Currently, standard treatment for port wine stain (PWS) birthmarks in the United States involves use of lasers or intense pulsed light to photocoagulate selectively the abnormal vasculature. With photothermal therapy, PWS often become lighter, but patients must undergo many treatments (15–20 are not uncommon; Koster et al, 2001). Furthermore, treatment of skin types IV–VI is difficult due to absorption of light by overlying epidermal melanin, limiting treatment safety and efficacy.
Photodynamic therapy (PDT), an alternative treatment option, involves optical excitation of an exogenous photosensitizer and subsequent energy transfer from the photosensitizer to oxygen to create cytotoxic singlet oxygen (Gorman et al, 2006). Excitation of photosensitizers localized primarily within the intravascular compartment, enables targeted vascular destruction. Treatment can be effective but is associated with prolonged photosensitivity and substantial scarring risk (Lu et al, 2010).
Talaporfin sodium (TS) is a photosensitizer with proven selective vascular effects in preclinical studies, an acceptable photosensitivity period of 5–7 days, and good safety data (Akimoto et al, 2012; Bromley et al 2011; Kujundzic et al 2007). Previously, we determined that the characteristic radiant exposure required for persistent vascular shutdown (RE50/7 value) for TS-mediated PDT, using a custom-built LED array (664nm, FWHM = 20nm), was 85J/cm2 (Moy et al, 2012). Based on these and other published data (Channual et al, 2008; Smith et al, 2006; Tournas et al, 2009), we hypothesized that dual phototherapy treatment with TS-mediated PDT and ensuing PDL therapy, will achieve persistent vascular shutdown with otherwise sub-therapeutic radiant exposures of PDT and PDL. To test this hypothesis, we performed studies to determine the RE50/7 to achieve persistent vascular shutdown with PDL irradiation and the associated RE50/7 values for dual phototherapy. We postulate that lower radiant exposures can be used for both TS-mediated PDT and ensuing PDL, minimizing adverse effects, allowing treatment of all skin types and potentially achieving enhanced treatment efficacy, compared to either alone.
Utilizing a UC Irvine Institutional Animal Care and Use Committee approved protocol, we installed dorsal window chambers (Moy et al, 2011) on adult C3H mice (25–30g, n=38) anesthetized with isoflurane. For PDT, we utilized a custom-built light emitting diode array centered at 664-nm excitation (FWHM=20nm). For TS-mediated PDT, we reconstituted TS (Light Sciences Oncology; Bellevue, Washington) using sterile saline to form a solution of 25 mg/mL. We injected TS (5 mg/kg) into the bloodstream via retro-orbital injection and began PDT immediately afterwards (irradiance 100 mW/cm2; radiant exposure 0–260 J/cm2). For PDL irradiation, we used a clinical 595-nm laser (Vbeam Perfecta, Candela Corporation, Wayland, MA; 10 mm diameter spot size, 1.5 ms pulse duration, radiant exposure 3.25–10.00 J/cm2). We randomized experiment order.
To test the hypothesis that the dual therapy protocol enables persistent vascular shutdown with lower radiant exposures of either PDT or PDL irradiation, we restricted our study to radiant exposure values of PDT (20–60 J/cm2) and PDL (4–6 J/cm2) that were below the associated RE50/7 values for either treatment alone. We performed PDL irradiation within 5 s after PDT.
To monitor blood-flow dynamics, we used Laser Speckle Imaging (LSI) (Moy et al, 2011). We used an experimental design based on dose-response analysis (Moy et al, 2012). We performed 19 experiments to establish a dose-response curve for PDL and 19 experiments for PDT+PDL. We collected raw speckle images before and at time points during the ensuing week (Choi et al, 2008). Five of the authors (BC, WJM, KMK, BSL, and JJM) independently reviewed the SFI images collected on Day 7 and graded them as “0” (no persistent vascular shutdown) or “1” (persistent vascular shutdown achieved). Prism (version 5.0d, GraphPad Software, San Diego, CA) was used to estimate the RE50/7 for each study. We used a F-test to compare the log (RE50/7) values determined from PDT (85J/cm2 from Moy et al., 2012) and PDT+PDL. Our null hypothesis was that the RE50/7 values for the two studies do not differ in a statistically significant manner.
