Abstract
BACKGROUND
Transcranial direct current stimulation (tDCS) may provide a safe, non-invasive technique for modulating neural excitability during neurorehabilitation.
OBJECTIVE
1) Assess feasibility and potential effectiveness of tDCS as an adjunct to standard upper extremity (UE) physical therapy (PT) for motor impairments resulting from neurological insult. 2) Determine sustainability of improvements over a six month period.
METHODS
Five participants with chronic neurologic insult (stroke or traumatic brain injury > 6 months prior) completed 24 sessions (40 minutes, three times/week) of UE-PT combined with bihemispheric tDCS delivered at 1.5mA over the motor cortex during the first 15 minutes of each PT session. Outcomes were assessed using clinical (UE Fugl-Meyer, Purdue Pegboard, Box and Block, Stroke Impact Scale) and robotic (unimanual and bimanual motor control) measures. Change in scores and associated effects sizes from Pre-test to Post-test and a six month Follow-up were calculated for each participant and group as a whole.
RESULTS
Scores on UE Fugl-Meyer, Box and Block, Purdue Pegboard, Stroke Impact Scale, and robotic measures improved from Pre- to Post-test. Improvements on UE Fugl-Meyer, Box and Block, and robotic measures were largely sustained at six months.
CONCLUSIONS
Combining bihemispheric tDCS with UE-PT in individuals with neurological insult warrants further investigation.
INTRODUCTION
Following neurological insult, sites in the intact hemisphere exert increased interhemispheric inhibition over homologous sites in the lesioned hemisphere. This inhibition may hinder functional recovery by further suppressing already reduced activity in the lesioned hemisphere and interfering with the normal operation of activity-dependent plasticity (Kidgell, Goodwill, Frazer, & Daly, 2013; Murase, Duque, Mazzocchio, & Cohen, 2004). Transcranial direct current stimulation (tDCS) may provide a safe, non-invasive (Russo, Wallace, Fitzgerald, & Cooper, 2013) technique for modulating neural excitability by exciting (anodal stimulation) or inhibiting (cathodal stimulation) neurons in targeted cortical areas (Kidgell et al., 2013; Pellicciari, Brignani, & Miniussi, 2013). Accordingly, tDCS may allow for better recovery by reducing interhemispheric imbalance (Bolognini, Pascual-Leone, & Fregni, 2009; Feng, Bowden, & Kautz, 2013; Murase et al., 2004; Nowak, Grefkes, Ameli, & Fink, 2009). In particular, bihemispheric stimulation, which involves placement of the source electrode over the damaged motor cortex and placement of the sink electrode over the undamaged motor cortex, may provide additional benefits over stimulation of a single hemisphere by simultaneously increasing excitability in weakened areas and decreasing excitability in regions that inhibit these areas (Vines, Cerruti, & Schlaug, 2008).
Bihemispheric tDCS holds potential as a feasible adjunct to standard rehabilitation because it is portable and requires minimal technical expertise, time, and monetary cost. Stimulation is delivered via a lightweight, portable unit that can be carried in a pocket. The unit is connected to two disposable sponge electrodes that can be held in place with a cap. Importantly, neither the unit nor the cap interferes with typical treatment activities. Placing the electrodes and programming the unit can be completed in approximately five minutes and requires minimal training. The units are also relatively inexpensive and, in many cases, already available for iontophoresis treatments in physical therapy (PT) clinics.
Multiple studies have been conducted to examine the immediate effects of a single bout of tDCS on individuals with stroke (Fusco et al., 2013; Giacobbe et al., 2013; Lefebvre et al., 2012; Lefebvre et al., 2013; O'Shea et al., 2013). However, few studies have used tDCS as an adjunct to upper extremity (UE) therapy for this population, and none have examined individuals with TBI. Most studies have included approximately five treatment sessions (Khedr et al., 2013; Lindenberg, Renga, Zhu, Nair, & Schlaug, 2010; Nair, Renga, Lindenberg, Zhu, & Schlaug, 2011; Ochi, Saeki, Oda, Matsushima, & Hachisuka, 2013), with a few longer studies including between 10 and 20 sessions (Bolognini et al., 2011; Wu et al., 2013). Follow-up periods have ranged from one week (Lindenberg et al., 2010; Nair et al., 2011) to three months (Khedr et al., 2013). As a result, numerous questions exist regarding the role of tDCS as an adjunct to PT over the course of an entire plan of care, which for UE PT, typically includes approximately 960 minutes dispersed over 24 treatment sessions (Birkenmeier, Prager, & Lang, 2010; Chang, Tung, Wu, Huang, & Su, 2007; Dickstein, Hocherman, Pillar, & Shaham, 1986; Kimberley, Samargia, Moore, Shakya, & Lang, 2010; Lang, MacDonald, & Gnip, 2007; Wang, Zhao, Zhu, Li, & Meng, 2011). Is providing stimulation throughout an entire plan of care feasible? Are there adverse effects? Does it elicit improvement in UE function? Are improvements maintained for extended periods of months and years following treatment?
Dobkin (2009) presented recommendations on staging of pilot studies in neurorehabilitation, which included guidelines for designing studies that build on each other and lead to effective multi-center randomized clinical trials (MRCT). Dobkin proposes that research on rehabilitation techniques should progress through four stages from consideration-of-concept studies (stage 1) through proof-of-concept MRCTs (stage 4) (Dobkin, 2009). As evidence is limited in regards to the use of bihemispheric tDCS as an adjunct to UE therapy in patients with neurological insult, consideration-of-concept studies are needed prior to moving on to more advanced stages. Our objective was to carry out a consideration-of-concept pilot study aimed at: 1) assessing the feasibility and potential effectiveness of tDCS paired with standard UE PT over an entire plan of care for individuals with motor impairments resulting from neurological insult, and 2) determining the sustainability of improvements over a six month period.
