Abstract
α-Synuclein is a key pathogenic protein in α-synucleinopathies including Parkinson’s disease (PD), and its over-expression and aggregation in model systems are associated with a neuroinflammatory response and increased oxidative stress. Apoptosis signal-regulating kinase 1 (ASK1) is activated upon stress signaling events such as oxidative stress, and is a central player linking oxidative stress with neuroinflammation. Here we demonstrate that over-expression of human α-synuclein activates ASK1 in both PC12 cells and in the brains of α-synuclein transgenic mice. Deleting ASK1 in mice mitigates the neuronal damage and neuroinflammation induced by α-synuclein and improves performance of the animals on the rotarod. ASK1 deletion does not impact the aggregation profile or phosphorylation state of α-synuclein in the mouse brain. These results collectively implicate ASK1 in the cascade of events triggered by α-synuclein overexpression, likely due to the inflammatory response and oxidative stress that lead to ASK1 activation. These conclusions raise the possibility that potent anti-oxidants and anti-inflammatory agents may ameliorate the phenotype of α-synucleinopathies.
Introduction
Abnormal protein aggregation and oxidative stress are consistent features of several neurodegenerative disorders including Parkinson’s disease (PD). Markers of oxidative damage to cellular proteins, lipids and DNA are well established (Dias, et al., 2013), and the number of nitric oxide synthase-containing glial cells is increased in the substantia nigra of PD affected brains (Hunot, et al., 1996). In addition to neuronal degeneration and nigrostriatal dopamine nerve terminal loss, a neuroinflammatory response is well documented in both postmortem studies and premortem investigations using PET imaging. Microglial activation has been demonstrated in midbrain, pons, basal ganglia and frontal and temporal cortical regions of PD patients using [11C]-(R)-PK11195, which is a ligand for the peripheral benzodiazepine receptors that are highly abundant in activated microglia (Gerhard, et al., 2006,Imamura, et al., 2003,McGeer, et al., 1988,Ouchi, et al., 2005). Accordingly, proinflammatory cytokines including IL-1beta, and IL-6 are elevated in affected brain regions of PD patients (Mogi, et al., 1994). Further support for the role of inflammation in the pathogenesis of PD comes from genetic association with the HLA region in Genome Wide Association Studies (Hamza, et al., 2010,International Parkinson Disease Genomics, et al., 2011).
Apoptosis signal-regulating kinase 1 (ASK1) belongs to the MAP3 kinase family that is activated by various stimuli including oxidative stress (Saitoh, et al., 1998, Song, et al., 2002) and TNF-α (Liu, et al., 2000), and relays those signals to JNK and p38 (Chang, et al., 1998) leading to apoptosis (Saitoh, et al., 1998). Challenging SH-SY5Y neuroblastoma cells with MPP+ or primary neurons in culture with hydrogen peroxide results in activation/phosphorylation of ASK1 (Lee, et al., 2012). In vivo, systemic exposure of wild-type mice to MPTP also results in activation of ASK1 in the substantia nigra (Lee, et al., 2012). Notably, ASK1 null mice are relatively resistant to MPTP as demonstrated by dampened dopaminergic neuronal loss, reduced inflammatory indicators, and less pronounced motor impairment, suggesting that ASK1 signaling is an important link between oxidative stress and the associated neuroinflammation in MPTP-induced toxicity in mice (Lee, et al., 2012). A similar trend of diminished toxicity of 6-hydroxydopamine in the mouse nigra occurs with ASK1 knockdown using shRNA (Hu, et al., 2011). Additionally, postmortem analysis of PD affected brains has revealed ASK1 to be activated in substantia nigra neurons compared to control brains (Hu, et al., 2011).
α-Synuclein is a key protein in PD and related disorders such as Dementia with Lewy Bodies as it accumulates in fibrillar form in hallmark pathologic inclusions known as Lewy bodies and Lewy neurites. In addition, multiplication of the α-synuclein gene is linked to rare dominantly inherited PD with a gene dosage effect (Ross, et al., 2008). Over-expression of α-synuclein in model organisms recapitulates many of the phenotypic features of PD including age dependent neurologic deficits and neuroinflammation (Lee, et al., 2011, Watson, et al., 2012). Mounting evidence suggests that accumulation of α-synuclein and its protofibrils stimulates microglial activation and cytokine production (Alvarez-Erviti, et al., 2011, Klegeris, et al., 2008, Lee, et al., 2010,Su, et al., 2008,Zhang, et al., 2005). α-Synuclein over-expression is also associated with increased indices of oxidative stress (Jiang, et al., 2007,Junn and Mouradian, 2002,Tanaka, et al., 2002,Turnbull, et al., 2001).
Considering that oxidative stress and neuroinflammation are associated with α-synuclein mediated pathology, and considering the role of ASK1 in linking these two important pathogenetic phenomena, the present study addresses the role of ASK1 in α-synuclein toxicity in cellular and mouse models of α-synucleinopathy. Our findings show that α-synuclein over-expression induces ASK1 activation, while ASK1 deletion mitigates α-synuclein phenotype. These observations suggest that activated ASK1 plays an important role in the neurodegeneration associated with α-synucleinopathy.
