Abstract
Background
Currently, there is no in vitro or ex vivo model that can isolate circulating tumor cells (CTCs). Recently, we developed a four-dimensional (4D) lung cancer model that allows for the isolation of CTCs. We postulated that these cells have different properties than parental (2D) cells.
Materials & Methods
We obtained CTCs by growing A549, H1299, 393P, and 344SQ cell lines on the 4D lung model. The CTCs were functionally characterized in vitro and gene expression of the cell adhesion molecules was compared with respective 2D cells. Integrin beta 4 (ITGB4) was further investigated by stably transfecting the A549 and H1299 cells.
Results
We found that all cell lines produced CTCs and that CTCs from the 4D model were less adherent to the plastic and have a slower growth rate than respective 2D cells (p < 0.01). Most of the cell adhesion molecules were downregulated (p < 0.05) in CTCs, and ITGB4 was the common molecule, significantly more underexpressed in CTCs from all cell lines than their respective 2D cells. The modulation of ITGB4 led to a differential function of 2D cells.
Conclusions
CTCs from the 4D model have different transcriptional, translational, and in vitro characteristics than the same cells grown on a petri dish, and these CTCs from the 4D model have the properties of CTCs that are responsible for metastasis.
Keywords: 4D model, ex vivo, lung cancer, circulating tumor cells
1. Introduction
Circulatory tumor cells (CTCs) play a crucial role in tumor metastasis. CTCs originate from the primary tumor site and then invade through the basement membrane, enter the vasculature and survive in circulation (1–3), and finally invade the organ at a distant site to form metastatic lesions (4). One of the hallmarks of CTCs in patients is their ability to float in the blood vessels (5–7). Understanding CTCs’ biology and characteristics is very important for the development of an effective therapeutic regimen for advanced lung cancer.
Recently, we have developed a 4D model that adds the additional dimension of “flow” to the traditional 3D model. Unlike 3D tumor models (8), the ex vivo 4D model keeps epithelial and vascular spaces separate from each other with an intact basement membrane (9) and allows oxygenated nutrients to travel through the vascular space and perfuse the epithelial space (10, 11). We have shown that our 4D model allows the growth of a perfusable nodule, with the tumor cells expressing matrix metalloproteinase similar to that of lung cancer patients (10). Moreover, the addition of flow has a significant impact on the biology of tumor growth. When we looked at the differential gene expression for tumor cells grown on the 3D and 4D models, we found that the 3D gene signature predicted good survival for lung cancer patients, whereas the 4D gene signature predicted poor survival (12). The tumor cells placed in a 3D model expressed genes that formed stable cancer nodules, whereas the same tumor cells in a 4D model expressed genes that formed aggressive tumor cells. Thus, the addition of “flow” to the 3D model completely changes the cancer cell behavior and makes them express genes that are associated with cancer progression and patient death.
Interestingly, we have observed the presence of tumor cells in the vasculature or live floating tumor cells in the circulating media in the 4D model. We postulated that these CTCs from the 4D model have different properties than the parent cells grown on a petri dish (2D) and they have the hallmark features of CTCs from patients with cancer with ability to float. We compared their appearance, phenotypic behavior, and gene expression pattern to the parent cells grown on a petri dish. We found that these cells are less adherent, with a decrease in the level of the cell adhesion molecule integrin beta 4 (ITGB4), than the parental cell.
2. Material and Methods
2.1 Animal handling and cell lines
The Institutional Animal Care and Use Committee at the Methodist Hospital Research Institute approved the protocols for animal experiments. All of the animal experiments were carried out in accordance with all applicable laws, regulations, guidelines, and policies governing the use of laboratory animals in research.
2.2 Cell culture (2D)
We used the human lung cancer cell lines A549 and H1299 (American Type Tissue Collection, Manassas, VA, USA), and mice cell lines 393P and 344SQ, kindly provided from Dr. Jonathan Kurie’s lab (The University of Texas MD Anderson Cancer Center, Houston, TX, USA). The H1299 cell line was derived from metastatic lymph nodes (13) with a deletion in the p53 gene, and the A549 cell line was derived from the primary adenocarcinoma of a lung with a wild-type p53 gene. The murine cell lines (344SQ and 393P) were derived from the KrasLA1/+ p53R172HΔG mouse model (14) and have been shown to recapitulate the human NSCLC metastasis on a transcriptional level (15). These cells were cultured in RPMI1640 (Hyclone, South Logan, UT, USA) with 10% fetal bovine serum (Lonza, Walkersville, MD, USA) and antibiotics (100 IU/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin; MP Biomedicals, Solon, OH, USA) at 37°C in 5% CO2.
2.3 Ex Vivo 4D model and CTCs
The Ex Vivo 4D model was created as previously described (11). Briefly, we removed all of the cells from the rat lung through decellularization process using SDS and Triton-X and recellularized the acellular lung with 25 million cells (A549, H1299, 393P, or 344SQ) diluted in 50 mL of complete media through the trachea (n = 3). The lung was placed in a bioreactor with pulmonary artery cannula connected to a pump and oxygenator. The media in the bioreactor ran through the pulmonary artery at 6 cc/ min for 24 hours and it was replaced with fresh media every day for 15 days. We isolated CTC by centrifuging (500g for 5 min) the media that circulated through the model for 24 hours, removing the supernatant and diluting it in 100 µl of complete media. The CTCs were counted and used in the experiments or stored in Trizol (Iso-RNA lysis reagent; 5 PRIME, Gaithersburg, MD, USA) at −80°C until needed. We used trypan blue staining to count the live cells.
