Abstract
Purpose
To examine the utility and reliability of obtaining early echocardiographic measurements of left ventricular (LV) remodeling as well as blood biomarkers of cardiac injury in asymptomatic childhood cancer survivors at risk for LV dysfunction and congestive heart failure due to past exposure to anthracycline chemotherapy.
Experimental Design
Using a cross-sectional design, anthracycline-exposed childhood cancer survivors with preserved EF (≥50%) were evaluated using early echocardiographic indices and blood biomarkers of LV dysfunction. Survivors treated with ≥300mg/m2 anthracyclines (high-risk [HR]: n=100) were compared with: i) those treated with <300 mg/m2 anthracyclines (low-risk [LR]: n=50) and matched healthy controls (HC: n=50). All echocardiograms were interpreted by an institutional cardiologist and a study cardiologist blinded to risk status.
Results
Time from diagnosis was comparable for HR (12.0y) and LR (13.2y, p=0.8) survivors. Echocardiograms: HR had lower LV thickness-dimension ratio (Z-score: HR: −0.62, LR: −0.03, HC: −0.02; p<0.001), increased LV wall stress (HR: 66.7 g/cm2, LR: 56.6 g/cm2, HC: 54.2 g/cm2; p<0.01) and higher myocardial performance index (HR: 0.51, LR: 0.46, HC: 0.46; P<0.01). Inter-observer correlation (clinical/blinded reading) for all echocardiographic indices was excellent (range: R=0.76-0.97, p<0.001). Blood biomarkers: With the exception of NT-proBNP (r=0.28, p<0.01), there was no correlation between blood biomarkers (BNP, Troponin-T, ST-2, Galectin-3) and LV dysfunction.
Conclusion
Childhood cancer survivors with preserved EF 10+years from anthracycline exposure had dose-dependent changes in echocardiographic markers of LV dysfunction.
INTRODUCTION
Anthracyclines are widely used in the treatment of childhood cancer.(1) However, there is clear evidence for a strong dose-dependent association between anthracyclines and congestive heart failure (CHF); it is estimated that 1 in 10 children treated with high dose anthracyclines (≥300mg/m2) will develop CHF.(2-6) Outcome following CHF is poor; 5-year survival rates are <50%.(7, 8) Detection of anthracycline-related cardiotoxicity has conventionally relied upon echocardiographic screening using resting left ventricular (LV) ejection fraction (EF) and fractional shortening (SF).(9) These parameters are derived from crude measurements of ventricular volume (EF) and size (SF), are load-dependent, may demonstrate intra-patient and inter-observer variability, and have increasingly been recognized as inadequate for detecting subtle changes in cardiac function.(9) Most importantly, at the point when changes in EF and SF are detected, functional deterioration is often irreversible, despite pharmacologic intervention.(10)
Previous research on anthracycline-related cardiotoxicity has revealed that cardiac remodeling after anthracycline exposure is associated with thinning of the LV wall, enlargement of LV diameter, and subsequent increase in LV end-systolic wall stress (ESWS).(2, 4) Other than EF and SF, ESWS is the best-studied echocardiographic index in childhood cancer survivors, and is a well-recognized precursor to anthracycline-related CHF.(2, 4, 11) However, the clinical application of ESWS is limited due to concerns regarding its reproducibility across different settings. Other echocardiographic indices such as Doppler-derived myocardial performance index (MPI) have been shown to prognosticate CHF in non-oncology populations,(12, 13) but have not been adequately studied in long-term survivors of childhood cancer. These gaps in knowledge are especially evident in childhood cancer survivors at highest risk of CHF (anthracycline dose ≥300mg/m2),(1, 2, 11) and have limited the use of these indices as much-needed early and robust endpoints for secondary prevention trials.
Established blood biomarkers of acute cardiac injury (i.e. cardiac troponins) and heart failure (i.e. natriuretic peptides) have been studied in non-oncology populations, but less is known regarding their ability to detect asymptomatic LV dysfunction years after completion of cardiotoxic cancer therapy. Emerging biomarkers of cardiac dysfunction (galectin-3, protein ST-2) have not been evaluated in this population.(1, 9) The current study examined the utility and reliability of early echocardiographic indices of LV remodeling as well as blood biomarkers of cardiac injury in long-term childhood cancer survivors exposed to anthracyclines, using a recruitment strategy that enriched for patients at highest risk of CHF.
METHODS
Study Participants
Study participants included cancer survivors and healthy controls. The survivor population was recruited from patients seen between October 2010 and September 2012 at the Childhood Cancer Survivorship Clinics at City of Hope (COH) and the Children's Hospital Los Angeles (CHLA). Eligibility criteria included: 1) cancer diagnosis before 22 years of age, irrespective of current age; and 2) two or more years since completion of cancer treatment; and 3) exposure to anthracyclines. Survivors at high risk (HR: cumulative anthracycline dose ≥300mg/m2) and low risk (LR: 1-300mg/m2) of developing CHF per the Children's Oncology Group Long Term Follow-up Guidelines(14) were targeted for recruitment. We excluded survivors who were actively being treated for cardiomyopathy, as echocardiographic indices or blood biomarkers in these individuals could have been altered by pharmacologic therapy. Healthy controls without a history of cancer or cardiomyopathy were recruited from the general population, matched to HR cancer survivors on sex and age at study participation. Patient recruitment disproportionately (2:1) targeted HR survivors over those who were LR or controls. The study was approved by the respective institutional review boards. All study participants or their parents/ legal guardians provided written informed consent.