Material and Methods
Cell culture and treatment
PC12 cells expressing inducible α-synuclein using the Tet-off system were cultured in 6 well plates (2×105 cells/ml) in Dulbecco’s modified eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco BRL), 5% horse serum (Gibco BRL) and 1ug/ml doxycycline (Dox) in a CO2 incubator at 37°C. Dox was withdrawn to induce transgene expression as described (Lee, et al., 2003). Recombinant human nerve growth factor (NGF) (50 ng/ml) was added to the medium for the indicated times to induce differentiation.
Animals
ASK1 knockout mice (Lee, et al., 2012, Tobiume, et al., 2001) and human wild-type α-synuclein transgenic mice under the control of the murine Thy-1 promoter (Lee, et al., 2011,Rockenstein, et al., 2002) are described previously. The latter line of mice exhibits sensorimotor deficits as early as 2 months of age, associated with α-synuclein aggregation, neuronal dysfunction, and a robust neuroinflammatory response (Chesselet, et al., 2012). To create α-synuclein transgenic/ASK1 null double mice (α-SynTg/ASK1−/−), male ASK1−/− mice were mated with female α-SynTg mice, and PCR-confirmed progeny were assessed at 3, 6 and 9 months of age. Animals were housed 2–5 / cage in an AAALAC approved facility in a temperature- and humidity-controlled environment under a 12-hour light/dark cycle and were maintained on a diet of lab chow and water ad libitum. All animals were handled in accordance with the Rutgers - Robert Wood Johnson Medical School Institutional Animal Care guidelines.
Western blot and immunohistochemistry
Western blotting and immunohistochemical analyses were performed as described previously (Lee, et al., 2011,Lee, et al., 2012). For Western blot analysis, brain tissue was homogenized in 4°C RIPA lysis buffer (50 mM Tri s, pH 8.0, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate) containing phosphatase inhibitor cocktail set II (Calbiochem, La Jolla, CA) and protease inhibitor cocktail set V (Calbiochem, La Jolla, CA), and then centrifuged at 14,000 rpm at 4°C for 30 min. Supernatants (soluble fraction) were saved, and pellets (insoluble fraction) were lysed with buffer (1% SDS in PBS) containing protease and phosphatase inhibitor cocktail. Each lane was loaded with 20 µg protein. Separated proteins were transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA), followed by blocking with 5% non-fat dry milk in Tris-buffered saline and 0.1% Tween 20. Immunoblots were developed using ECL plus (PerkinElmer, Boston, MA). For immunohistochemistry, mice were perfused transcardially with PBS, and the brains were removed and post-fixed in 4% paraformaldehyde at 4°C overnight. For staining with single antibody, brains were sectioned coronally in 40 µm thickness using a vibratome, and free-floating sections were blocked by 5% BSA. Biotinylated HRP complex (Vector Laboratories, Burlingame, CA) and 3.3'-diaminobenzidine were used for color development. For image analyses, computer-assisted image analysis (Eclipse 55i microscope and NIS Elements D3.2 software, Nikon) was used. For double immunofluorescence staining, 20 µm thick cryostat sections were permeabilized with 1 % BSA and 0.2 % Triton X-100, followed by blocking with 5% normal donkey and goat serum. Sections were then incubated with unconjugated anti-mouse IgG to block the binding of mouse secondary antibody to endogenous mouse IgG. Images were captured using Carl Zeiss axiovert 200 microscope. Primary antibodies used were: ASK1 (H-300) (Santa Cruz Biotechnology), rabbit polyclonal phospho-ASK1 generated against the ASK1 epitope that contains p-Thr845 (Tobiume, et al., 2002) (Lee, et al., 2012), Syn1 for α-synuclein (BD bioscience), LB509 for human α-synuclein (Zymed), anti-phospho-Ser129-α-synuclein (WAKO), NeuN (Chemicon), GFAP (Millipore or DAKO), CD11b (Serotec), Iba-1 (WAKO), Calbindin (Sigma) and β-actin (Sigma). Fluorescent secondary antibodies used were: FITC-conjugated rabbit IgG (green fluorescence for pASK1 detection), TRITC-conjugated mouse IgG (red fluorescence for NeuN and GFAP detection), or TRITC-conjugated rat IgG (for CD11b detection).
Behavioral assessments
Motor coordination was measured by the rotarod. Mice were placed on a rotating cylinder (diameter = 4.5 cm) with a coarse surface for firm grip and tested for three trials with an accelerating speed of 0.2 rpm/second, increasing from 4 to 40 rpm. A cut-off time of 3 min and an inter-trial interval of 60 min were used. Latency on the rod before falling was measured.
Statistical analysis
Data are presented as means ± S.E.M. and analyzed by one-way analysis of variance (ANOVA) followed by the Newman-Keuls multiple range test. Significance was determined at p < 0.05.