2.4 RNA extraction and quantitative reverse transcription polymerase chain reaction (RT-PCR) of CTCs versus 2D cultures
The total RNA was extracted from 2D monolayer cell cultures or CTCs from 4D model with Isol-RNA lysis reagent (5 PRIME) followed by the SurePrep RNA cleanup and concentration kit (Fisher Scientific, Pittsburgh, PA, USA). RNA was treated with the Ambion DNA-free kit (Applied Biosystems, Carlsbad, CA, USA) as per the manufacturer’s instructions. The cDNA was prepared using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) with 500 ng of total RNA, and the real-time PCR assay was prepared with SensiFast SYBR green reagent (Bioline USA Inc., Taunton, MA, USA). The relative gene expression of target genes was determined against the reference genes L32 (16) and 18s (17) for mice and humans, respectively. The primers for human and mice target genes were designed using Primer 3.0 online software (SimGene.com) (18) (Table 1). We calculated the relative fold of gene expression using 2−(ΔΔCt) formula.
Table 1.
Human and mouse primer sequence of cell adhesion molecules.
| GENE NAME | PRIMER SEQUENCE (5'-3') |
|---|---|
| H 18s F | TTTTCGGAACTGAGGCCATG |
| H 18s R | CTTGGCAAATGCTTTCGCTC |
| H NCAM1 F | CGGCATTTACAAGTGTGTGG |
| H NCAM1 R | CACACAATCACGGCATCTTC |
| H VCAM1 F | TGAGGATGGAAGATTCTGGA |
| H VCAM1 R | GTCTCCAATCTGAGCAGCAA |
| H ITGB2 F | AGCTGTCCCCACAAAAAGTG |
| H ITGB2 R | TGAGGTCATCAAGCATGGAG |
| H ITGB4 F | CGGAATAAACTGCAGGGAGA |
| H ITGB4 R | GCTGACTCGGTGGAGAAGAC |
| H CDH11 F | CGTTTGAAATCACAACGGACT |
| H CDH11 R | GAAAGGGCCATTGCTGATAA |
| H CDH4 F | GACAGCCAGACAGCAGAGAA |
| H CDH4 R | GGCAGCAGGGTGTCCTTC |
| H SELL F | TCAGCTCACAGTGTGCCTTC |
| H SELL R | CCCCAAATCTGGTGCTGATA |
| H CEACAM5 F | ATACGTGCCAAGCCCATAAC |
| H CEACAM5 R | GTTGGAGTTGTTGCTGGTGA |
| H CEACAM7 F | CAGAACGTCACCCACAATGA |
| H CEACAM7 R | CTCCACCGGATTGAAGTTGT |
| M L32 F | GGAGAAGGTTCAAGGGCCAG |
| M L32 R | TGCTCCCATAACCGATGTTTG |
| M NCAM1 F | CATCTACAACGCCAACATCG |
| M NCAM1 R | TTAAACTCCTGTGGGGTTGG |
| M VCAM1 F | TGAGGATGGAAGACTCTGGA |
| M VCAM1 R | ACTTGTGCAGCCACCTGAG |
| M ITGB2 F | TGATGACCTCAACAACGTCAA |
| M ITGB2 R | CAGCTTCTCAGGATGGGTGT |
| M ITGB4 F | ACTGCAAGGAGAACGCATCT |
| M ITGB4 R | CAGACTCGGTGGAGAACACC |
H- Human primer
M- Mouse primer
2.5 Cell adhesion assay
We compared the cell adhesion of CTCs from the 4D model with the respective cells grown on plastic (2D). In 96 well plates, 25,000 cells of either CTCs or 2D in 150 µL complete media were incubated for 3, 6, 12, or 24 hours at 37°C, along with negative control of complete media (n = 3). After each incubation period, plates were kept on the shaker for 10 to 15 seconds at 2000 rpm and then washed with washing buffer (0.1% bovine serum albumin in RPMI 1640) three times. Cells were fixed with 4% paraformaldehyde (Sigma, St. Louis, MO, USA) for 15 minutes at room temperature and were again washed with the washing buffer. We stained the cells with crystal violet for 10 minutes and washed them with water three times. The cell culture plates were completely air-dried; 2% SDS was then added to dissolve the stain. The mixture was further incubated for 30 minutes at room temperature on the shaker at 2000 rpm. The plate was read at 550 µm and 750 µm (background). For each well, the optical density was corrected by subtracting the background optical density at 750 µm.
2.6 Cell growth assay
We conducted the cell growth assay in 12-well cell culture plates by seeding 100,000 cells/well of CTCs from the 4D model seeded with 393P, 344SQ, A549, or H1299 cell lines. The respective 2D cells were cultured for 2, 4, and 6 days in the incubators (n = 3). After each incubation period, floating cells in media and adherent cells in the well were counted using the BioRad TC10 cell counter (Bio Rad, Hercules, CA, USA) following trypsinization. We determined the number of viable cells using trypan blue exclusion.