METHODS
Participants
All participants signed an Informed Consent form approved by the Institutional Review Board of the University of XXX. Inclusion and exclusion criteria are presented in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|
| Exclusion Criteria |
|
Intervention
During the intervention phase of the study, participants completed 24 sessions (40 minutes, three times per week) of UE PT combined with bihemispheric tDCS. The first 10 minutes of each session were dedicated to standardized strengthening activities, and the final 30 minutes were spent performing three, separate functional activities for 10 minutes each. All activities were tailored to the appropriate level for each participant with the goal of maintaining a degree of difficulty sufficient to produce adaptation. The treatment clock was started at the beginning of the session and the session was terminated once 40 minutes had elapsed, similar to the time demands in a clinical setting. A physical therapist or doctoral student in physical therapy delivered all treatments.
Strengthening Phase (10 minutes)
Participants performed one minute of five UE exercises in circuit format completed twice. Standardized exercises consisted of push-ups, pronation/supination of the affected UE, and UE therapy-ball exercises (lifting ball up/down, moving ball side-to-side, and rotating ball). Push-ups were progressed by transitioning from wall push-ups to push-ups on an elevated mat table. Forearm pronation/supination was progressed by changing the weight or lever arm of the object held by the participant. Therapy ball exercises were progressed by increasing the size of the ball (e.g. 45cm diameter to 55cm). During each strengthening activity, continuous performance for one minute was encouraged.
Functional Activities Phase (30 minutes)
Although the structure of delivery of the functional activities was standardized (i.e. three activities for 10 minutes), activities were participant-specific and modeled on clinical practice. By organizing functional activities into 10 minute blocks, we were able to address different impairments, maximize active time, and minimize time lost transitioning between activities. Activities ranged from gross to fine motor depending on participant deficits. Examples of gross motor activities included reaching for items on shelves, hitting a balloon with a racquet or hand, and simulating household chores. Activities were progressed by changing the direction or height of reaching and hitting motions, incorporating heavier objects, or increasing the complexity of chores. Examples of fine motor tasks included flipping playing cards, manipulating small change, and writing. These were progressed by adding speed and/or accuracy components.
tDCS Parameters
During the first 15 minutes of each treatment session (10 minutes of strengthening and first five minutes of functional activities), participants received 1.5 mA of bihemispheric tDCS delivered via a dual-channel constant-current stimulator (Chattanooga Ionto Dual Channel Drug Delivery Phoresor Device, Iomed® Inc., Salt Lake City, Utah). In order to determine location of motor cortices and maintain electrode contact, the participants were fitted with a standard EEG cap (International EEG system). Saline soaked 2”× 2” sponge electrodes were then placed under the C3 and C4 electrode locations on the cap, which correspond to the left and right motor cortices, respectively. Cap sizing and electrode placement were in accordance with the international 10–20 system (Homan, Herman, & Purdy, 1987; Okamoto et al., 2004). The anodal electrode was centered over the ipsilesional motor cortex and cathodal electrode over the contralesional motor cortex. Previous studies involving individuals with chronic stroke have applied tDCS ranging from 1 to 2 mA delivered for between 10 and 40 minutes (Ang et al., 2012; Bolognini et al., 2011; Lefebvre et al., 2012; Lindenberg et al., 2010; Ochi et al., 2013). The dosage selected for this study falls within this range and has been shown to alter excitability of underlying cortical regions for 60 to 90 minutes (Nitsche & Paulus, 2001).
Outcome Measures
A unique feature of this study was supplementing traditional clinical measures with more sensitive robotic measures of UE function. Robotic assessments provide objective measures that can accurately quantify spatial and temporal parameters of UE movements in neurological populations (Balasubramanian, Colombo, Sterpi, Sanguineti, & Burdet, 2012; Debert, Herter, Scott, & Dukelow, 2012; Einav, Geva, Yoeli, Kerzhner, & Mauritz, 2011; Zollo, Gallotta, Guglielmelli, & Sterzi, 2011). Such objective measures are particularly valuable for avoiding biases that can influence longitudinal assessments used for intervention studies (Scott & Dukelow, 2011).
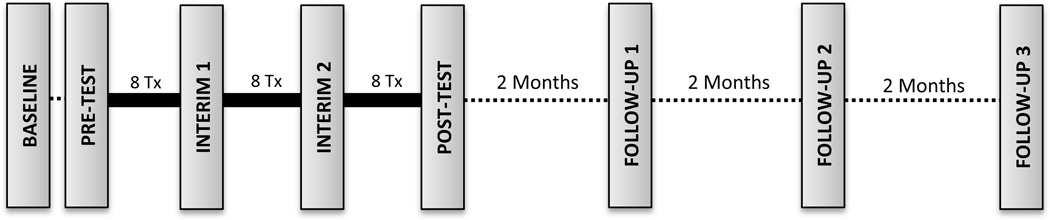
The schedule of assessment sessions is presented in Figure 1. Both clinical and robotic data were collected at each time point. Pre-intervention testing, Post-test, and Follow-up sessions were comprehensive, while only a subset of the clinical measures (i.e. Box and Block Test, Purdue Pegboard Test, and robotic measures) were administered during interim testing sessions. All testing was conducted by a licensed physical therapist who received training on the standardized protocols.
Figure 1.