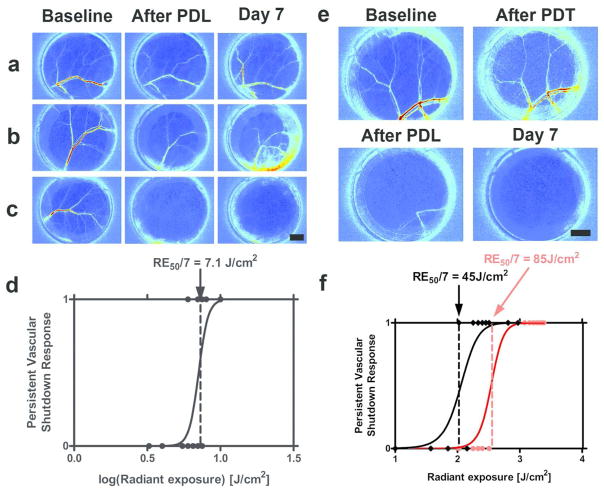
We observed three dose-dependent responses: 1) minimal acute change in blood flow and no persistent vascular shutdown (Figure 1A); 2) marked acute change in blood flow, followed by partial-to-full recovery of blood flow and no persistent vascular shutdown (Figure 1B); and 3) marked acute or delayed reduction in blood flow, followed by complete vascular shutdown at Day 7 (Figure 1C). With application of dose-response methodology, we estimated a RE50/7 of 7.1J/cm2 required to induce persistent vascular shutdown with PDL irradiation (Figure 1D). With PDT+PDL, the characteristic PDT radiant exposure required to achieve persistent vascular shutdown, decreased from 85 to 45J/cm2 (Figure 1E). This difference in PDT RE50/7 was found to be statistically significant (p=0.0002).
Figure 1. The combination of TS-mediated PDT and PDL irradiation leads to a significant reduction in the characteristic PDT radiant exposure required to achieve persistent vascular shutdown.
We first determined the characteristic radiant exposures associated with persistent vascular shutdown following 595-nm pulsed dye laser (PDL) irradiation. We performed PDL on the epidermal side of window chambers and imaged blood-flow using Laser Speckle Imaging (LSI). We assessed persistent vascular shutdown on Day 7. We assigned a “0” score if some evidence of blood flow was present, and a “1” score if flow was no longer evident. We used dose-response analysis to calculate a characteristic radiant exposure (RE50/7) at which 50% of irradiated window chambers are expected to have vascular shutdown on Day 7. (A–C) Representative LSI data associated with 595-nm PDL irradiation, in which persistent vascular shutdown was not (A,B) and was (C) achieved, using radiant exposures of (A) 4, (B) 6, and (C) 10 J/cm2, respectively. (D) Based on data from 19 experiments, we identified a RE50/7 of 7.1 J/cm2 for PDL irradiation. We then studied the combination of TS-mediated PDT and PDL irradiation. In this set of experiments, we used PDT (20 to 60 J/cm2) and PDL radiant exposures (4 to 6 J/cm2) that were below the RE50/7 values of 85 J/cm2 (Moy et al., 2012) and 7.1 J/cm2 (Figure 1D), respectfully. (E) Representative maps of blood flow that demonstrate persistent vascular shutdown at Day 7. In this specific example, we applied PDT (60 J/cm2) followed by PDL (6 J/cm2). This combination resulted in marked acute vascular shutdown, which persisted through Day 7. (F) Based on data from 30 experiments, we determined that the characteristic radiant exposure required to achieve persistent vascular shutdown, decreased from 85 J/cm2 with PDT alone to 45 J/cm2 for the combined PDT+PDL protocol. Scale bars = 2mm.
We evaluated the degree of synergy between PDT and PDL vascular effects with dual phototherapy (Madsen et al., 2002):
| (1) |
where fPDT and fPDL are the fractions of single phototherapy experiments and fPDT+PDL is the fraction of combined experiments, which do not induce persistent vascular shutdown. An additive (or absence of any) effect is indicated by α=1, α>1 indicates a synergistic effect, and α<1 indicates an antagonistic effect. Our data (Table 1) suggest the synergistic nature (α=2.7) of PDT+PDL. Collectively, our results reveal that PDT+PDL reduces the PDT light dose required to achieve persistent vascular shutdown, even at low PDL radiant exposures. We hypothesize that TS-mediated PDT enhances persistent vascular shutdown achieved with ensuing PDL therapy, primarily via endothelial cell damage (Mitra and Foster 2008); mechanistic studies currently are underway.
Table 1.
Summary of observations of persistent vascular shutdown for experiments in which the PDT radiant exposure was 20–60J/cm2 and/or the PDL radiant exposure was 4–6J/cm2. The PDT data is taken from Moy et al. (2012). Based on these data and Eq. 1, the degree of interaction is 2.7, suggesting that PDT and PDL irradiation together achieve a synergistic shutdown effect.
| Experimental condition | Number of experiments meeting criterion | Number of occurrences of persistent vascular shutdown | fPDT, fPDL, or fPDT+PDL |
|---|---|---|---|
| PDT | 4 | 0 | 100% |
| PDL | 7 | 1 | 86% |
| PDT+PDL | 19 | 13 | 32% |
Dual phototherapy represents a potential new approach for more effective treatment of PWS birthmarks. We have initiated an Investigational Review Board approved trial to evaluate intravenously administered TS/664 nm laser light mediated dual phototherapy for PWS treatment. Treatment has been painless and notable lesion lightening has been achieved with both PDT and PDT+PDL in a single session. Patients are photosensitive for 5–7 days post-procedure and for the first 72 hours must remain indoors with lights dimmed. Completion of this study will determine if lesion lightening is greater with dual phototherapy than PDL alone. It is our intent that this combined low energy dual phototherapy will offer clinicians and patients of all skin types improved lesion lightening in fewer treatments.