2.7 ITGB4 small interfering RNA (siRNA) transfection
We performed the knockdown of the ITBG4 gene by ITGB4 siRNA transfection (Santa Cruz Biotechnology, Dallas, TX, USA) and scrambled sequence (SCR) siRNA as a control (Santa Cruz Biotechnology). We used the Lipofectamine RNAiMAX transfection kit (Life Technologies, Grand Island, NY, USA) for transfection in a 6-well plate. We plated 100,000 cells per well for 24 hours and then transfected with ITGB4 siRNA and SCR siRNA. We trypsinized the cells after 24 hours of transfection and studied the effects on its gene expression, protein expression, and cell adherence.
2.8 ITGB4 overexpression
Full-length cDNA encoding ITGB4 (NM_001009731.1 SNP 5426T>C) was amplified from human lung cDNA (Human Total RNA Master Panel II Clontech #636643, Clontech, Mountain View, CA, USA) and transferred to the viral vector pLenti63/V5 DEST (Invitrogen, Carlsbad, CA, USA) via Gateway recombination and virus production following the manufacturer’s recommendations. Lentivirus was prepared by co-transfecting 293T cells with the above-mentioned vector backbones and standard virus packaging systems for the subsequent collection of viral supernatants. All over-expression studies were performed with the newly transduced stable cells lines.
2.9 Western analysis
We performed western analysis for the ITGB4 gene (Santa Cruz Biotechnology) on the 2D model versus the CTCs, siITGB4 versus the control siRNA transfection in human cell lines (A549 and H1299) and ITGB4 overexpression cells (A549 and H1299) versus the control. Cells from the 2D model, the CTCs, and the transfection were lysed on ice in a RIPA lysis buffer containing protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and Western analysis was performed as described previously (12). The band intensities were quantified using ImageJ 1.46r software (National Institutes of Health, Bethesda, MD, USA).
2.10 Statistical analysis
All of the statistical analyses were performed using Prism software. Data are expressed as mean ± standard error of mean following a student’s t-test (two-tailed with unequal variance).
3. Results
3.1 Ex vivo 4D model produces circulating tumor cells
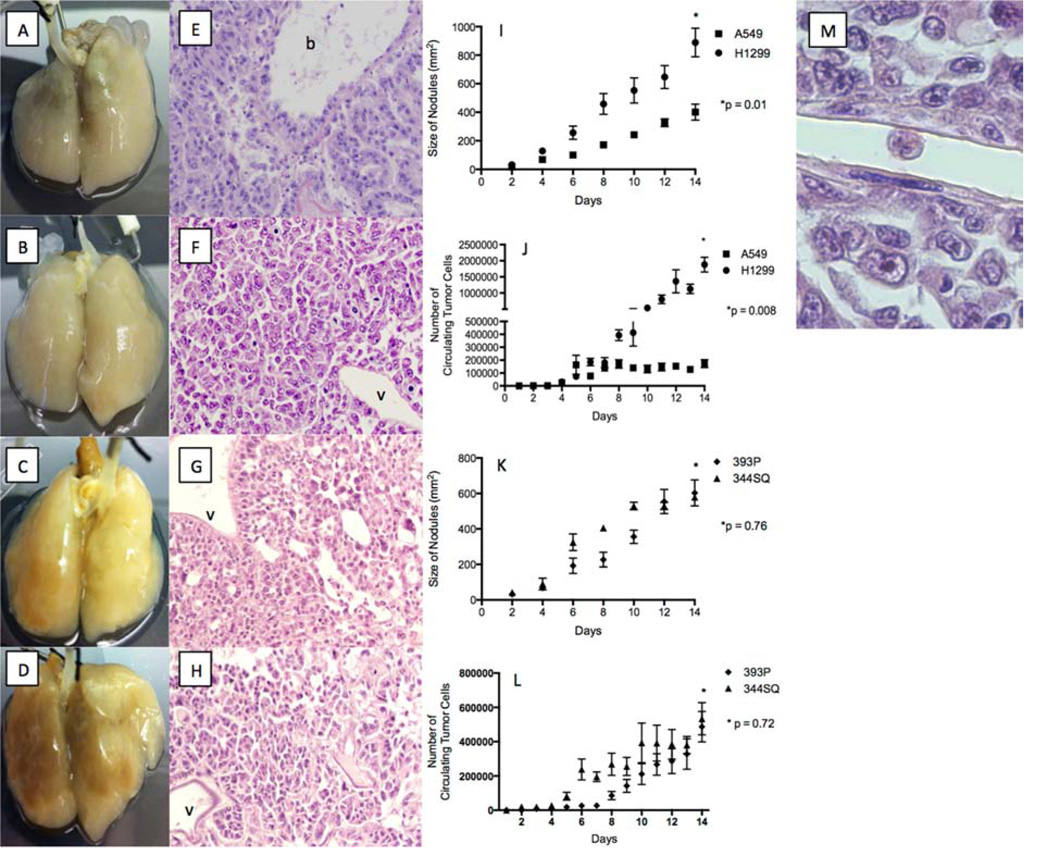
All cell lines formed a tumor nodule on the acellular rat lung matrix by day 2 (4D) and grew over time (Fig. 1A–I, K). No tumor cells grew in the model’s vasculature (v), whereas there was a robust growth in the epithelial space on hematoxylin and eosin staining (Fig. 1E–H). As the tumor nodules formed in the 4D model (Fig. 1I, K), there were live CTCs in the media (Fig. 1J, L). The number of CTCs increased over time. The H1299 cells had greater tumor growth (Fig. 1I) and more CTCs (Fig. 1J) than the A549 cells. In the murine cell lines, the 344SQ cells initially had faster tumor growth (Fig. 1K) and more CTCs (Fig. 1L) than the 393P cells, but there were no differences by day 14. The hematoxylin and eosin staining of the lung tissue from the 4D model shows a live tumor cell in a vessel (Fig. 1M) that looks very different than the same cells grown on a petri dish. The tumor cell in the vessel is oval without any cytoplasmic projections and with a high nucleus-to-cytoplasm ratio, irregular nuclear contours, and clumpy chromatin.