Flow of assessment and treatment sessions. Tx, treatment sessions
Clinical Measures
Fugl-Meyer Assessment of Sensorimotor Impairment UE section (UE-FM) The UE-FM is a valid and reliable measure of UE impairment following stroke and is recommended for assessing change in this population (Gladstone, Danells, & Black, 2002; J. H. Lin et al., 2009). The UE-FM assesses reflexes, range of motion, pain, light touch sensation, proprioception, movements in and out of synergy, grasp, and coordination on a 3-point ordinal scale (0 = cannot perform, 1 = performs partially, 2 = performs fully) with a maximum score of 66 points (Duncan, Propst, & Nelson, 1983). For individuals with chronic stroke, change scores of 4.25 to 7.25 points indicate that a clinically important change has occurred (Page, Fulk, & Boyne, 2012). The UE-FM was administered at Baseline, Post-test, and all Follow-up testing sessions.
Box and Block Test (BBT)
The BBT assesses manual dexterity by requiring participants to move as many 2.5 cm blocks as possible in one minute. Participants move the blocks over a partition separating two sides of a standardized test box. Normative data regarding the number of blocks moved for five-year age groups has been established (Mathiowetz, Volland, Kashman, & Weber, 1985). The measure is valid (K. C. Lin, Chuang, Wu, Hsieh, & Chang, 2010) and reliable (Chen, Chen, Hsueh, Huang, & Hsieh, 2009) for assessing UE dexterity following stroke with a minimal detectable change (MDC) for this population of 5.5 blocks (Chen et al., 2009). The BBT was administered at all assessment sessions.
Purdue Pegboard Test (PPT)
The PPT is a reliable measure of fine manual dexterity that requires participants to pick up pins one at a time out of a well and place them consecutively in a row of holes (Desrosiers, Hebert, Bravo, & Dutil, 1995; Tiffin & Asher, 1948). The goal is to successfully place as many pins as possible in 30 seconds. Normative data for this measure have been established (Desrosiers et al., 1995; Tiffin & Asher, 1948). The PPT was administered at all assessment sessions.
Stroke Impact Scale-16 (SIS-16)
The SIS-16 is a valid and reliable assessment of self-perceived physical function (Duncan, Lai, Bode, Perera, & DeRosa, 2003) with a minimal clinically important difference value of 9.4–14.1 points (Fulk et al., 2010). Domains covered on the SIS-16 include hand function, strength, mobility, and ADL/IADL (Duncan et al., 2003). The participant scores each item on a 1 to 5 Likert scale with higher scores indicating better self-perceived function. The SIS was administered at Baseline, Post-test, and all Follow-up testing sessions.
Robotic Measures
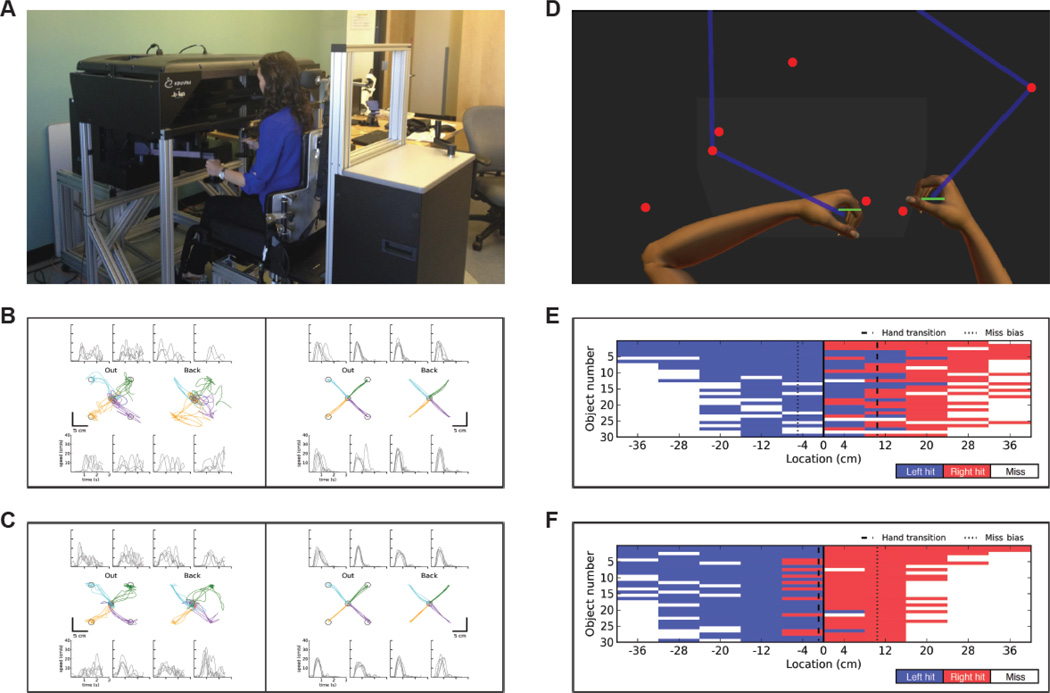
Robotic assessments were performed using the KINARM End-Point Lab (BKIN Technologies, Kingston, Ontario, Canada). Participants sat at the robot and grasped handles linked to robotic motors with each hand (see Figure 2A). Arm movements were performed in a horizontal plane in response to targets presented with an augmented reality display. Robotic measures were collected at all assessment sessions. The following standardized tasks were used to quantify the participants’ UE sensorimotor functioning.
Figure 2.