Cardiac evaluation
Study participants underwent a clinical cardiac assessment by their healthcare provider within the respective Survivorship Clinics. The evaluation included a detailed physical examination, with special attention to signs and symptoms of CHF. Echocardiograms were performed on the day of clinical evaluation by a designated study technician, and consisted of complete two-dimensional (2D), M-mode, and Doppler evaluations, per the American Heart Association/American College of Cardiology (AHA/ACC) task force practice guidelines for the clinical application of echocardiography.(15) Identical ultrasound machines (General Electric Vivid-7 echocardiography machine; General Electric) were used for all study-related echocardiographic evaluations at the two institutions. LV end diastolic/ systolic diameters (LVEDD, LVESD), and LV posterior wall thickness in diastole and systole (LVPWD, LVPWS) were measured on M-mode recordings obtained from a standard LV parasternal view. LV ESWS was calculated using the formula (1.35 × MAP × LVESD)/ ((4 × LVPWS) (1+LVPWS/LVESD)),(16, 17) where MAP is the mean arterial pressure as obtained by Dinamap blood pressure machine. LV EF was calculated from the apical 4- and 2-chamber views using a modified Simpson biplane method.(15) LV mass was calculated from the recommended AHA/ ACC formula(15) that takes into consideration LV dimension, posterior wall and intraventricular septal thickness, and correction factors derived from regression analysis, divided by body surface area. Corrected velocity of circumferential fiber shortening (VCFc) was calculated using the following formula: (LVEDD-LVESD)/(LVEDD × ETc), where ETc is the heart rate corrected ejection time measured from the onset to the end of LV outflow velocity pattern.(18)
Measurements of LV diastolic function included pulsed Doppler measurement of mitral valve inflow, peak velocities of the early filling (E-wave) and filling during atrial systole (A-wave), and mitral E/A ratio. LV isovolumic contraction time (IVCT) was measured as the interval between mitral valve closing and aortic valve opening.(10) LV isovolumic relaxation time (IVRT) was measured as the interval between aortic valve closure and the onset of mitral flow.(10) MPI(19) was calculated using the following formula: (IVCT+IVRT)/ET. All Doppler measurements were the average of three consecutive cycles. Study echocardiograms were first interpreted by a designated cardiologist at each institution (COH: VK, CHLA: JDM). Then, an anonymized copy of the digitized echocardiogram was sent to the study core cardiology laboratory (University of Michigan, Ann Arbor, MI) where all study endpoints were re-measured by a single cardiologist (SG) who was blinded to the risk or control status of the study participant.
Blood biomarkers
Blood samples were collected on the day of the echocardiographic assessment. Troponin-T was determined using the Elecsys 2010 system (Roche Diagnostics, Indianapolis, Indiana: Elecsys Troponin sandwich immunoassay); measurable range: 0.01 to 25.00 ng/ml. B-type natriuretic peptide (BNP) was measured using the Triage BNP test (Beckman Coulter, Fullerton, California); measurable range: 2.0 pg/mL to 5000 pg/mL. NT proBrain Natriuretic Peptide (NT-proBNP) was measured using the Elecsys proBNP II test (Roche Diagnostics, Indianapolis, Indiana); lower limit of detection: 5 pg/mL. Galectin-3 was measured using a research use only assay (BG Medicine, Waltham, MA), which is a sandwich enzyme-linked immunosorbent assay (ELISA) that measures quantitative galectin-3 in plasma or serum samples; measurable range: 1.35 to 79.9 ng/mL. Protein ST2 was measured using the Presage ST2 ELISA (Critical Diagnostics, New York, NY) test; lower limit of detection: 2.0 U/mL.
Clinical data collection
Self-reported questionnaires were used to obtain baseline data on demographics. Medical records provided the following information: date of diagnosis, type of cancer, stage of disease (if applicable), cumulative dose of anthracycline exposure and receipt of chest-directed radiation therapy. Lifetime cumulative anthracycline dose was calculated by multiplying the total dose of each anthracycline (doxorubicin, daunorubicin, epirubicin, idarubicin, and mitoxantrone) by a factor that reflects the cardiotoxic potential of each drug, and then summing the individual doses.(9)
Statistical analysis
The primary endpoint for this study was LV dysfunction, defined as abnormal ESWS (>2SD norm). ESWS was selected because of the pre-existing clinical experience with this index in anthracycline-exposed childhood cancer survivors. Median ESWS was projected to be 50±10 g/cm2 for HC, 60±10 g/cm2 for the LR survivors, and 70±10 g/cm2 for HR survivors.(2, 11) Assuming a Type I error of 0.025 (controlling for conducting two t-tests: HR vs. HC, and HR vs. LR), enrolling approximately 100 HR survivors, 50 LR survivors, and 50 HCs provided 80% power to detect a significant difference in ESWS between the groups.