Figure 1. Tumor nodule and CTC formation on 4D model.
(A–D) Image of A549 (A), H1299 (B), 393P (C), and 344SQ (D) cells grown on the 4D model on day 14 showing the presence of tumor nodules. (E–H) Hemotoxyline and eosin staining of the lung tissue from the 4D model on day 14 seeded with A549 (E), H1299 (F), 393P (G), and 344SQ (H) cells show a lack of tumor growth in vasculature (v) and the presence of tumors in the bronchus (b). (I) Bigger tumor nodules on the 4D model seeded with H1299 cells on day 14 compared to the 4D model seeded with A549 cells (p = 0.01). (J) Higher number of live CTCs in the 4D model seeded with H1299 tumor cells as compared to those seeded with A549 tumor cells on day 14 (p < 0.008). (K) No significant difference in tumor nodule size on day 14 between the 4D model seeded with 344SQ and 393P cells (p = 0.76). (L) No significant difference in the number of CTCs on day 14 between the 4D model seeded with 344SQ and 393P cells (p = 0.72). The error bar represents the standard error of mean. (M) Hemotoxyline and eosin staining of lung tissue from the 4D model seeded with H1299 cells, with the live tumor cell in the vasculature. The tumor cell is oval in shape; without any cytoplasmic extension; and with a high nucleus-to-cytoplasm ratio, irregular nuclear contours, and clumpy chromatin.
3.2 CTCs have different in vitro characteristics than the same cells grown on a 2D model
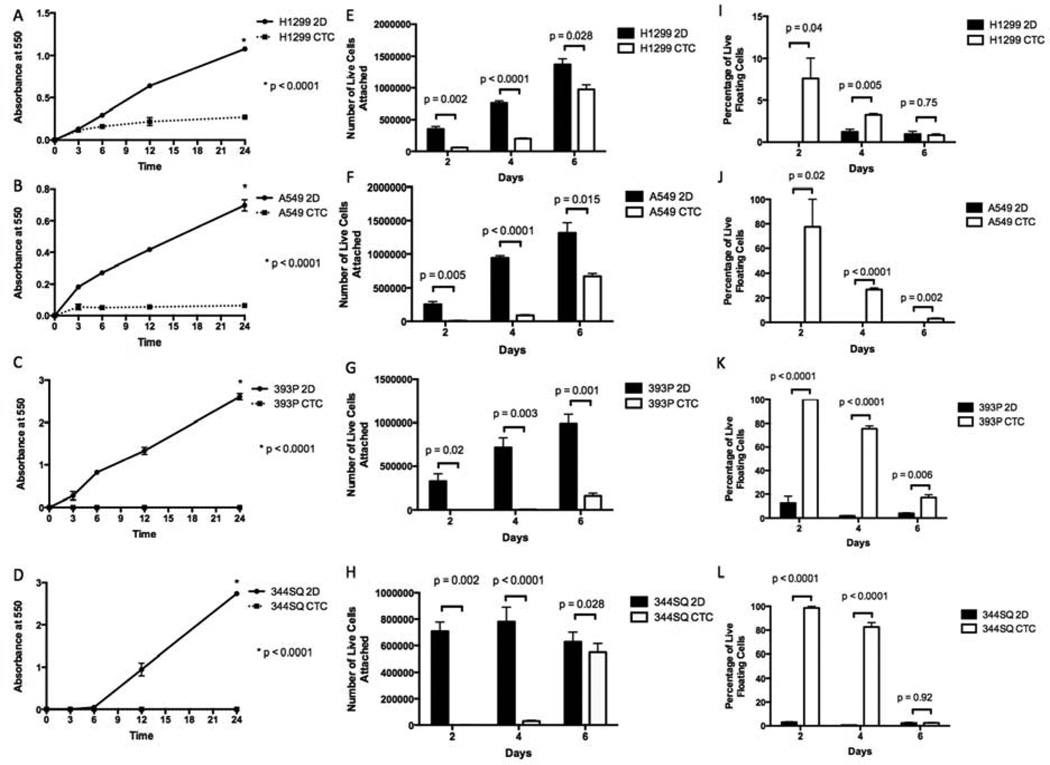
We compared the in vitro characteristics of CTCs from the 4D model and the respective 2D cells. We found that, for all four cell lines, all CTCs adhered significantly less to the plastic than the same cells from the 2D culture (Fig. 2A–D). Moreover, the CTCs from the 4D model attached and grew significantly more slowly on the 2D culture than the parental 2D cells (Fig. 2E–H). Finally, a significantly higher percentage of live tumor cells were floating in the media for the CTCs from the 4D model than from the parental 2D cells (Fig. 2I–L).
Figure 2. Comparison of in vitro characteristics of 2D versus CTCs.