Results
α-synuclein overexpression induces phosphorylation/activation of ASK1
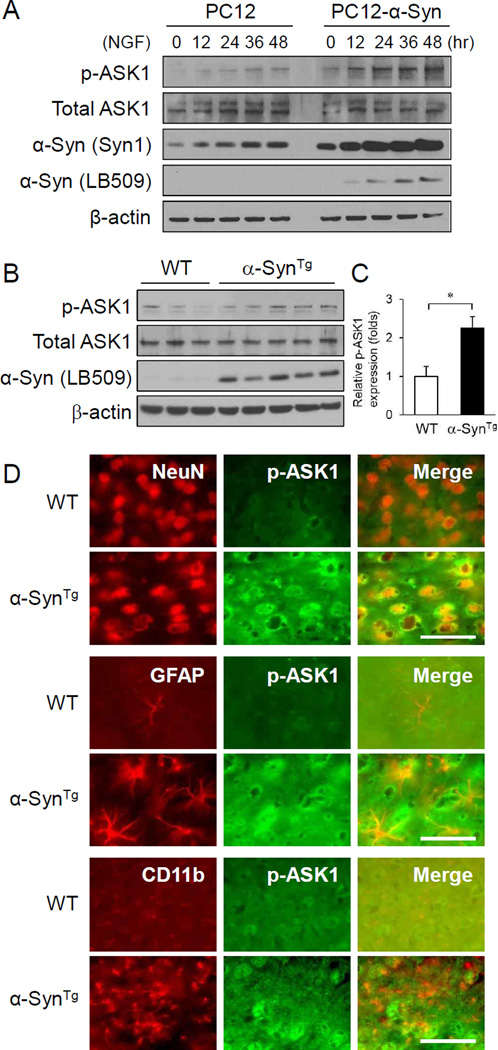
To examine whether α-synuclein expression level affects ASK1 activation state, PC12 cells engineered to express α-synuclein upon withdrawal of doxycycline were differentiated with nerve growth factor (NGF) for 12, 24, 36, and 48 hr, and lysates were assessed for ASK1 and α-synuclein expression. As expected, removal of Dox from the culture medium induced α-synuclein expression, but also increased phospho-ASK1 expression with no appreciable change in total ASK1 levels (Figure 1A). NGF treatment alone also increased α-synuclein level, consistent with the known transcriptional up-regulation of the α-synuclein gene by NGF in PC12 cells and primary neurons through MAP/ERK-dependent parallel pathways (Clough and Stefanis, 2007,Stefanis, et al., 2001). These results indicate that α-synuclein over-expression leads to ASK1 phosphorylation/activation in a simple cellular model. Next, we explored the state of ASK1 activation in the cortex of 3-month old transgenic mice over-expressing human α-synuclein. The levels of phosphorylated ASK1 were increased more than 2 fold in α-synuclein transgenic mice compared with wild-type animals (Fig 1B,C). This was also confirmed by fluorescent immunohistochemistry of cortical tissue sections with phospho-ASK1 antibody (Figure 1D). Notably, the induction of phospho-ASK1 was detected within neurons of α-synuclein transgenic mice compared to wild-type littermates but not in astrocytes or microglia (Figure 1D). Total ASK1 expression was no different between the two mouse groups (Figure 1B). These results collectively indicate that α-synuclein over-expression leads to ASK1 activation.
Figure 1.
α-Synuclein over-expression leads to activation/phosphorylation of ASK1. (A) NGF-induced differentiation of native PC12 cells and PC12 cells engineered to over-express α-synuclein upon withdrawal of doxycyclin enhances ASK1 phosphorylation. Total ASK1 expression is unchanged. (B) Cerebral cortical tissue lysates of 3-month old α-synuclein transgenic mice have increased p-ASK1 expression compared to wild-type mice. (C) Quantification of p-ASK1 band intensities relative to total ASK1 shown in panel B. *P <0.05. (D) Double immunofluorescence staining of p-ASK1 with NeuN, GFAP (Millipore) and CD11b in the cortex of 3-month old α-synuclein transgenic mice compared to wild-type mice. Increased p-ASK1 signal is detected in neurons but not in astrocytes or microglia in transgenic animals. Scale bar = 50 µm.