In order to standardize LV chamber size and wall thickness data by age or body surface area, we expressed LV diastolic (LVEDD, LVEWD) and systolic (LVESD, LVEWS) dimensions in Z scores, using established normative data for children and adults.(20, 21) The remaining echocardiographic indices are independent of age and body growth;(20) their raw values were used in the analyses.
Descriptive statistics for clinical variables, echocardiographic indices, and blood biomarkers were generated for all three groups. Categorical variables were compared using Χ2 tests. Continuous variables were compared using independent two-sample t-tests or analysis of variance (ANOVA). Inter-observer variability for each echocardiographic index was reported as the mean of the difference between values obtained from readings at the primary site (COH or CHLA) and the core cardiology lab (UM). However, only the measurements obtained from the core cardiology lab were used in our comparison of echocardiographic indices across the three groups. Pearson's correlation was calculated between continuous variables as well as for assessment of inter-observer variability. Multivariable unconditional logistic regression was used to identify variables that were associated with LV dysfunction. The dependent variable was LV dysfunction (>2SD ESWS). The independent variables included clinical and treatment-related factors that differed between the three groups (p<0.1), as well as variables thought to impact ESWS, and included: sex, age at examination (≤18 years, 19-29 years, ≥30 years), ethnicity/race (non-Hispanic white, Hispanic, other), chest radiation exposure (yes/no), and risk status (HC, LR, HR); a separate regression model was created to evaluate the association between ESWS and anthracycline dose (0, 1-99 mg/m2, 100-299 mg/m2, 300-399 mg/m2, ≥400 mg/m2). Data were analyzed using SPSS Version 18.0 (IBM, Armonk, NY). All statistical tests were 2-sided, and P<0.05 were considered statistically significant.
RESULTS
Patient characteristics
Two hundred and three individuals were approached to participate in the study. One individual (HR group) refused participation, and 2 others (1 HR, 1 control) did not complete the full battery of echocardiographic and blood biomarker testing. The current report includes results from 200 study participants who completed all study measurements (participation rate: 98.5%). Table 1 summarizes the participants’ characteristics. Due to previously established matching criteria, there were no statistically significant differences by sex, race/ethnicity and age at study enrollment between HR participants and healthy controls. Because of study design, HR survivors were more likely to have received a higher anthracycline dose than LR survivors. In addition, HR survivors were significantly more likely to have been treated for a non-hematologic malignancy, received chest radiation, and were older at cancer diagnosis as well as at the time of study enrollment when compared to LR survivors. There were no differences by sex, race/ethnicity, treatment era, and time from cancer diagnosis to study enrollment between the two survivor populations. Importantly, none of the survivors had clinical signs or symptoms of cardiac dysfunction at the time of study evaluation.
Table 1.
Patient and treatment characteristics
| Characteristics | High Risk (N=100) | Low Risk (N=50) | P-Value | Controls (N=50)* | P-Value |
| Sex, No. (%) | |||||
| Male | 55 (55.0) | 30 (60.0) | 27 (54.0) | ||
| Female | 45 (45.0) | 20 (40.0) | 0.56 | 23 (46.0) | 0.91 |
| Race/Ethnicity, No. (%) | |||||
| Non-Hispanic white | 40 (40.0) | 14 (28.0) | 20 (40.0) | ||
| Hispanic | 46 (46.0) | 27 (54.0) | 16 (32.0) | ||
| Other | 14 (14.0) | 9 (18.0) | 0.35 | 14 (28.0) | 0.08 |
| Age at examination, Years | |||||
| Median, range | 26.6 (6.7-55.8) | 20.8 (10.2-44.4) | <0.01 | 28.6 (14.9-57.9) | 0.07 |
