111/j.1749-6632.2001.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: Results from a systematic review and meta-analysis. Int J Biol Markers. 2014;29:e21–9. doi: 10.5301/jbm.5000047. [DOI] [PubMed] [Google Scholar]
- 45.Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol. 1998;12:399–406. doi: 10.3109/09513599809012842. [DOI] [PubMed] [Google Scholar]
- 46.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump V. Metformin is a potent inhibitor of endometrial cancer cell proliferation-implications for a novel treatment strategy. Gynecol Oncol. 2010;116:92–8. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Dong L, Sui L, Yang Y, Liu X, Yu Y, et al. Metformin reverses progestin resistance in endometrial cancer cells by downregulating GloI expression. Int J Gynecol Cancer. 2011;21:213–21. doi: 10.1097/IGC.0b013e318207dac7. [DOI] [PubMed] [Google Scholar]
- 48.Hanna RK, Zhou C, Malloy KM, Sun L, Zhong Y, Gehrig PA, et al. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation on the mTOR pathway. Gynecol Oncol. 2012;125:458–69. doi: 10.1016/j.ygyno.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iglesias DA, Yates MS, van der Hoeven D, Rodkey TL, Zhang Q, Co NN, et al. Another surprise from Metformin: Novel mechanism of action via K-Ras influences endometrial cancer response to therapy. Mol Cancer Ther. 2013;12:2847–56. doi: 10.1158/1535-7163.MCT-13-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarfstein R, Friedman Y, Attias-Geva Z, Fishman A, Bruchim I, Werner H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PLoS One. 2013;8:e61537. doi: 10.1371/journal.pone.0061537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, et al. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014;14:53. doi: 10.1186/1475-2867-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan BK, Adya R, Chen J, Lehnert H, Sant Cassia LJ, Randeva HS. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2011;96:808–16. doi: 10.1210/jc.2010-1803. [DOI] [PubMed] [Google Scholar]
- 53.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 54.Terakawa N, Kanamori Y, Yoshida S. Loss of PTEN expression followed by Akt phosphorylation is a poor prognostic factor for patients with endometrial cancer. Endocr Relat Cancer. 2003;10:203–8. doi: 10.1677/erc.0.0100203. [DOI] [PubMed] [Google Scholar]
- 55.Hudes G, Carducci MA, Tomczac P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, Interferon Alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;365:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 56.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of Everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 57.Temkin SM, Yamada SD, Fleming GF. A phase I study of weekly temsirolimus and topotecan in the treatment of advanced and/or recurrent gynecologic malignancies. Gynecol Oncol. 2010;117:473–6. doi: 10.1016/j.ygyno.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 58.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–85. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, et al. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–20. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, Celestino J, Schmandt R, McCampbell AS, Urbauer DL, Meyer LA, et al. Chemopreventive effects of metformin on obesity-associated endometrial proliferation. Am J Obstet Gynecol. 2013;209:24, e1–e12. doi: 10.1016/j.ajog.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker C, Jick SS, Meier CR, Bodmer M. Metformin and the risk of endometrial cancer: A case-control analysis. Gynecol Oncol. 2013;129:565–9. doi: 10.1016/j.ygyno.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Luo J, Beresford S, Chen C, Chlebowski R, Garcia L, Kuller L, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014;111:1432–9. doi: 10.1038/bjc.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko EM, Walter P, Jackson A, Clark L, Franasiak J, Bolac C, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol. 2014;132:438–42. doi: 10.1016/j.ygyno.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, et al. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132:236–40. doi: 10.1016/j.ygyno.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuler KM, Rambally BS, Difurio MJ, Gehrig PA, Bae-Jump V. A preoperative window study of metformin for the treatment of endometrial cancer. J Clin Oncol. 2013;(Supplemental) abstract 5519. [Google Scholar]
- 66.Soliman PT, Broaddus R, Westin S, Iglesias D, Runsell M, Schmandt R. Phase 0 study: Prospective evaluation of the molecular effects of metformin on the endometrium in women with newly diagnosed endometrial cancer. Society of Gynecologic Oncologists. 2013;(Supplemental) doi: 10.1016/j.ygyno.2016.10.011. 15-abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laskov I, Drudi L, Beauchamp MC, Yasmeen A, Ferenczy A, Pollak M, et al. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecol Oncol. 2014;134:607–14. doi: 10.1016/j.ygyno.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: A preoperative prospective trial. Cancer. 2014 doi: 10.1002/cncr.28853. doi: 10.1002/cncr.28853. [DOI] [PubMed] [Google Scholar]
- 69.GLOBOCAN 2012 . Internatonal Agency for Researach on Cancer. World Health Organization; 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012. [Google Scholar]
- 70.Gibb RK, Martens MG. The impact of liquid-based cytology in decreasing the incidence of cervical cancer. Rev Obstet Gynecol. 2011;4:S2–S11. [PMC free article] [PubMed] [Google Scholar]
- 71.Kurtz JE, Hardy-Bessard AC, Deslandres M, Lavau-Denes S, Largillier R, Roemer-Becuwe C, et al. Cetuximab, topotecan and cisplatin for the treatment of advanced cervical cancer: A phase II GINECO trial. Gynecol Oncol. 2009;113:16–20. doi: 10.1016/j.ygyno.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 72.Faried LS, Faried A, Kanuma T, Sano T, Nakazato T, Tamura T, et al. Predictive and prognostic role of activated mammalian target of rapamycin in cervical cancer treated with cisplatin-based neoadjuvant chemotherapy. Oncol Rep. 2006;16:57–63. [PubMed] [Google Scholar]
- 73.Faried LS, Faried A, Kanuma T, Aoki H, Sano T, Nakazato T, et al. Expression of an activated mammalian target of rapamycin in adenocarcinoma of the cervix: A potential biomarker and molecular target therapy. Mol Carcinog. 2008;47:446–57. doi: 10.1002/mc.20402. [DOI] [PubMed] [Google Scholar]
- 74.Kim MK, Kim TJ, Sung CO, Choi CH, Lee JW, Kim BG, et al. High expression of mTOR is associated with radiation resistance in cervical cancer. J Gynecol Oncol. 2010;21:181–5. doi: 10.3802/jgo.2010.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tinker AV, Ellard S, Welch S, Moens F, Allo G, Tsao MS, et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199). Gynecol Oncol. 2013;130:269–74. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, et al. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol. 2012;127:249–55. doi: 10.1016/j.ygyno.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, et al. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/beta-catenin signaling activity. PLoS One. 2013;8:e53597. doi: 10.1371/journal.pone.0053597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yung MM, Chan DW, Liu VW, Yao KM, Ngan HY. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 2013;13:1–8. doi: 10.1186/1471-2407-13-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Do MT, Kim HG, Khanal T, Choi JH, Kim DH, Jeong TC, et al. Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol Appl Pharmacol. 2013;271:229–38. doi: 10.1016/j.taap.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 81.NIH [2014 October]; http://clinicaltrials.gov/.
- 82.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the anti-diabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 83.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–12. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–31. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niraula S, Dowling R, Ennis M, Chang MC, Done SJ, Hood N, et al. Metformin in early breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135:821–30. doi: 10.1007/s10549-012-2223-1. [DOI] [PubMed] [Google Scholar]