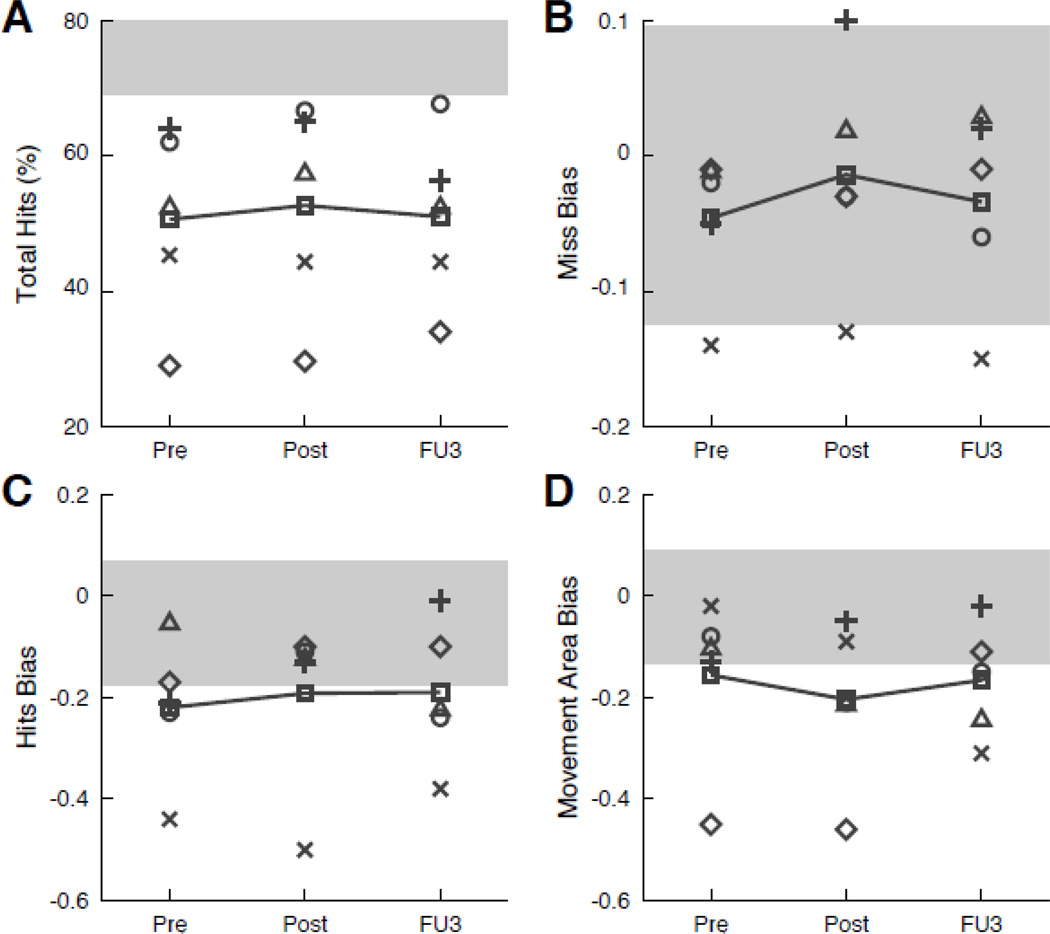
Robotic Assessment. A, Individual seated at the KINARM End-Point Lab (BKIN Technologies, Kingston, Ontario, Canada). While sitting in a modified wheelchair base that can be raised and lowered with a hydraulic system, participants grasp two handles linked to robotic motors that can apply loads to either hand. Small discs are mounted underneath each handle to provide the hands with gravitational support. A video monitor and semitransparent mirror located above the hands are used to project visual targets onto the same plane as the hands. B,C, Hand paths and hand-speed profiles of Participant 5 during performance of the visually guided reaching task with the affected left hand (left half) and less affected right hand (right half) at Pre-test (B) and Post-test (C). Hand paths (center row) depict each reaching movements from the central target out to the four peripheral targets (first and third plots) and from the peripheral targets back to the central target (second and fourth plots). The hand-speed profiles (top and bottom rows) correspond to reaching movements to and from the closest peripheral target in each plot. Each line on the graph represents a single trial. D, Overhead reproduction of Participant 2 (right upper extremity affected) performing the object hit task. Participants use paddles (5 cm) attached to each hand to hit away as many balls as possible as they move towards them from the top of the screen. E, F, Performance of Participant 2 on the object hit task at Pre-test (E) and Post-test (F). Balls move along 10 vertical paths (columns) with 30 balls (rows) in each path for a total of 300 balls. Blue areas represent hits with the left hand (less affected) and red areas represent hits with the right hand (affected). White areas represent misses. At Pre-test, Participant 2 frequently uses her left hand to hit balls on the right side of the screen (E), whereas hand and use is more symmetrical at Post-test (F). This is shown measured by the hand transition parameter (dashed line), which is on the right during the Pre-test and relatively central during the Post-test.
Visually Guided Reaching Task
The visually guided reaching task is a reliable, sensitive measure of UE visuomotor control following stroke (Coderre et al., 2010). Participants generated reaching movements from a central target to four peripheral targets (2.0 cm diameter circles) located 10 cm away (see Figure 2 B and C). Participants were instructed to reach as quickly and accurately as possible to the targets as they appear. Targets were presented in a block design with five randomized blocks for a total of 40 trials. Each participant repeated the task twice, once with each hand. Four measures were used to quantify distinct aspects of task performance: speed maxima count, path length ratio, movement time, and initial direction error. These are defined in Table 2.
Table 2.