(A–D) CTCs from the 4D model seeded with H1299 (A), A549 (B), 393P (C), and 344SQ (D) cells were significantly less adherent than respective cell lines cultured on a petri dish (2D). (E–H) CTCs from the 4D model (CTC) seeded with H1299 (E), A549 (F), 393P (G), and 344SQ (H) cells had significantly fewer cells that were attached to the petri dish on days 2 and 4 compared to the same cells grown on a petri dish (2D). (I–L) Total number of the live cells that were floating in the supernatant in the CTCs from the 4D model seeded with H1299 (I), A549 (J), 393P (K), and 344SQ (L) cells on days 2 and 4 were significantly higher compared to the cells grown on a petri dish (2D). The error bar represents the standard error of mean.
3.3 CTCs have downregulation of cell adhesion molecules
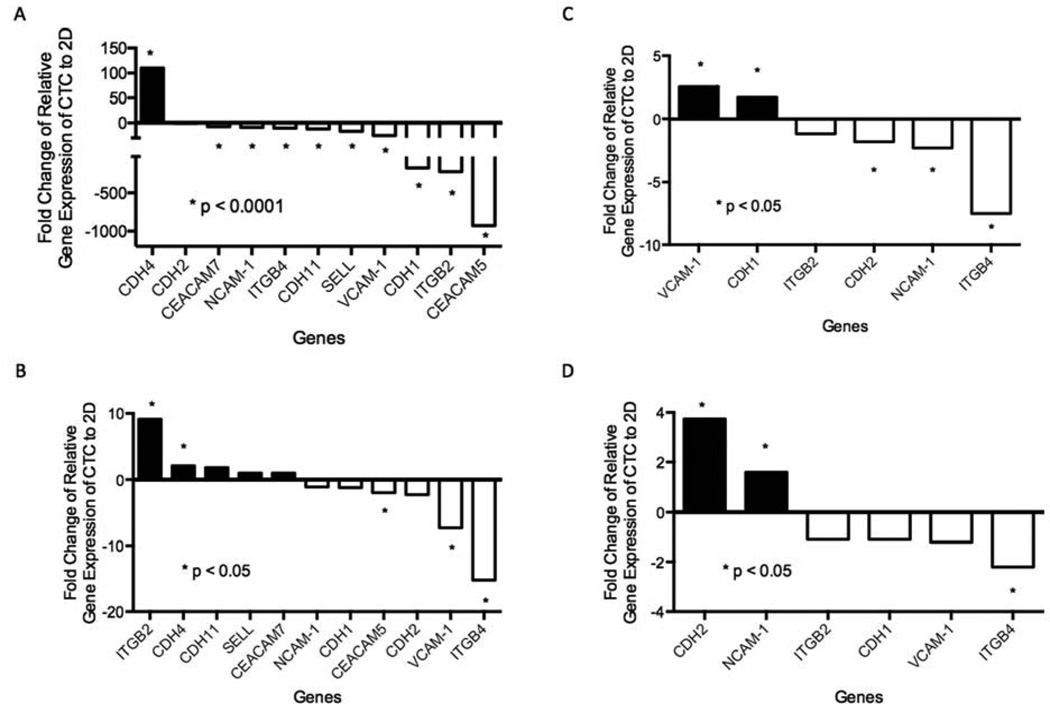
We postulated that the in vitro differences between the CTCs from the 4D model and the parental 2D cells might be due to the downregulation of adhesion molecules in the CTCs from the 4D model compared to a lack of downregulation of adhesion molecules in the same cells grown in the 2D model. We selected a panel of cell adhesion molecules (CDH1, CDH2, CDH4, CDH11, NACM-1, VCAM-1, CEACAM7, ITGB2, ITGB4, SELL, and CEACAM5) to determine the level of messenger RNA (mRNA) using RT-PCR. We compared the gene expression of these cell adhesion molecules in CTCs to their respective parental 2D cells from all four cell lines. We analyzed only those cell adhesion molecules that were present at a detectable level in both 2D and CTC cells. Most of these genes were detected and showed a significant downregulation in the CTCs from the 4D model seeded with A549 compared to parental 2D cells (p < 0.0001, Fig. 3A). Only ITGB4, VCAM-1, and CEACAM5 were downregulated in the CTCs from the 4D model seeded with H1299 compared to the parental 2D cells (p < 0.05, Fig 3B). Moreover, CDH2, NCAM-1, and ITGB4 were downregulated in the CTCs from the 4D model seeded with 393P compared to the parental 2D cells (p < 0.05, Fig. 3C). Finally, only ITGB4 was significantly downregulated in the CTCs from the 4D model seeded with 344SQ compared to the parental 2D cells (p < 0.05, Fig. 3D). Among all the cell lines (human and murine), ITGB4 was significantly downregulated in the CTCs from the 4D model compared to the respective 2D cells at the transcriptional level.
Figure 3. RT-PCR of cell adhesion molecules.
(A–D) Relative gene expression of cell adhesion genes in CTCs from the 4D model seeded with A549 (A), H1299 (B), 393P (C), or 344SQ (D) cells compared to the respective 2D cells. RT-PCR shows that only ITGB4 was significantly downregulated in CTCs compared to the 2D model in all four cell lines.