ASK1 deletion attenuates the neuroinflammation in α-synuclein transgenic mice
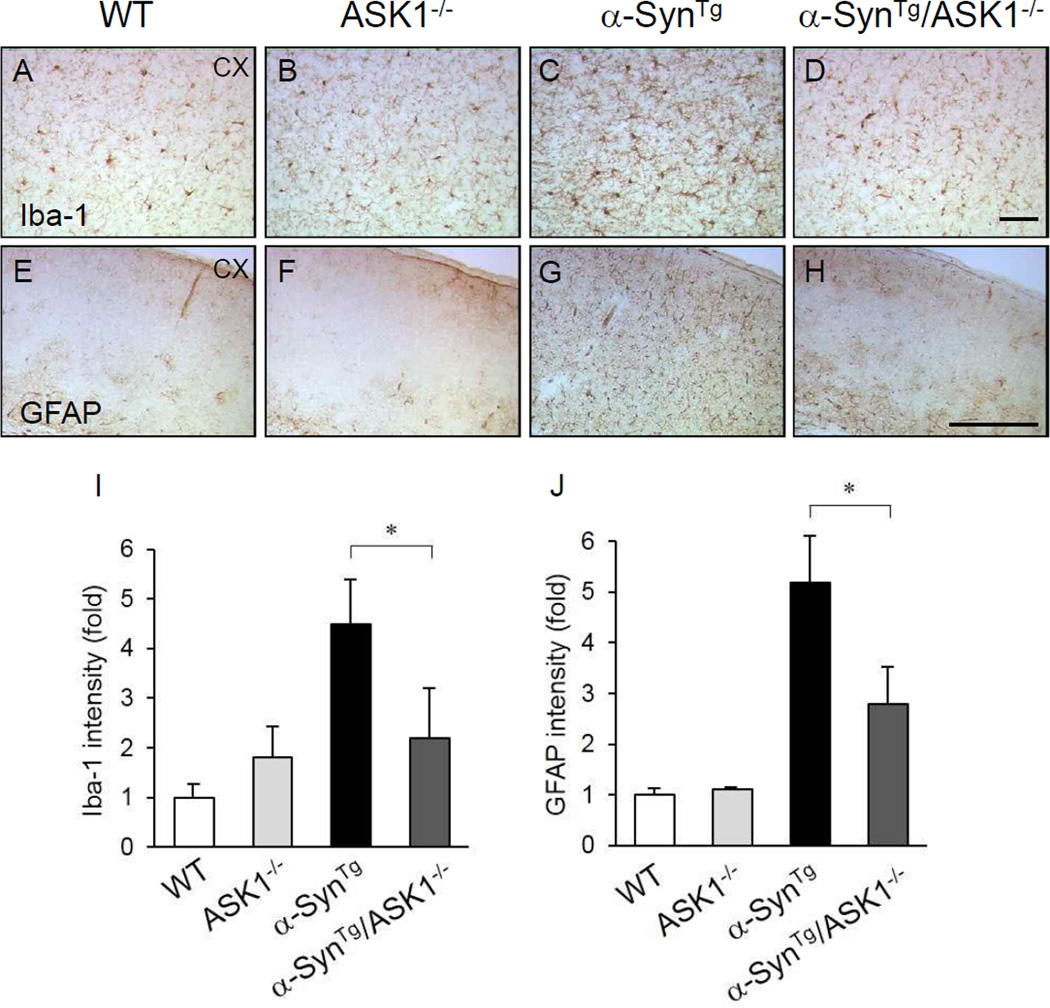
α-Synuclein transgenic mice exhibit robust astroglial and microglial activation compared with wild-type mice (Figure 1D) (Lee, et al., 2011,Watson, et al., 2012). To investigate the impact of ASK1 on these pathological features, brains of 9-month old mice with four genotypes (wild-type, ASK1−/−, α-SynTg, α-SynTg/ASK1−/−) were studied immunohistochemically with the microglial and astroglial markers, Iba-1 and GFAP, respectively. Compared with the increased number of reactive microglia (Figure 2A–D, I) and astrocytes (Figure 2E–H, J) analyzed in the cerebral cortex, α-SynTg/ASK1−/− mice showed diminished levels of microgliosis and astrogliosis. This finding suggests that ASK1 deletion mitigates the neuroinflammatory response to α-synuclein overexpression.
Figure 2.
ASK1 deletion suppresses the glial activation seen in the brains of α-synuclein transgenic mice. Immunohistochemistry with the microglial marker Iba-1 in cortex (A–D). Immunohistochemistry for the astrocytic marker GFAP (DAKO) in cortex (E–H). (I and J) Quantification of microglial and astroglial activation (n: wild-type mice = 5; ASK1−/− = 3; α-SynTg = 4; α-SynTg /ASK1−/− = 3). *P < 0.05. Scale bar = 200 µm.