Parameters of Robotic Assessment
| Parameter | Definition | Direction of Improvement |
|
|---|---|---|---|
| Visually Guided Reaching | Speed Maxima Count | Number of speed peaks between two targets. | Count closer to 1 |
| Path Length Ratio | Distance hand travels to reach a target divided by straight-line distance to target. | Ratio closer to 1 | |
| Movement Time | Time from movement initiation to termination. | Decreased time | |
| Initial Direction Error | Angle between line representative of straight path between targets and line corresponding to actual direction hand travels on initiation of reaching motion. | Angle closer to 0° | |
| Object Hit | Total Hits | Number of balls successfully hit away with paddle. | Increased hits |
| Miss Bias | Indication of location of misses. Negative- more misses on side of screen corresponding to affected UE and vice versa. Calculated as the mean of the distance of each missed ball from center. | Distance closer to 0 | |
| Hit Bias | Indication of hand use. Calculated as (hits with affected − hits with unaffected)/(hits with affected + hits with unaffected). | Ratio closer to 0 | |
| Movement Area Bias | Indication of amount of area covered by one hand relative to the other. Calculated as (area covered by affected − area covered by unaffected)/(area covered by affected + area covered by unaffected). | Ratio closer to 0 | |
Object Hit Task
The object hit task is designed to assess bimanual coordination and spatial awareness (Tyryshkin et al., 2014). Participants used 5 cm paddles displayed on top of the robot handles to hit away balls (2.0 cm diameter circles) moving towards them from the top of the workspace. Participants were instructed to use both hands to hit as many balls away as possible with the paddles. The workspace (80 cm wide) was divided into 10 bins containing 30 balls each for a total of 300 balls. The time and location that balls were released was randomized, but the speed each ball moved was constant. The task took a fixed amount of time, and difficulty increased over time by progressively increasing the number of balls and their speed (See Figure 2 D–F). Four measures were used to quantify distinct aspects of task performance: total hits, hit bias, miss bias, and movement area bias (Table 2).
Data Analysis
Change in outcome measure scores from Pre-test to Post-test and Pre-test to Follow-up 3 were calculated for each participant and the group as a whole. Effect size data was included as this may help inform sample sizes of future studies. To maintain consistency of effect size interpretation across all measures, positive effect size values represent improvement and negative values represent decline. For measures in which a positive effect size indicated decline, the sign on the effect size was reversed.
RESULTS
Participants
Eight individuals who met all inclusion and exclusion criteria participated in the study. Three participants did not complete the intervention; one dropped out due to unrelated health issues and two dropped out due to unexpected scheduling/transportation issues. Baseline characteristics of the remaining five participants are presented in Table 3.
Table 3.
Baseline Characteristics of Study Sample
| Participant | Diagnosis | Age (years) |
Time since Stroke/TBI (months) |
Affected UE |
Gender | MAS elbow flexors |
MAS wrist flexors |
MOCA |
|---|---|---|---|---|---|---|---|---|
| 1 | Stroke | 57 | 15 | R | F | 0 | 0 | 21 |
| 2 | Stroke | 69 | 50 | R | F | 0 | 0 | 23 |
| 3 | Stroke | 59 | 253 | R | F | 3 | 1+ | 11 |
| 4 | TBI | 39 | 206 | L | M | 1+ | 0 | 23 |
| 5 | TBI+Stroke | 38 | 9 | L | M | 0 | 1 | 24 |
TBI, traumatic brain injury; UE, upper extremity; MAS, Modified Ashworth Scale; MOCA, Montreal Cognitive Assessment; R, right; L, left; F, female; M, male
Follow-up Assessment Sessions
Table 4 shows the timing of the Follow-up assessment sessions of each participant. Although follow-up testing was planned for two, four, and six months following completion of intervention, scheduling difficulties resulted in a number of minor deviations from the planned protocol. Notably, Participant 1 completed Follow-up 2 at five months post-intervention and was unable to complete Follow-up 3. Because of the proximity in time (see Table 4), data from Follow-up 2 was carried forward and included in the data analysis for Follow-up 3 for this patient only. Participant 5 completed 14 sessions of an unrelated sensory intervention study focused solely on proprioception between Follow-ups 2 and 3.
Table 4.
Timing of Follow-up Assessment Sessions
| Participant | Post to FU1 | FU1 to FU2 | FU2 to FU3 | Post to FU3 |
|---|---|---|---|---|
| 1 | 77 | 80 | − | 157* |
| 2 | 71 | 77 | 62 | 210 |
| 3 | 98 | 56 | 64 | 218 |
| 4 | 58 | 56 | 63 | 177 |
| 5 | 44 | 77 | 63 | 184 |
| AVERAGE | 70 | 69 | 63† | 197.25 |
Results presented in days.
Abbreviations: Post, post-test; FU, follow-up
Post to FU2 presented for participant 1
Average for participants 2–5
Individual Participant and Group Outcomes
Due to the small diverse sample, results are presented for each participant separately, as well as for the group as a whole. Data from Pre-test, Post-test, and Follow-up 3 were selected for presentation. These time points provide information on participant performance prior to, immediately following, and six months following intervention.