3.4 CTCs from the 4D model have less ITGB4 than the parental 2D cells and the modulation of ITGB4 in 2D cells associated with differential cell adhesion
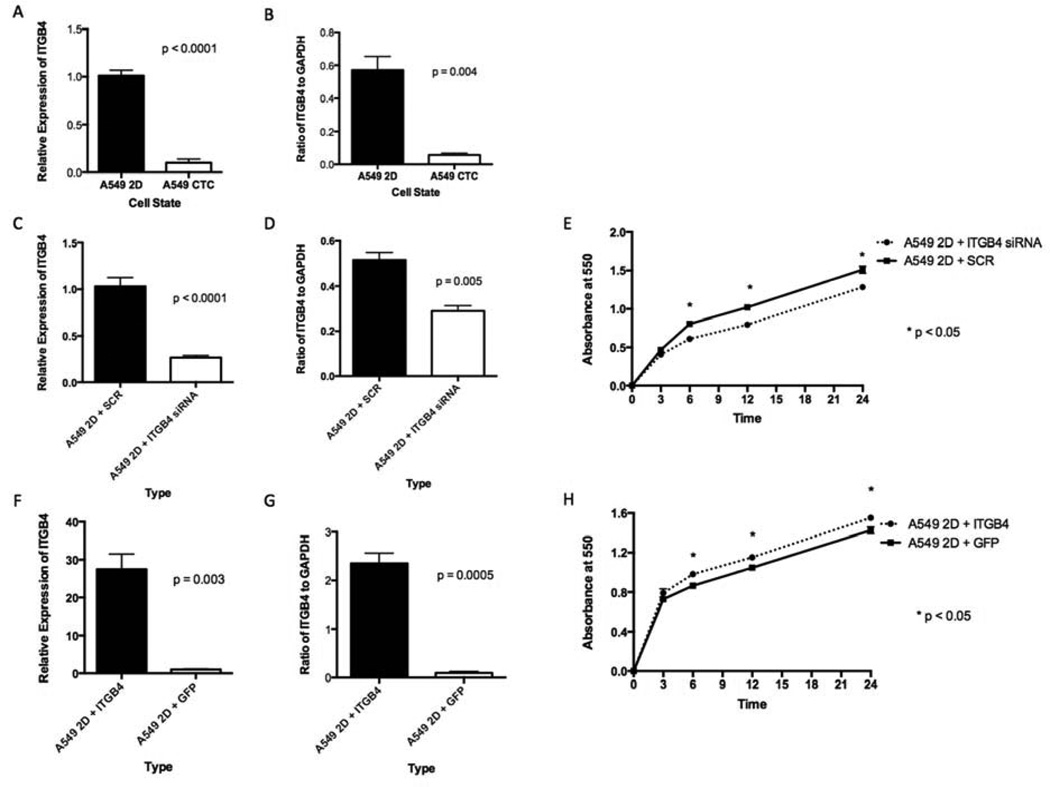
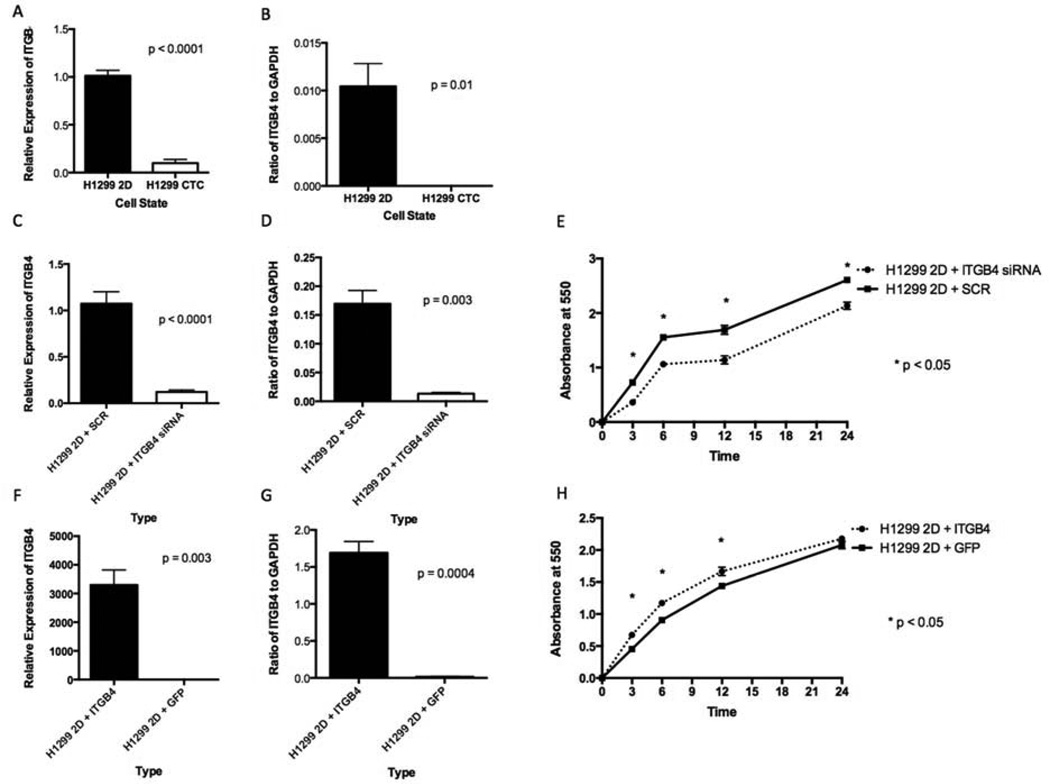
ITGB4 is a transmembrane glycoprotein receptor for laminins. It mediates the cell-matrix or cell-cell adhesion and transduces signals that regulate gene expression and cell growth. ITGB4 mRNA level (Fig. 4A) and protein level (Fig. 4B) were significantly lower in the CTCs from the 4D model seeded with A549 than in the respective parental 2D cells. We incubated parental A549 cells grown on a petri dish (A549 2D) with siRNA and SCR and found less ITGB4 mRNA (Fig. 4C) and protein (Fig. 4D) with the siRNA than with the SCR. The cells with siRNA had significantly less cell adhesion to the plastic than the SCR (Fig. 4E). We then overexpressed ITGB4 and compared it to the green fluorescent protein (GFP) control. We found significantly higher levels of ITGB4 mRNA (Fig. 4F) and protein (Fig. 4G) with the ITGB4 transfection than with the GFP vector. Tumor cells with an overexpression of ITGB4 had significantly more cell adhesion to the plastic than GFP control (Fig. 4H). We obtained similar results with the H1299 cells (Fig. 5A–H).