| BMI at examination, Kg/m2 | |||||
| <25 | 57 (57.0) | 29 (58.0) | 29 (59.2) | ||
| ≥25 | 43 (43.0) | 21 (42.0) | 0.91 | 20 (40.8) | 0.80 |
| On anti-hypertensive medications at the time of assessment, No. (°/o)¶ | |||||
| Any | 10 (10%) | 3 (6%) | 0.42 | 1 (2%) | 0.08 |
| Diagnosis, No. (%)¶¶ | |||||
| Hematologic malignancy | 49 (49.0) | 46 (92.0) | - | ||
| Acute Lymphoblastic Leukemia | 13 (13.0) | 40 (80.0) | - | ||
| Acute Myeloid Leukemia | 18 (18.0) | 0 | - | ||
| Lymphoma | 18 (18.0) | 6 (12.0) | - | ||
| Solid tumor | 51 (51.0) | 4 (8.0) | <0.01 | - | |
| Ewing Sarcoma | 18 (18.0) | 1 (2.0) | - | ||
| Osteosarcoma | 13 (13.0) | 1 (2.0) | - | ||
| Soft tissue sarcoma | 16 (16.0) | 0 | - | ||
| Other | 4 (4.0) | 2 (4.0) | - | ||
| Age at diagnosis, Years | |||||
| Median, range | 13.1 (0.4-21.7) | 6.1 (1.2-21.9) | <0.01 | - | |
| Date of diagnosis | |||||
| ≤1994 | 31 (31.0) | 17 (34.0) | |||
| 1995-2001 | 36 (36.0) | 19 (38.0) | |||
| ≥2002 | 33 (33.0) | 14 (28.0) | 0.83 | ||
| Lifetime Anthracycline, mg/m2 | |||||
| Median, range | 375 (300-642) | 120 (25-225) | <0.01 | - | |
| Chest radiation, No. (%) | |||||
| Any | 16 (16.0) | 0 | <0.01 | - | |
| Time since diagnosis, Years | |||||
| Median, range | 12.0 (2.6-37.9) | 13.2 (5.3-28.6) | 0.76 | - | |
Matched to high risk: Sex, Age at examination
Angiotensin converting enzyme inhibitor or beta-blocker
Analyzed as hematologic malignancy vs. solid tumor
Echocardiographic assessment
As seen in Table 2, inter-observer correlation was excellent across all echocardiographic indices ranging from R=0.76, p<0.001 (IVRT, mean difference: 2.55 [-55-38]) to R=0.97, p<0.001 (LVEDD, mean difference -0.09 [0.55-0.81]). A comparison of the echocardiographic measurements of LV systolic and diastolic function between HR and LR survivors as well as HCs is presented in Table 3. All study participants had a normal EF (≥50%) at the time of study enrollment. However, HR survivors were significantly more likely to have an enlarged LV diameter in diastole/systole, as well as decreased wall-thickness in diastole/systole, resulting in a significantly decreased LV thickness-dimension ratio. In addition, median ESWS and MPI were significantly higher for HR survivors when compared to the other two populations. There were no differences in MAP, LV mass, VCFc, and indices of diastolic function such as E/A ratio, and IVRT between the three groups.
Table 2.
Inter-observer correlation for LV echocardiographic indices.
| Characteristics | Mean Difference* (Range) | Correlation coefficient (R) | P-Value |
|---|---|---|---|
| End-diastolic diameter, systole | −0.05 (−0.610.29) | 0.91 | <0.001 |
| End-diastolic diameter, diastole | −0.09 (−0.550.81) | 0.97 | <0.001 |
| Posterior wall thickness, systole | 0.00 (−0.310.46) | 0.89 | <0.001 |
| Posterior wall thickness, diastole | 0.02 (−0.230.41) | 0.83 | <0.001 |
| Thickness-dimension ratio | 0.00 (−0.04-0.07) | 0.90 | <0.001 |
| End systolic wall stress | 0.40 (−24.60-25.10) | 0.85 | <0.001 |
| LV Mass | 0.12 (−12.30-18.60) | 0.94 | <0.001 |
| Isolvolumic relaxation time | 2.55 (−55.00-38.00) | 0.76 | <0.001 |
| Isovolumic contraction time | 1.81 (−44.00-27.00) | 0.78 | <0.001 |
| Ejection time | 1.62 (−46.00-34.80 | 0.88 | <0.001 |
| Myocardial performance index | 2.11 (−8.20-12.30) | 0.82 | <0.001 |
| E wave | 0.03 (−0.07-0.44) | 0.95 | <0.001 |
| A wave | 0.01 (−0.19-0.11) | 0.96 | <0.001 |
| E/A ratio | 0.02 (−0.62-0.90) | 0.93 | <0.001 |
Calculated as the mean difference between values obtained from echocardiographic reading at the clinical site (COH or CHLA) and the core cardiology lab (UM).
Table 3.
Cardiac echocardiographic and blood biomarkers
| Characteristics | High Risk (N=100) | Low Risk (N=50) | P-Value* | Controls (N=50)* | P-Value** |
|---|---|---|---|---|---|
| Echocardiographic indices | |||||
| LV end-systolic diameter | |||||