Deficiency of ASK1 improves neuronal integrity that is lost in α-synuclein transgenic mice
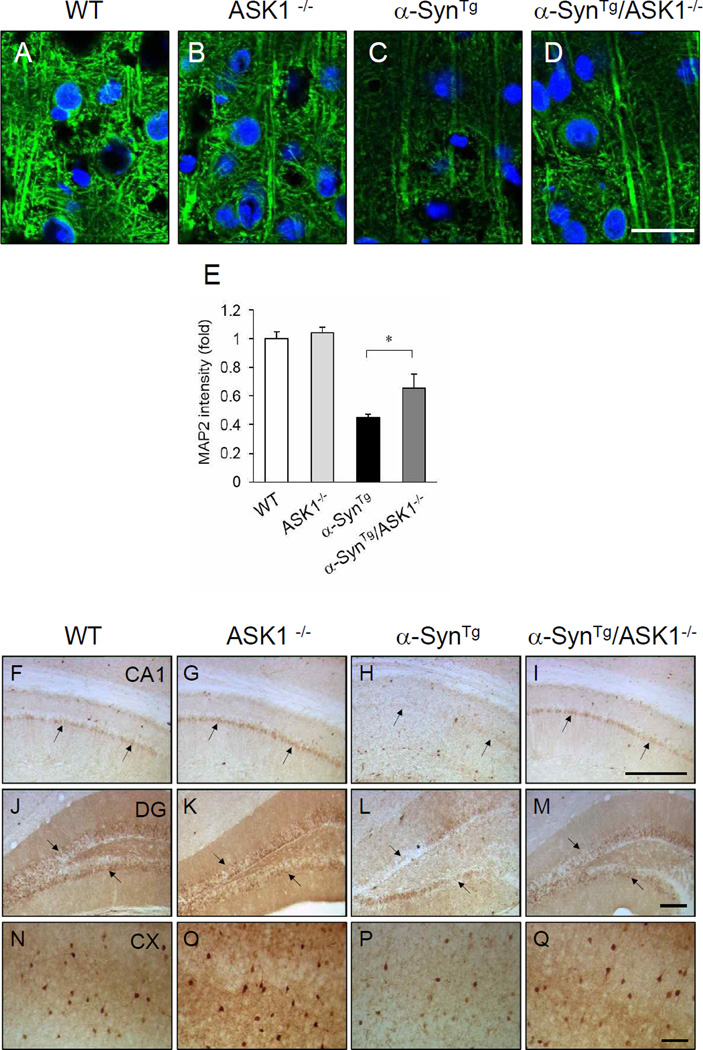
α-Synuclein over-expression in the mouse brain is associated with diminished neuronal dendritic arborizations compared with wild-type animals (Lee, et al., 2011). Here, analysis of pyramidal neurons in the cerebral cortex using MAP2 staining showed that the marked diminution seen in α-SynTg mice is substantially restored in α-SynTg/ASK1−/− mice (Figure 3A–E). We also assessed these brains for the calcium binding protein calbindin, which is important for intracellular calcium homeostasis and helps maintain neuronal functions including synaptic plasticity. Accordingly, PD afflicted brains have been shown to express reduced levels of calcium-binding proteins (Iacopino and Christakos, 1990). In the present study, immunohistochemical analysis indicated that calbindin expression is markedly reduced in the CA1 region (Figure 3F–I) and dentate gyrus (Figure 3J–M) of hippocampus as well as in the cortex of α-SynTg mice compared with wild-type littermates (Fig. 3N–Q). On the other hand, calbindin expression is enhanced in α-SynTg/ASK1−/− mice compared with α-SynTg animals. These results suggest that α-synuclein over-expression impairs neuronal integrity and down-regulates calbindin protein expression, while deficiency of ASK1 prevents these negative consequences of α-synuclein.
Figure 3.
Improvement of dendritic arborization and restoration of calbindin immunoreactive neurons in α-SynTg /ASK1−/− mice compared to α-SynTg mice. (A–D) Immunofluorescence staining of MAP2 (green) in the cortex. Nuclei are stained blue with DAPI. Scale bar = 100 µm. (E) Quantification of MAP2 intensity (n: wild-type mice = 3; ASK1−/− = 3; α-SynTg = 4; α-SynTg /ASK1−/− = 3). *P<0.05. (F–Q) Immunohistochemistry for calbindin in CA1 (F–I) and dentate gyrus (J–M) of the hippocampus, and in cortex (N–Q). Scale bar = 200 µm.
Deleting ASK1 improves the motor impairment of α-SynTg mice
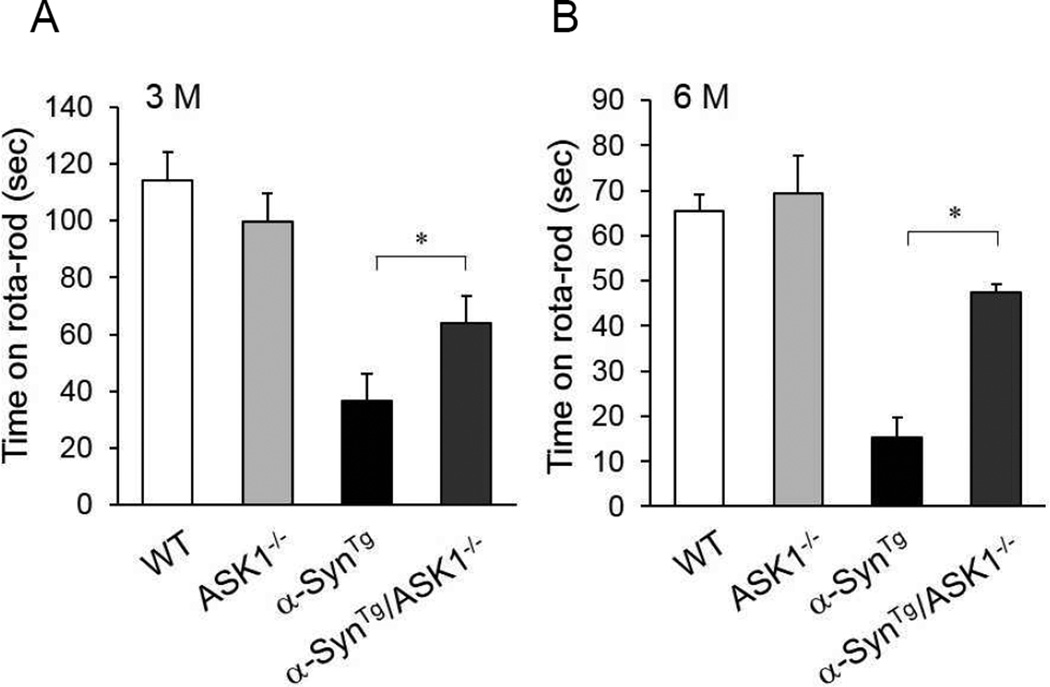
Performance of α-SynTg mice on the rotarod is markedly impaired compared to wild-type and ASK1−/− mice at 3 and 6 months of age (Fig. 4). However, α-SynTg/ASK1−/− mice perform significantly better allowing them to stay on the rotating rod three times longer than α-SynTg mice. This behavioral improvement is consistent with the histopathological data in Figures 2–3.