Acknowledgments
Work was supported in part by grants obtained from National Institutes of Health (R01 HD065536), National Institutes of Health Laser Microbeam and Medical Program (P41 EB015890), and Arnold and Mabel Beckman Foundation. Authors thank Light Sciences Oncology for providing us with TS and Dr. Tom Foster (University of Rochester) for discussions involving TS-mediated PDT.
ABBREVIATIONS
- α
degree of interaction
- BPD
Benzoporphyrin derivative monoacid ring A
- C(t)
fluorescence emission
- fPDT
fraction of photodynamic therapy experiments
- fPDL
fraction of pulsed dye laser experiments
- fPDT+PDL
fraction of combined experiments
- LSI
Laser speckle imaging
- PDL
Pulsed dye laser
- PDT
Photodynamic Therapy
- PDT+PDL
Photodynamic therapy followed by pulsed dye laser
- PWS
Port Wine Stain
- RE50/7
Characteristic radiant exposure required to achieve persistent vascular shutdown on Day 7 following phototherapy
- SFI
Speckle flow index
- TS
Talaporfin sodium
Footnotes
CONFLICT OF INTEREST
Light Sciences Oncology provided TS for this research.
References
- Akimoto J, Haraoka J, Aizawa K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagnosis Photodyn Ther. 2012;9:91–99. doi: 10.1016/j.pdpdt.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Bromley E, Briggs B, Keltner L, et al. Characterization of cutaneous photosensitivity in healthy volunteers receiving talaporfin sodium. Photodermatol Photoimmunol Photomed. 2011;27:85–89. doi: 10.1111/j.1600-0781.2011.00573.x. [DOI] [PubMed] [Google Scholar]
- Channual J, Choi B, Osann K, et al. Vascular effects of photodynamic and pulsed dye laser therapy protocols. Lasers Surg Med. 2008;40(9):644–50. doi: 10.1002/lsm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Jia W, Channual J, et al. The importance of long-term monitoring to evaluate the microvascular response to light-based therapies. J Invest Dermatol. 2008;128:485–488. doi: 10.1038/sj.jid.5700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman SA, Brown SB, Griffiths J. An overview of synthetic approaches to porphyrin, phthalocyanine and phenothiazine photosesnsitizers for photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:79–108. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Koster PH, van der Horst CM, Bossuyt PM, et al. Predictin of portwine stain clerance and required number of flashlamp pumped pulsed dye laser treatments. Lasers Surg Med. 2001;29:151–5. doi: 10.1002/lsm.1102. [DOI] [PubMed] [Google Scholar]
- Kujundzic M, Vogl TJ, Stimac D, et al. A phase II safety and effect on time to tumor progression study of intratumoral light infusion technology using talaporfin sodium in patients with metastatic colorectal cancer. J Surg Oncol. 2007;96:518–524. doi: 10.1002/jso.20832. [DOI] [PubMed] [Google Scholar]
- Lu YG, Wu JJ, Yang YD, et al. Photodynamic therapy of port-wine stains. J Dermatolog Treat. 2010;21:240–244. doi: 10.1080/09546630903200604. [DOI] [PubMed] [Google Scholar]
- Madsen SJ. Effects of combined photodynamic therapy and ionizing radiation on human glioma spheroids. Photochemistry and Photobiology. 2002;76(4):411–416. doi: 10.1562/0031-8655(2002)076<0411:eocpta>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitra S, Foster TH. In vivo confocal fluorescence imaging of the intratumor distribution of the photosensitizer mono-L-aspartylchlorin-e6. Neoplasia. 2008;10(5):429–438. doi: 10.1593/neo.08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy AJ, White SM, Indrawan ES, et al. Wide-field functional imaging of blood flow and hemoglobin oxygen saturation in the rodent dorsal window chamber. Microvasc Res. 2011;82:199–209. doi: 10.1016/j.mvr.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy WJ, Patel SJ, Lertsakdadet BS, et al. Pre-clinical in vivo evaluation of NPe6-mediated photodynamic therapy on normal vasculature. Laser Surg Med 2012. 2012;44:158–162. doi: 10.1002/lsm.21155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Choi B, Ramirez-San-Juan JC, et al. Microvascular blood flow dynamics associated with photodynamic therapy, pulsed dye laser irradiation and combined regimens. Lasers Surg Med. 2006;38:532–539. doi: 10.1002/lsm.20335. [DOI] [PubMed] [Google Scholar]
- Tournas JA, Lai J, Truitt A, et al. Combined benzoporphyrin derivative monoacid ring photodynamic therapy and pulsed dye laser for port wine stain birthmarks. Photodiagnosis Photodyn Ther. 2009;6:195–199. doi: 10.1016/j.pdpdt.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]