| Z-score, median | 0.80 | 0.36 | <0.01 | 0.02 | <0.01 |
| Range | −2.0-5.7 | −1.8-3.3 | −2.3-2.7 | ||
| LV end-diastolic diameter | |||||
| Z-score, median | 0.75 | 0.25 | 0.01 | 0.01 | <0.01 |
| Range | −2.8-5.0 | −1.8-3.25 | −2.5-2.5 | ||
| LV Posterior wall thickness, systole | |||||
| Z-score, median | −0.58 | −0.08 | <0.01 | 0.01 | <0.01 |
| Range | −2.4-1.7 | −2.1-2.5 | −2.4-2.5 | ||
| LV Posterior wall thickness, diastole | |||||
| Z-score, median | −0.63 | −0.04 | <0.01 | 0.03 | <0.01 |
| Range | −3.1-2.2 | −2.0-3.8 | −2.5-2.6 | ||
| LV Thickness-Dimension ratio | |||||
| Z-score, median | −0.62 | −0.03 | 0.02 | −0.02 | <0.01 |
| Range | −3.0-2.4 | −1.8-2.7 | −1.4-2.9 | ||
| Mean arterial pressure (mm Hg) | |||||
| Median | 82 (63-135) | 81 (58-122) | 0.23 | 83 (68-108) | 0.45 |
| End systolic wall stress (g/cm2) | |||||
| Median, range | 66.7 (34-116) | 56.6 (28-82) | <0.01 | 54.2 (34-109) | <0.01 |
| LV Mass (g/m2) | |||||
| Median, range | 56.7 (25-121) | 53.4 (34-110) | 0.19 | 56.0 (30-82) | 0.28 |
| Velocity of circumferential shortening (c/s) | |||||
| Median, range | 1.11 (0.69-1.69) | 1.16 (0.86-1.48) | 0.16 | 1.16 (0.77-1.52) | 0.11 |
| Myocardial performance index | 0.51 (0.26-0.81) | 0.46 (0.24-0.67) | <0.01 | 0.46 (0.32-0.68) | <0.01 |
| IVRT, ms | 80 (20-123) | 72 (44-106) | 0.07 | 78 (49-109) | 0.36 |
| IVCT, ms | 66 (20-123) | 61 (37-100) | 0.18 | 62 (37-88) | 0.09 |
| Ejection time, ms | 290 (241-355) | 298 (241-350) | <0.01 | 304 (250-343) | <0.01 |
| E/A ratio | 1.5 (0.7-2.7) | 1.6 (0.9-2.5) | 0.10 | 1.5 (0.8-2.6) | 0.41 |
| E Wave, ms | 0.9 (0.5-2.0) | 1.1 (0.7-1.5) | 0.08 | 1.0 (0.6-1.5) | 0.13 |
| A Wave, ms | 0.6 (0.3-1.5) | 0.6 (0.3-0.9) | 0.39 | 0.6 (0.4-1.0) | 0.90 |
| Blood biomarkers | |||||
| BNP (pg/dL) | |||||
| Median, range | 15.8 (0-225) | 13.7 (0-168) | 0.06 | 18 (0-92) | 0.17 |
| NT-proBNP (pg/dL) | |||||
| Median, range | 71 (6-804) | 37 (6-552) | 0.01 | 26 (8-151) | <0.01 |
| Troponin-T (ng/mL) | |||||
| Median, range | 0 (0-0.02) | 0 (0-0.03) | 0.37 | 0 (0-0.02) | 0.45 |
| Galectin-3 (ng/mL) | |||||
| Median, range | 10.8 (5.2-20.6) | 9.2 (5.4-19.7) | 0.11 | 9.7 (6.8-18.4) | 0.40 |
| ST-2 (U/mL) | |||||
| Median, range | 26 (11.1-59.0) | 25.6 (13.8-60.0) | 0.69 | 26.1 (12.0-62.0) | 0.78 |
High risk vs. standard risk
High risk vs. controls. Abbreviations
IVRT, isolvolumic relaxation time; IVCT, isovolumic contraction time; BNP, B-type natriuretic peptide; NT-pro-BNP, N-terminal prohormone of brain natriuretic peptide
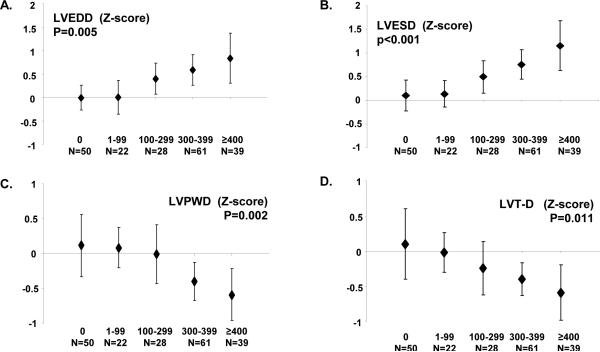
Figures 1 and 2 depict the dose-dependent association between LV echocardiographic indices and cumulative anthracycline dose. There was a significant increase in median LV diameter in diastole/systole, ESWS, and MPI by increasing anthracycline dose; there was a corresponding decrease in LV wall-thickness and thickness-dimension ratio by anthracycline dose as well. There were no dose-dependent changes in other echocardiographic indices such as LV mass, VCFc, E/A ratio, and IVRT (data not shown). In multivariable logistic regression analysis adjusting for sex, age at examination, race/ethnicity, and chest radiation exposure, HR survivors had a greater than eight-fold risk of having LV dysfunction (controls, referent group with no exposure to anthracyclines or chest radiation): HR, Odds Ratio (OR)=8.15 (p<0.01); LR, OR=2.13 (p=0.36). When examining this association by anthracycline dose, there was a dose-dependent increasing risk by cumulative exposure: 1-99 mg/m2, OR=1.43; 100-299 mg/m2, OR=2.71; 300-399 mg/m2, OR=4.13; ≥400 mg/m2, OR=12.81; p=0.01 (trend).
Figure 1.
Left ventricular (LV) echocardiographic indices by cumulative anthracycline dose (mg/m2). A) End diastolic diameter (LVEDD); B) End-systolic diameter (LVESD); C) Posterior wall thickness in diastole (LVPWD); D) Thickness-dimension ratio (LV T-D).
Figure 2.