Figure 4.
Improved motor behavior of α-SynTg /ASK1−/− mice compared with α-SynTg animals. (A) Performance on the rotarod at 3 months of age (n: wild-type mice = 7; ASK1−/− = 6; α-SynTg = 6; α-SynTg /ASK1−/− = 6). (B) Performance on the rotarod at 6 months of age (n: wild-type mice = 5; ASK1−/− = 7; α-SynTg = 5; α-SynTg /ASK1−/− = 3). *<0.05.
Genetic deletion of ASK1 does not alter α-synuclein aggregation or phosphorylation in α-synuclein transgenic mice
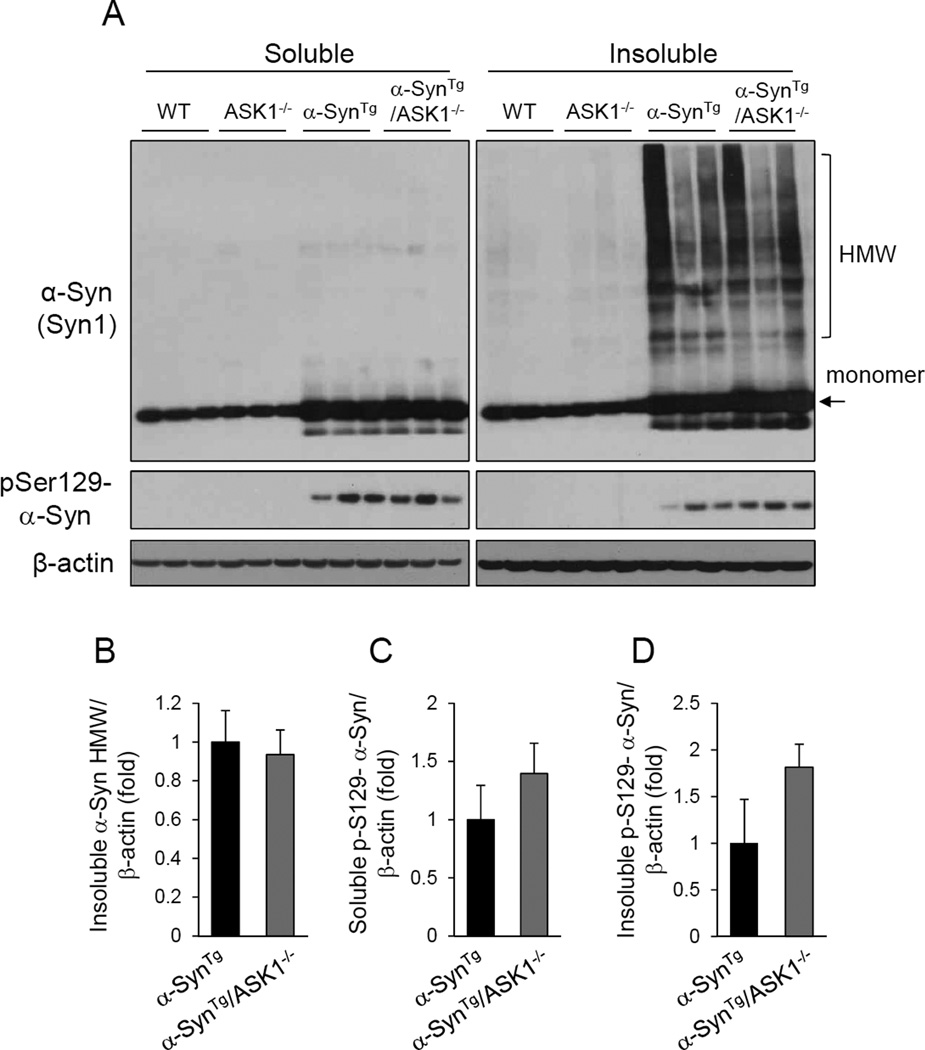
To examine whether ASK1 impacts α-synuclein aggregation or phosphorylation in vivo, cortical tissue lysates from 9 month old α-synuclein transgenic mice (SynTg) were compared with mice that also had a homozygous deletion of ASK1 (SynTg/ASK1−/−). As expected, insoluble high molecular weight species of α-synuclein was abundantly present in the brains of α-SynTg mice and this was no different in animals that also lacked ASK1 (Figure 5). Similarly, the levels of phosphorylated α-synuclein detected in α-SynTg mice were unchanged by ASK1 deletion. These results indicate that ASK1 has no impact on α-synuclein aggregation or phosphorylation in vivo.