Clinical Outcomes
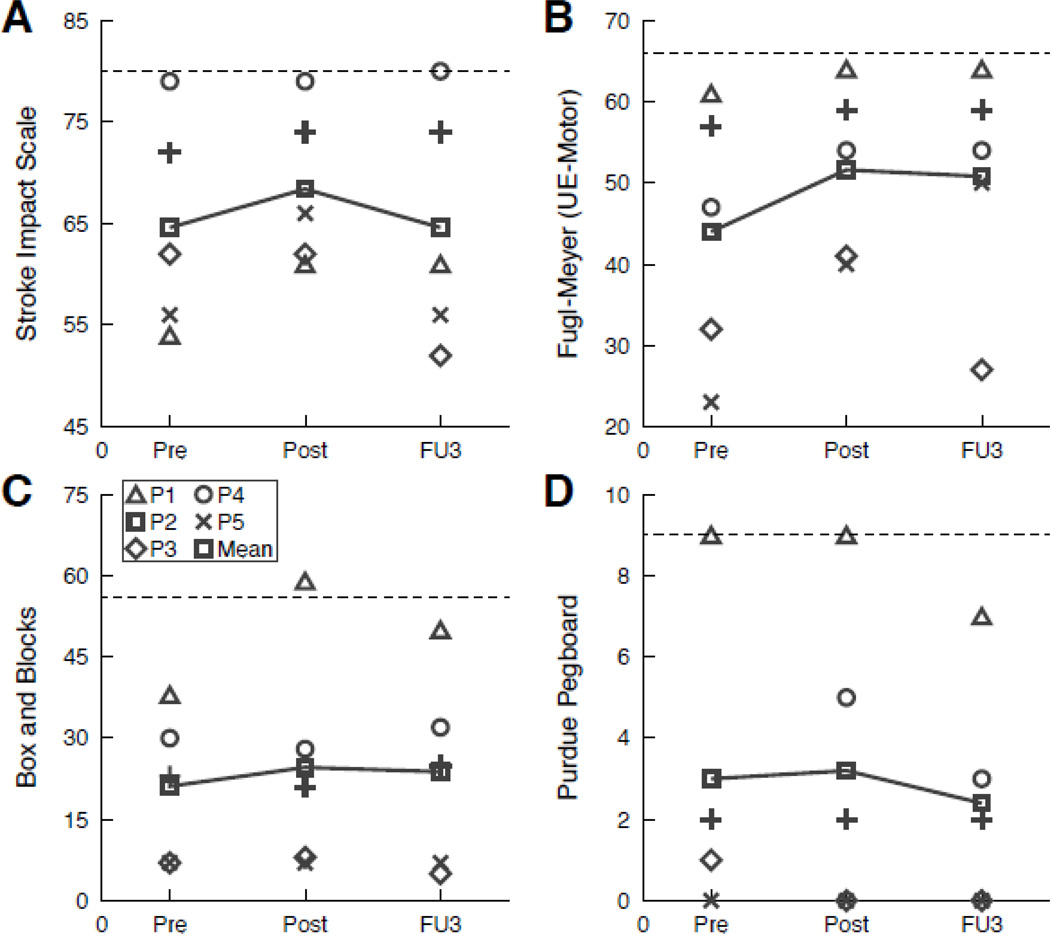
Table 5 and Figure 3 show individual and group data on clinical outcome measures. The greatest improvement immediately following intervention was seen on the UE-FM (mean change = 7.6, effect size = 0.47). Individual participant results on the clinical measures were variable, with some participants demonstrating change on specific measures and others demonstrating little to no change on the same measure.
Table 5.
Participant and Group Outcomes on Clinical Measures
| Participant | Pre | Post | FU 3* | Δ Pre to Post {Effect Size} |
Δ Pre to FU 3 {Effect Size} |
|
|---|---|---|---|---|---|---|
| Stroke Impact Scale | 1 | 54 | 61 | 61 | 7 | 7 |
| 2 | 72 | 74 | 74 | 2 | 2 | |
| 3 | 62 | 62 | 52 | 0 | −10 | |
| 4 | 79 | 79 | 80 | 0 | 1 | |
| 5 | 56 | 66 | 56 | 10 | 0 | |
| GROUP | 64.6 (10.67) | 68.4 (7.83) | 64.6 (11.95) | 3.8 {0.36} | 0 {0} | |
| Fugl-Meyer (UE-Motor) | 1 | 61 | 64 | 64 | 3 | 3 |
| 2 | 57 | 59 | 59 | 2 | 2 | |
| 3 | 32 | 41 | 27 | 9 | −5 | |
| 4 | 47 | 54 | 54 | 7 | 7 | |
| 5 | 23 | 40 | 50 | 17 | 27 | |
| GROUP | 44 (16.22) | 51.6 (10.74) | 50.8 (14.31) | 7.6 {0.47} | 6.8 {0.42} | |
| Box and Block (Affected UE) | 1 | 38 | 59 | 50 | 21 | 12 |
| 2 | 23 | 21 | 25 | −2 | 2 | |
| 3 | 7 | 8 | 5 | 1 | −2 | |
| 4 | 30 | 28 | 32 | −2 | 2 | |
| 5 | 7 | 7 | 7 | 0 | 0 | |
| GROUP | 21 (13.84) | 24.6 (21.17) | 23.8(18.65) | 3.6 {0.26} | 2.8 {0.20} | |
| Purdue Pegboard (Affected UE) | 1 | 9 | 9 | 7 | 0 | −2 |
| 2 | 2 | 2 | 2 | 0 | 0 | |
| 3 | 1 | 0 | 0 | −1 | −1 | |
| 4 | 3 | 5 | 3 | 2 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| GROUP | 3 (2.30) | 3.2 (3.83) | 2.4 (2.88) | 0.2 {0.06} | −0.6 {−0.17} | |
Pre, pre-test; Post, post-test; FU3, follow-up 3; ∆, change; UE, upper extremity
FU3 results for Participant 1 are FU2 results carried forward
Participant 5 participated in 14 sessions of a sensory intervention between post-test and
Figure 3.
Individual and group outcomes on clinical measures. Results presented for Stroke Impact Scale (A), Fugl-Meyer Assessment of Sensorimotor Impairment UE section (B), Box and Blocks Test (C), and Purdue Pegboard Test (D). Dashed lines in A and B show maximum attainable score (normal). Dashed lines in C and D show the lower limit of normal range. For all measures, increases in score indicate improvement. Abbreviations: UE, upper extremity; Pre, Pre-test; Post, Post-test; FU 3, Follow-up 3.