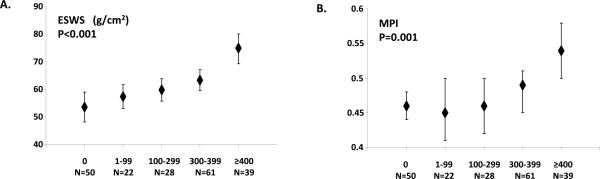
Left ventricular End systolic wall stress (ESWS) and Myocardial performance index (MPI) by cumulative anthracycline dose (mg/m2).
Blood biomarkers
Candidate biomarkers of cardiac injury and remodeling were compared across all three groups (Table 1). HR survivors had significantly higher serum NT-proBNP levels (71 pg/dL) when compared to LR survivors (37 pg/dL, p=0.02) and controls (26 pg/dL, p<0.01). There were no differences in BNP, Troponin-T, galectin-3 and ST-2 levels among the 3 groups. With the exception of NT-proBNP (r=0.28, p<0.01), there was no correlation between the blood biomarkers and ESWS: BNP (R=0.16, p=0.06), Troponin-T (R=0.03, p=0.66), ST-2 (-0.13, p=0.24), and Galectin-3 (0.14, p=0.10).
DISCUSSION
It is well-recognized that there is a clear dose-response relation between anthracyclines and CHF, and that there is often a long latency between exposure to anthracyclines and clinically overt disease.(1, 4) Several randomized clinical trials conducted mostly in adults with cancer have found that empiric intervention (during cancer treatment) with a beta-blocker and/or ACE-inhibitor,(21-24), use of liposomal formulation of anthracyclines(25), or iron chelators such as dexrazoxane(26) can decrease the risk of anthracycline-related left ventricular dysfunction. However, a growing concern among oncologists is whether the cardioprotective effects of these agents will interfere with the tumoricidal activity of anthracyclines.(26) This concern is especially pervasive in the pediatric oncology community where, despite data supporting the cardioprotective efficacy of dexrazoxane, the drug has yet to be incorporated as standard of care.(27) As a result, current clinical practice includes echocardiographic screening after treatment completion, and initiation of pharmacologic therapy (ACE inhibition and/or beta-blockade) only after abnormal EF or SF are identified.(8) Furthermore, while data from adult non-oncology populations support efficacy of pharmacologic therapy in this setting,(28) the beneficial effects of these treatments in childhood cancer survivors has been shown to be short lived.(9) This is thought to be due to the progressive irreversible chronic cardiac remodeling and fibrosis which inevitably follows detectable change in EF or SF, highlighting the importance of early echocardiographic biomarkers of LV dysfunction and remodeling that precede detectable change in EF or SF.
Early intervention with afterload reduction, prior to decrease in EF or SF, has been an effective strategy for CHF risk reduction in children with progressive neuromuscular disorders such as Duchene muscular dystrophy.(22, 23) There is a paucity of information on early markers of LV dysfunction that can be used reliably in secondary prevention studies for childhood cancer survivors at high risk of CHF. To date, studies in these survivors have relied on time- and labor-intensive echocardiographic measurements to describe asymptomatic cardiac dysfunction.(11, 24, 25) These measurements have generally been obtained relatively soon after completion of cardiotoxic cancer therapy,(26, 27) and often include small numbers of survivors exposed to high anthracycline doses and therefore at the highest risk of CHF.(11, 16, 28) The current study overcomes these limitations by reporting on echocardiographic measurements that can be obtained reliably from routine 2D, M-mode, and Doppler-based echocardiograms, long after completion of cardiotoxic therapy, in a population enriched for survivors at highest risk for CHF. Overall, inter-observer correlation for these echocardiographic indices was excellent, with the highest correlation seen for LV chamber diameter in diastole (R=0.97), and the lowest for isolvolumic relaxation time (R=0.76). These findings demonstrate that despite excellent correlation across the measured indices, there may be variance in reproducibility, suggesting that certain indices are more reliably obtained than others in a multi-center setting.(29)
Anthracycline cardiotoxicity is thought to be due to formation of free radicals and cardiotoxic alcohol metabolites that result in direct cardiac injury.(1) With enough cardiac damage, the heart expands in size and the chamber walls become thinner, creating a clinical picture similar to dilated cardiomyopathy.(4) The current study confirms these findings, by reporting on clear dose-dependent changes in LV chamber diameter and wall-thickness, resulting in an increase in ESWS. Measurement of ESWS had, until recently, relied on simultaneous acquisition of an M-mode echocardiogram, carotid pulse tracing, phonocardiogram, and blood pressure measurement.(11, 12) Obtaining a carotid artery tracing can be especially difficult in the pediatric population due to the shortness of the neck, discomfort, and heart rate variability. Aggarwal and colleagues(17) found that substitution of MAP for carotid pulse tracing-based measurements had excellent diagnostic accuracy (sensitivity 95%, specificity 96%) for ESWS, which is the approach utilized in the current study. We successfully obtained complete echocardiographic measurements, including ESWS, for nearly all study participants, with excellent inter-observer correlation, demonstrating clearly the feasibility of using these indices to monitor anthracycline-related LV dysfunction.