Figure 5.
ASK1 deletion does not alter the aggregation or phosphorylation of α-synuclein in the cortex of α-SynTg /ASK1−/− double mice. (A) Soluble and insoluble fractions of cortical lysates from mice of the indicated four genotypes were subjected to Western blot analyses. (B) Quantification of insoluble high molecular weight (HMW) α-synuclein aggregates. (C) Phospho-Serine 129 α-synuclein in the soluble fraction. (D) Phospho-Serine 129 α-synuclein in the insoluble fraction. None of the differences were significant.
Discussion
The results of the present investigation show that α-synuclein over-expression leads to activation of ASK1 in both cultured PC12 cells and in the brains of transgenic mice. Importantly, deleting ASK1 mitigates the phenotype of α-synuclein transgenic mice on a number of outcome measures including markedly repressed inflammatory response to the α-synuclein transgene as well as preserved neuronal integrity and function. These neuropathological indices of protection translate to improved motor coordination. Notably, manipulating ASK1 expression does not impact the phosphorylation state or aggregation potential of α-synuclein in the brain. These observations together indicate that ASK1 activation is downstream of the insults that are initiated by α-synuclein over-expression.
The site of α-synuclein induced ASK1 activation appears to be within neurons where α-synuclein is expressed, since we detect no ASK1 activation in astrocytes or microglia of α-synuclein transgenic mice. The reported finding of ASK1 activation in substantia nigra neurons of PD affected brains (Hu, et al., 2011) is consistent with this notion. A potent activator of ASK1 is oxidative stress (Saitoh, et al., 1998,Song, et al., 2002), and α-synuclein over-expression and protofibrils induce reactive oxygen species generation in vitro and in cultured primary neurons and cell lines (Jiang, et al., 2007,Junn and Mouradian, 2002,Tanaka, et al., 2002,Turnbull, et al., 2001). One mechanism offered for these observations is that α-synuclein protofibrils can form pore-like structures that permeabilize membranes including those of vesicles (Volles and Lansbury, 2002) resulting in leakage of catecholamines to the cytosol (Mosharov, et al., 2006) where they can contribute to oxidative stress. Interestingly, although ASK1 is expressed in immune cells including in resting primary astrocytes and microglia (Lee, et al., 2012), it is not activated in these neuroinflammatory cells by intraneuronal α-synuclein overexpression.
The role of α-synuclein in inducing a neuroinflammatory response is well documented. Activated microglia are present and many inflammatory cytokines are elevated in mouse models of PD where α-synuclein is over-expressed either diffusely in the brains of transgenic mice (Lee, et al., 2011,Watson, et al., 2012), in dopaminergic neurons using TH promoter driven transgene expression (Su, et al., 2008), or through localized over-expression of α-synculein using AAV vector mediated delivery (Chung, et al., 2009,Sanchez-Guajardo, et al., 2010,Theodore, et al., 2008). Stereotactic injection of α-synuclein protofibrils into the substantia nigra of adult rats also results in neuroinflammation and neuronal loss (Wilms, et al., 2009). Additionally, α-synuclein has been shown to up-regulate the expression of factors, such as cell adhesion molecules and matrix metalloproteinase, that result in the release of microglia from the surrounding extracellular matrix allowing their migration into the substantia nigra in the 6-hydroxydopamine mouse model of PD (Kim, et al., 2009). Our present data show that, in the absence of ASK1, the neuroinflammatory response to neuronal α-synuclein over-expression in transgenic mice is attenuated, and suggest that ASK1 activation within neurons is required for this response in vivo. The mechanism by which ASK1 promotes α-synuclein-induced neuroinflammation remains to be investigated.
The therapeutic implications of the present findings are clear. If ASK1 mediates and promotes α-synuclein induced pathology, including both neuronal damage and neuroinflammation, then inhibiting this kinase may ameliorate the toxicity of α-synuclein. Alternatively, potent anti-oxidants and anti-inflammatory agents that are effective in the brain, perhaps in combination, may have to be given further consideration.
Highlights.