Robotic Outcomes
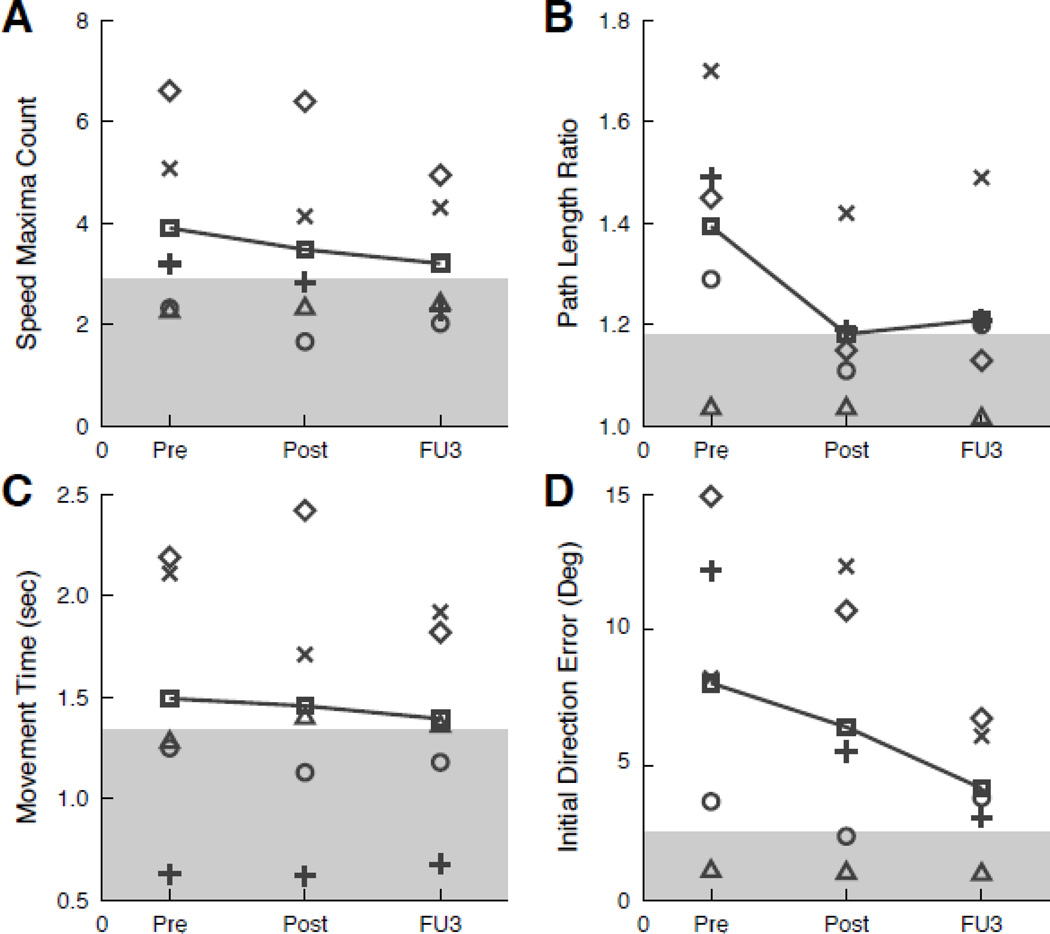
Table 6 and Figures 4 and 5 show individual and group data on robotic outcome measures. At the group level, the participants exhibited consistent Pre- to Post-test improvements on the reaching task and object hit task. Despite their heterogeneity, individual participants showed improvements in performance on the reaching and/or object hit task that were consistent with the changes in the group data.
Table 6.
Participant and Group Outcomes on Robotic Measures
| Participant | Pre | Post | FU 3*† | Δ Pre to Post {Effect Size} |
Δ Pre to FU 3 {Effect Size} |
||
|---|---|---|---|---|---|---|---|
| OBJECT HIT TASK (BILATERAL) | Total Hits | 1 | 158 | 173 | 158 | 15 | 0 |
| 2 | 192 | 195 | 169 | 3 | −23 | ||
| 3 | 87 | 89 | 102 | 2 | 15 | ||
| 4 | 186 | 200 | 203 | 14 | 17 | ||
| 5 | 136 | 133 | 133 | −3 | −3 | ||
| GROUP | 151.8 (42.64) | 158 (46.75) | 153 (38.02) | 6.2 {0.15} | 1.2 {0.03} | ||
| Miss Bias | 1 | −0.01 | 0.02 | 0.03 | 0.03 | 0.04 | |
| 2 | −0.05 | 0.10 | 0.02 | 0.15 | 0.07 | ||
| 3 | −0.01 | −0.03 | −0.01 | −0.02 | 0.01 | ||
| 4 | −0.02 | −0.03 | −0.06 | −0.01 | −0.04 | ||
| 5 | −0.14 | −0.13 | −0.15 | 0.01 | −0.01 | ||
| GROUP | −0.05 (0.05) | −0.01 (0.09) | −0.04 (0.08) | 0.03 {0.63} | 0.01 {0.24} | ||
| Hit Bias | 1 | −0.05 | −0.12 | −0.22 | −0.07 | −0.17 | |
| 2 | −0.21 | −0.13 | −0.01 | 0.08 | 0.20 | ||
| 3 | −0.17 | −0.10 | −0.10 | 0.07 | 0.07 | ||
| 4 | −0.23 | −0.11 | −0.24 | 0.12 | −0.02 | ||
| 5 | −0.44 | −0.50 | −0.38 | −0.06 | 0.06 | ||
| GROUP | −0.22 (0.14) | −0.19 (0.17) | −0.19 (0.14) | 0.03 {0.19} | 0.03 {0.21} | ||
| Movement Area Bias | 1 | −0.10 | −0.21 | −0.24 | −0.11 | −0.14 | |
| 2 | −0.13 | −0.05 | −0.02 | 0.08 | 0.10 | ||
| 3 | −0.45 | −0.46 | −0.11 | −0.01 | 0.34 | ||
| 4 | −0.08 | −0.21 | −0.15 | −0.13 | −0.07 | ||
| 5 | −0.02 | −0.09 | −0.31 | −0.07 | −0.29 | ||
| GROUP | −0.15 (0.17) | −0.20 (0.16) | −0.15 (0.12) | −0.05 {−0.30} | 0.01 {0.04} | ||
| REACHING TASK (AFFECTED UE) | Speed Maxima Count | 1 | 2.31 | 2.37 | 2.46 | 0.06 | 0.15 |
| 2 | 3.20 | 2.84 | 2.30 | −0.36 | −0.90 | ||
| 3 | 6.61 | 6.40 | 4.95 | −0.21 | −1.67 | ||
| 4 | 2.33 | 1.67 | 2.03 | −0.66 | −0.31 | ||
| 5 | 5.08 | 4.14 | 4.31 | −0.94 | −0.77 | ||
| GROUP | 3.91 (1.89) | 3.49 (1.86) | 3.21 (1.32) | −0.42 {0.22} | −0.70 {0.37} | ||
| Path Length Ratio | 1 | 1.04 | 1.04 | 1.02 | 0.00 | −0.02 | |
| 2 | 1.49 | 1.19 | 1.21 | −0.30 | −0.28 | ||
| 3 | 1.45 | 1.15 | 1.13 | −0.30 | −0.32 | ||
| 4 | 1.29 | 1.11 | 1.20 | −0.18 | −0.09 | ||
| 5 | 1.70 | 1.42 | 1.49 | −0.28 | −0.20 | ||
| GROUP | 1.39 (0.24) | 1.18 (0.14) | 1.21 (0.18) | −0.21 {0.85} | −0.18 {0.74} | ||
| Movement Time (sec) | 1 | 1.29 | 1.41 | 1.37 | 0.12 | 0.08 | |
| 2 | 0.63 | 0.62 | 0.68 | −0.01 | 0.05 | ||
| 3 | 2.19 | 2.42 | 1.82 | 0.23 | −0.37 | ||
| 4 | 1.25 | 1.13 | 1.18 | −0.12 | −0.07 | ||
| 5 | 2.11 | 1.71 | 1.92 | −0.40 | −0.19 | ||
| GROUP | 1.49 (0.65) | 1.46 (0.67) | 1.39 0.50 | −0.04 {0.06} | −0.10 {0.15} | ||
| Initial Direction Error (degrees) | 1 | 1.17 | 1.10 | 1.06 | −0.07 | −0.11 | |