In addition to ESWS, we found a dose-dependent increase in MPI, a Doppler-derived global marker of cardiac function that is independent of heart rate, intravascular volume, and blood pressure. MPI does not rely on geometric assumptions, and has been shown to be predictive of CHF and cardiac death in non-oncology populations, irrespective of other echocardiographic indices of CHF risk such as depressed EF.(13, 30, 31) Studies in small numbers of childhood cancer survivors treated with anthracyclines have shown that prolongation of MPI can be seen soon after completion of cancer therapy.(16, 32, 33) The current study builds on these findings by demonstrating elevation in MPI within a large population of survivors, several years after completion of anthracycline therapy. It is important to note, however, that the long-term prognostic implications of persistent elevation in MPI as well as other indices such as ESWS in anthracycline-exposed childhood cancer survivors are not known. Longitudinal studies are needed to evaluate the association between these echocardiographic indices and subsequent CHF risk.
Serum cardiac troponins are sensitive and specific biomarkers for cardiac cell injury, and have successfully been used to monitor cardiac injury during and shortly after anthracycline administration.(1, 9) However, in the current study, all participants were ≥2 years from exposure to cardiotoxic therapies, making it unlikely that there would be significant elevation in cardiac troponins in the setting of chronic LV dysfunction. Unlike the situation with cardiac troponins, studies have been more consistent regarding the diagnostic value of natriuretic peptides in anthracycline-related CHF monitoring.(1, 9) Several studies have documented that BNP and NT-proBNP levels, if persistently elevated, correlate well with echocardiographic indices of cardiac dysfunction.(34-36) In the current study, even though there were detectable differences in median NT-ProBNP by risk group, for the most part, the association between ESWS and natriuretic peptides (BNP, NT-ProBNP), as well as newer biomarkers such as galectin-3 and ST-2 was weak. It remains to be seen what role, if any, blood biomarkers may play in screening for cardiac dysfunction after anthracycline therapy. Current long-term follow-up guidelines for survivors of childhood cancer(9, 37) do not recommend routine surveillance using these blood biomarkers. This is largely due to the wide inter-patient variability of these biomarkers in individuals with asymptomatic LV dysfunction as measured by EF, as well as difficulties with establishment of an appropriate cutoff for abnormal values in the asymptomatic setting.(34-36)
Unlike previous studies,(16, 28, 36) we did not find a significant association between anthracycline dose and spectral Doppler echocardiographic indices of LV diastolic dysfunction. This may be because diastolic dysfunction can be transient, occurring shortly after anthracycline exposure, and is largely due to exposure of cardiac tissues to radiation.(4) Our study included survivors who were on average 12 years from diagnosis and only 10% of the population had received chest-directed radiation therapy. Lastly, there is emerging data that newer imaging modalities such as cardiac magnetic resonance imaging, “speckle tracking”, and 3-D echocardiography may facilitate early identification of cardiac dysfunction prior to changes in EF.(38, 39) However, these modalities are not uniformly available across cancer follow-up centers, and lack of longitudinal follow-up studies in childhood cancer survivors precludes their routine use for primary surveillance at the current time. The current study relied on measurements that can be obtained from routine 2D, M-mode, and Doppler echocardiograms and included indices with clear prognostic implications in pediatric as well as adult populations at risk for cardiac compromise.
In summary, comprehensive profiling of anthracycline-exposed childhood cancer survivors with preserved EF revealed dose-dependent changes in LV function more than 10 years from cancer diagnosis. These indices were readily obtained from standard echocardiograms performed as part of clinical care, and there was excellent correlation between the clinical reading and the established core cardiology laboratory. The findings from the current study may facilitate the development of early pharmacologic interventions aimed at progression or potential reversal of cardiac remodeling, and therefore, prevention of overt CHF in anthracycline-exposed childhood cancer survivors.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Cardiovascular complications such as anthracycline-related congestive heart failure (CHF) have emerged as a leading cause of morbidity and mortality in survivors of childhood cancer. Outcome following CHF is poor, emphasizing the importance of early detection, prior to onset of symptomatic heart disease. Screening for asymptomatic disease using conventional echocardiographic indices such as left ventricular ejection fraction (EF) precludes intervention, because EF decline is a late event in the progression to CHF. The current study describes how novel echocardiographic indices of early cardiac dysfunction can be reliably obtained from long-term survivors of childhood cancer, using routine echocardiograms that can be ordered as part of clinical care. Importantly, we found clear dose-dependent changes in these echocardiographic indices by anthracycline dose. These findings may facilitate the development of early detection strategies as well as interventions aimed at progression or potential reversal of cardiac injury, and therefore, prevention of overt CHF in these survivors.
Acknowledgments
Funding source: NIH/NCI: 2 K12 CA001727-14, STOP Cancer Foundation.
Financial support. All authors: no financial disclosures.
Footnotes
Financial disclosures. All authors: no financial disclosures.
Presented, in part, at the International Conference on Long Term Complications of Treatment of Children and Adolescents for Cancer and the American Society of Clinical Oncology, 2013.