α-Synuclein overexpression in neurons leads to activation of Apoptosis Signal Regulating Kinase 1 (ASK1) in culture and in the mouse brain
Deleting the ASK1 gene mitigates the phenotype of α-synuclein transgenic mice including the neuronal damage, neuroinflammation and motor impairment
These findings implicate ASK1 in the cascade of events triggered by α-synuclein overexpression
Acknowledgements
The authors thank Eliezer Masliah for providing α-synuclein transgenic mice. This work was supported by National Institutes of Health (NIH) grants NS059869 and NS053517 to M.M.M. who is the William Dow Lovett Professor of Neurology. E.J. is supported by NIH grant NS070898.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no relevant conflict of interest
References
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69(4):337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281(5384):1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson's disease: the Thy1-aSyn ("Line 61") mice. Neurotherapeutics. 2012;9(2):297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29(11):3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough RL, Stefanis L. A novel pathway for transcriptional regulation of alpha-synuclein. FASEB J. 2007;21(2):596–607. doi: 10.1096/fj.06-7111com. [DOI] [PubMed] [Google Scholar]
- Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. Journal of Parkinson's disease. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21(2):404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42(9):781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J, Hastings T, Greenamyre JT, Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31(1):247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72(2):355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci U S A. 1990;87(11):4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics, C. Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wu YC, Nakamura M, Liang Y, Tanaka Y, Holmes S, Dawson VL, Dawson TM, Ross CA, Smith WW. Parkinson's disease genetic mutations increase cell susceptibility to stress: mutant alpha-synuclein enhances H2O2- and Sin-1-induced cell death. Neurobiol Aging. 2007;28(11):1709–1717. doi: 10.1016/j.neurobiolaging.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Junn E, Mouradian MM. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett. 2002;320(3):146–150. doi: 10.1016/s0304-3940(02)00016-2. [DOI] [PubMed] [Google Scholar]
- Kim S, Cho SH, Kim KY, Shin KY, Kim HS, Park CH, Chang KA, Lee SH, Cho D, Suh YH. Alpha-synuclein induces migration of BV-2 microglial cells by up-regulation of CD44 and MT1-MMP. J Neurochem. 2009;109(5):1483–1496. doi: 10.1111/j.1471-4159.2009.06075.x. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29(5):739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185(1):615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng X, Ray N, Fernandez JR, Chao Y, Masliah E, Voronkov M, Braithwaite SP, Stock JB, Mouradian MM. Enhanced phosphatase activity attenuates alpha-Synucleinopathy in a mouse model. J Neurosci. 2011;31(19):6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Zhao X, Im JY, Grosso H, Jang WH, Chan TW, Sonsalla PK, German DC, Ichijo H, Junn E, Mouradian MM. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS One. 2012;7(1):e29935. doi: 10.1371/journal.pone.0029935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Kim YM, Junn E, Lee G, Park KH, Tanaka M, Ronchetti RD, Quezado MM, Mouradian MM. Cell cycle aberrations by alpha-synuclein over-expression and cyclin B immunoreactivity in Lewy bodies. Neurobiol Aging. 2003;24(5):687–696. doi: 10.1016/s0197-4580(02)00196-3. [DOI] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20(6):2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180(2):147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Staal RG, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen KE, Moore CM, Troyer MD, Edwards RH, Przedborski S, Sulzer D. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006;26(36):9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57(2):168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68(5):568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, Nishioka K, Fuchs J, Gasser T, Maraganore DM, Adler CH, Larvor L, Chartier-Harlin MC, Nilsson C, Langston JW, Gwinn K, Hattori N, Farrer MJ. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63(6):743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson's disease. PLoS One. 2010;5(1):e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277(48):46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- Stefanis L, Kholodilov N, Rideout HJ, Burke RE, Greene LA. Synuclein-1 is selectively up-regulated in response to nerve growth factor treatment in PC12 cells. J Neurochem. 2001;76(4):1165–1176. doi: 10.1046/j.1471-4159.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29(11):1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Takehashi M, Matoh N, Iida S, Suzuki T, Futaki S, Hamada H, Masliah E, Sugiura Y, Ueda K. Generation of reactive oxygen species and activation of NF-kappaB by non-Abeta component of Alzheimer's disease amyloid. J Neurochem. 2002;82(2):305–315. doi: 10.1046/j.1471-4159.2002.00958.x. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67(12):1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2(3):222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. Journal of cellular physiology. 2002;191(1):95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson's disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med. 2001;30(10):1163–1170. doi: 10.1016/s0891-5849(01)00513-5. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41(14):4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237(2):318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Romero-Ramos M, Arlt A, Schafer H, Seegert D, Kahle PJ, Odoy S, Claasen JH, Holzknecht C, Brandenburg LO, Deuschl G, Schreiber S, Kirik D, Lucius R. Suppression of MAP kinases inhibits microglial activation and attenuates neuronal cell death induced by alpha-synuclein protofibrils. Int J Immunopathol Pharmacol. 2009;22(4):897–909. doi: 10.1177/039463200902200405. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]