Figure 4. ITGB4 expression in 2D vs CTCs from the 4D model (A549) and the effect on cell adhesion.
In the A549 cells, the CTCs had significantly lower mRNA expression (A) and protein expression (B) than the same cells in the 2D culture. A549 cells grown in the 2D culture treated with ITGB4 siRNA had significantly less mRNA (C) and less protein (D) than the cells treated with SCR. A549 cells grown in the 2D culture treated with ITGB4 siRNA had significantly less adhesion to the petri dish than the same cells treated with SCR (E). A549 cells grown in the 2D culture transfected with ITGB4 had significantly more mRNA (F) and more protein (G) than the cells transfected with GFP vector. A549 cells grown in the 2D culture transfected with ITGB4 had significantly more adhesion to the petri dish than the same cells transfected with the GFP vector (H). The error bar represents the standard error of mean.
Figure 5. ITGB4 expression in 2D vs CTCs from the 4D model (H1299) and the effect on cell adhesion.
In the H1299 cells, the CTCs had significantly lower mRNA expression (A) and protein expression (B) than the same cells in the 2D culture. H1299 cells grown in the 2D culture treated with ITGB4 siRNA had significantly less mRNA (C) and less protein (D) than the cells treated with SCR. H1299 cells grown in the 2D culture treated with ITGB4 siRNA had significantly less adhesion to the petri dish than the same cells treated with SCR (E). H1299 cells grown in the 2D culture transfected with ITGB4 had significantly more mRNA (F) and more protein (G) than the cells transfected with the GFP vector. H1299 cells grown on the2D culture transfected with ITGB4 had significantly more adhesion to the petri dish than the same cells transfected with the GFP vector (H). The error bar represents the standard error of mean.
4. Discussion
In patients with an epithelial malignancy such as breast, colon, or prostate cancer, epithelial cell adhesion molecule-based searches for the tumor cells have yielded the identification of tumor cells in circulation (19, 20). Moreover, the higher number of CTCs identified in patients with metastatic cancer has been correlated with poor patient survival (21, 22). Animal model studies showed that < 0.1% of tumor cells in the bloodstream are thought to be capable of secondary tumor formation (23) and that existing techniques have limitations in the form of patient sampling and CTC-separation, owing to their extremely rare occurrence (1–100 CTCs per 109 blood cells).
CTCs are also being studied in metastatic mouse cancer models in relation to particular pathways such as epithelial-to-mesenchymal transition (16), but it is difficult to determine if this is the only mechanism of metastasis and if it applies to other mouse cancer cells lines or even human cancer. There are orthotopic mouse models for cancer studies that can identify CTCs in circulation in real time (24); however, the difficulty lies in our ability to isolate rare CTCs in metastatic mouse models that are undetectable with routine diagnostic modalities but are responsible for micrometastasis.
The use of an acellular natural matrix that separates the epithelial and vascular space allows us for the first time to isolate CTCs that are difficult to identify and characterize in vivo. The lack of cellular components such as endothelial cells and fibroblasts allow for high number of CTCs to extravasate into the circulation. Thus providing a high number of CTC compared to the amount that are isolated in patients in lung cancer. There are no other ex vivo models that can isolate CTCs using murine or human cancer cell lines. We found that all four tumor cells formed CTCs. The CTCs formed on the model after the development of tumor nodules. Thus, after forming a nodule, a subpopulation of cells broke the cell-cell and cell-matrix adhesions and entered circulation. We further compared the in vitro characteristics of the CTCs and the respective 2D cells. We found that the CTCs are less adherent than the respective 2D cells. The likely reason that the CTCs are less adherent than the same cells that were grown on a 2D model is that, in the 2D culture, the tumor cells become a single cell after trypsinization, whereas in the 4D model, CTCs are formed by the biological process of forming a single cell. The ability to not adhere readily to its environment is one of the hallmarks of CTCs in patients with lung cancer. Thus, the CTCs from the 4D model share this distinct feature with the CTCs from patients with lung cancer.
The common difference between the CTCs from the 4D model and the parental 2D cells was the downregulation of ITGB4, which allows the formation of attachment to the surrounding matrix structures. All four cell lines had downregulation of this protein in the CTCs compared to the respective 2D cells. The overexpression of this protein led to an increase in the adhesion of the cells to the petri dish, and the inhibition of this protein led to a decrease in the adhesion of the cells to the petri dish. This may be an important cell adhesion protein that may be involved in tumor progression.