References
- 1.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A Scientific Statement From the American Heart Association. Circulation. 2013;128:1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. The New England journal of medicine. 1995;332:1738–43. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 4.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatric blood & cancer. 2005;44:600–6. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 5.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–8. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D'Ascenzo F, Malavasi V, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. The American journal of cardiology. 2013;112:1980–4. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. The New England journal of medicine. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 8.Armenian SH, Sun CL, Francisco L, Steinberger J, Kurian S, Wong FL, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–43. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–96. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Lipsitz SR, Sallan SE, Simbre VC, 2nd, Shaikh SL, Mone SM, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–22. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MM, Rai SN, Nunez C, Merchant TE, Marina NM, Zalamea N, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–43. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 12.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. Journal of cardiology. 1995;26:357–66. [PubMed] [Google Scholar]
- 13.Arnlov J, Ingelsson E, Riserus U, Andren B, Lind L. Myocardial performance index, a Doppler-derived index of global left ventricular function, predicts congestive heart failure in elderly men. European heart journal. 2004;25:2220–5. doi: 10.1016/j.ehj.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Children's Oncology Group . Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers. 3.0 ed. Children's Oncology Group; Arcadia, CA: 2008. [Google Scholar]
- 15.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Journal of the American College of Cardiology. 2003;42:954–70. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 16.Ganame J, Claus P, Uyttebroeck A, Renard M, D'Hooge J, Bijnens B, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20:1351–8. doi: 10.1016/j.echo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal S, Pettersen MD, Gurckzynski J, L'Ecuyer T. Measuring stress velocity index using mean blood pressure: simple yet accurate? Pediatric cardiology. 2008;29:108–12. doi: 10.1007/s00246-007-9101-3. [DOI] [PubMed] [Google Scholar]
- 18.Ruschhaupt DG, Sodt PC, Hutcheon NA, Arcilla RA. Estimation of circumferential fiber shortening velocity by echocardiography. Journal of the American College of Cardiology. 1983;2:77–84. doi: 10.1016/s0735-1097(83)80379-9. [DOI] [PubMed] [Google Scholar]
- 19.Vantrimpont P, Rouleau JL, Wun CC, Ciampi A, Klein M, Sussex B, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. Journal of the American College of Cardiology. 1997;29:229–36. doi: 10.1016/s0735-1097(96)00489-5. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21:922–34. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years' follow-up. American heart journal. 2007;154:596–602. doi: 10.1016/j.ahj.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. Journal of the American College of Cardiology. 2005;45:855–7. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003;97:1991–8. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 25.Pein F, Sakiroglu O, Dahan M, Lebidois J, Merlet P, Shamsaldin A, et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. British journal of cancer. 2004;91:37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. The lancet oncology. 2010;11:950–61. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulides M, Kremers A, Stohr W, Bielack S, Jurgens H, Treuner J, et al. Prospective longitudinal evaluation of doxorubicin-induced cardiomyopathy in sarcoma patients: a report of the late effects surveillance system (LESS). Pediatric blood & cancer. 2006;46:489–95. doi: 10.1002/pbc.20492. [DOI] [PubMed] [Google Scholar]
- 28.Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–29. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 29.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, et al. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–54. e6. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnlov J, Lind L, Andren B, Riserus U, Berglund L, Lithell H. A Doppler-derived index of combined left ventricular systolic and diastolic function is an independent predictor of cardiovascular mortality in elderly men. American heart journal. 2005;149:902–7. doi: 10.1016/j.ahj.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–85. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 32.Eidem BW, Sapp BG, Suarez CR, Cetta F. Usefulness of the myocardial performance index for early detection of anthracycline-induced cardiotoxicity in children. The American journal of cardiology. 2001;87:1120–2. A9. doi: 10.1016/s0002-9149(01)01476-x. [DOI] [PubMed] [Google Scholar]
- 33.Karakurt C, Kocak G, Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue Doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography (Mount Kisco, NY. 2008;25:880–7. doi: 10.1111/j.1540-8175.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Pettersen MD, Bhambhani K, Gurczynski J, Thomas R, L'Ecuyer T. B-type natriuretic peptide as a marker for cardiac dysfunction in anthracycline-treated children. Pediatric blood & cancer. 2007;49:812–6. doi: 10.1002/pbc.21100. [DOI] [PubMed] [Google Scholar]
- 35.Germanakis I, Kalmanti M, Parthenakis F, Nikitovic D, Stiakaki E, Patrianakos A, et al. Correlation of plasma N-terminal pro-brain natriuretic peptide levels with left ventricle mass in children treated with anthracyclines. International journal of cardiology. 2006;108:212–5. doi: 10.1016/j.ijcard.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Pinarli FG, Oguz A, Tunaoglu FS, Karadeniz C, Gokcora N, Elbeg S. Late cardiac evaluation of children with solid tumors after anthracycline chemotherapy. Pediatric blood & cancer. 2005;44:370–7. doi: 10.1002/pbc.20281. [DOI] [PubMed] [Google Scholar]
- 37.Sieswerda E, Postma A, van Dalen EC, van der Pal HJ, Tissing WJ, Rammeloo LA, et al. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23:2191–8. doi: 10.1093/annonc/mdr595. [DOI] [PubMed] [Google Scholar]
- 38.Monsuez JJ. Detection and prevention of cardiac complications of cancer chemotherapy. Archives of cardiovascular diseases. 2012;105:593–604. doi: 10.1016/j.acvd.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]