We have shown that the 4D model can form CTCs from both mouse and human lung cancer cells and that these cells have different genotypic and phenotypic characteristics compared to the 2D cells, in part due to the presence of ITGB4. These data suggest that the CTCs from the 4D model may be the cells that are responsible for metastatic lung cancer and the ex vivo 4D model may provide a new tool to study the biology of metastasis.
Highlights.
Hallmark of circulating tumor cells in patients are its ability to be less adherent to its environment.
Circulating tumor cells from 4D model are less adherent to its environment compared to parental cells.
Circulating tumor cells from 4D model expression less ITGB4, a cell adhesion molecule, compared to parental cells.
The 4D model may mimic the biology of tumor progression by forming circulating tumor cells.
Acknowledgments
Funding:
MPK received grant support from the Second John W. Kirklin Research Scholarship from the American Association for Thoracic Surgery Graham Research Foundation. MPK received funding from the Houston Methodist Foundation with a donation from J. Michael Jusbasche. KLS and JMW were supported in part by the Cancer Target Discovery and Development Network grant IU01CA16839401. No funding sources had any involvement in the study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: DKM and MPK were involved in study design; collection, analysis, and interpretation of data; and writing of the report. KLS, JMW, and MT were involved in study design and collection, analysis, and interpretation of data. All authors were involved in the decision to submit the article.
Disclosure:
MPK has applied for a patent for the 4D model.
References
- 1.Marco RA, Diaz-Montero CM, Wygant JN, Kleinerman ES, McIntyre BW. Alpha 4 integrin increases anoikis of human osteosarcoma cells. Journal of cellular biochemistry. 2003;88:1038–1047. doi: 10.1002/jcb.10465. [DOI] [PubMed] [Google Scholar]
- 2.Helzer KT, Barnes HE, Day L, Harvey J, Billings PR, et al. Circulating tumor cells are transcriptionally similar to the primary tumor in a murine prostate model. Cancer research. 2009;69:7860–7866. doi: 10.1158/0008-5472.CAN-09-0801. [DOI] [PubMed] [Google Scholar]
- 3.Howard EW, Leung SC, Yuen HF, Chua CW, Lee DT, et al. Decreased adhesiveness, resistance to anoikis and suppression of GRP94 are integral to the survival of circulating tumor cells in prostate cancer. Clinical & experimental metastasis. 2008;25:497–508. doi: 10.1007/s10585-008-9157-3. [DOI] [PubMed] [Google Scholar]
- 4.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer research. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: principles and methods. Biotechnology advances. 2013;31:1063–1084. doi: 10.1016/j.biotechadv.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Phillips KG, Kolatkar A, Rees KJ, Rigg R, Marrinucci D, et al. Quantification of cellular volume and sub-cellular density fluctuations: comparison of normal peripheral blood cells and circulating tumor cells identified in a breast cancer patient. Frontiers in oncology. 2012;2:96. doi: 10.3389/fonc.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips KG, Velasco CR, Li J, Kolatkar A, Luttgen M, et al. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Frontiers in oncology. 2012;2:72. doi: 10.3389/fonc.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 10.Mishra DK, Sakamoto JH, Thrall MJ, Baird BN, Blackmon SH, et al. Human lung cancer cells grown in an ex vivo 3D lung model produce matrix metalloproteinases not produced in 2D culture. PloS one. 2012;7:e45308. doi: 10.1371/journal.pone.0045308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra DK, Thrall MJ, Baird BN, Ott HC, Blackmon SH, et al. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. The Annals of thoracic surgery. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra DK, Creighton CJ, Zhang Y, Gibbons DL, Kurie JM, et al. Gene expression profile of A549 cells from tissue of 4D model predicts poor prognosis in lung cancer patients. International journal of cancer. Journal international du cancer. 2013 doi: 10.1002/ijc.28428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giaccone G, Battey J, Gazdar AF, Oie H, Draoui M, et al. Neuromedin B is present in lung cancer cell lines. Cancer research. 1992;52:2732s–2736s. [PubMed] [Google Scholar]
- 14.Zheng S, El-Naggar AK, Kim ES, Kurie JM, Lozano G. A genetic mouse model for metastatic lung cancer with gender differences in survival. Oncogene. 2007;26:6896–6904. doi: 10.1038/sj.onc.1210493. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons DL, Lin W, Creighton CJ, Zheng S, Berel D, et al. Expression signatures of metastatic capacity in a genetic mouse model of lung adenocarcinoma. PloS one. 2009;4:e5401. doi: 10.1371/journal.pone.0005401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes & development. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohira T. WNT7a induces E-cadherin in lung cancer cells. Proceedings of the National Academy of Sciences. 2003;100:10429–10434. doi: 10.1073/pnas.1734137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, et al. Primer3Plus, an enhanced web interface to Primer3. Nucleic acids research. 2007;35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 20.Vona G, Sabile A, Louha M, Sitruk V, Romana S, et al. Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. The American journal of pathology. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 22.Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Therapeutic advances in medical oncology. 2010;2:351–365. doi: 10.1177/1758834010378414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature reviews. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 24.Fan ZC, Yan J, Liu GD, Tan XY, Weng XF, et al. Real-time monitoring of rare circulating hepatocellular carcinoma cells in an orthotopic model by in vivo flow cytometry assesses resection on metastasis. Cancer research. 2012;72:2683–2691. doi: 10.1158/0008-5472.CAN-11-3733. [DOI] [PubMed] [Google Scholar]