| 2 | 12.18 | 5.50 | 3.06 | −6.68 | −9.12 | ||
| 3 | 14.92 | 10.70 | 6.72 | −4.22 | −8.20 | ||
| 4 | 3.65 | 2.37 | 3.79 | −1.29 | 0.14 | ||
| 5 | 8.21 | 12.34 | 6.07 | 4.13 | −2.14 | ||
| GROUP | 8.03 (5.72) | 6.40 (4.97) | 4.14 2.30 | −1.62 {0.28} | −3.89 {0.68} | ||
Pre, pre-test; Post, post-test; FU3, follow-up 3; ∆, change; UE, upper extremity; sec, seconds
FU3 results for Participant 1 are FU2 results carried forward
Participant 5 participated in 14 sessions of a sensory intervention between post-test and FU3
Figure 4.
Individual and group outcomes on visually guided reaching task. Results presented for speed maxima count (A), path length ratio (B), movement time (C), and initial direction error (D). For all measures, movement towards normal range (grey shaded areas) is indicative of improvement. Abbreviations and symbols same as Figure 2.
Figure 5.
Individual and group outcomes on object hit task. Results presented for total hits (A), miss bias (B), hit bias (C), and movement area bias (D). For all measures, movement towards normal range (grey shaded areas) is indicative of improvement. Abbreviations and symbols same as Figure 2.
DISCUSSION
This consideration-of-concept pilot study provides preliminary information regarding the feasibility and potential effectiveness of bihemispheric tDCS as an adjunct to UE therapy for patients with a chronic neurological insult. Feasibility of the approach was demonstrated by the easy incorporation into treatment sessions. The delivery of stimulation did not hinder participants’ ability to engage in our treatment activities, nor did it result in complaints of skin irritation or discomfort. We cannot conclude, however, whether daily treatments would be as well-tolerated.
In addition to feasibility, another goal of consideration-of-concept pilot studies is to determine if novel intervention strategies warrant further investigation (Dobkin, 2009); in other words, does it demonstrate the potential to provide an effective approach. As the effect sizes for a majority of the clinical and robotic measures were in a direction indicating improvement, tDCS demonstrates potential and warrants further investigation. This is particularly true given that the treatment combination that can be easily implemented into clinical practice.
Clinical Outcomes
The largest improvement on the clinical measures was seen on the UE-FM. Using a clinically important difference estimate of 4.25 points (Page et al., 2012), three participants demonstrated a meaningful change immediately following the intervention and two maintained the improvements at six months. Gross manual dexterity improved slightly over the course of the intervention and was largely maintained at six months, as seen by performance on the BBT. In contrast, fine manual dexterity did not improve. Scores for all participants on the PPT were largely unchanged at Post-Test and Follow-up 3 compared to performance prior to the intervention. The differences observed between gross and fine manual dexterity may be due to the selected treatment activities. A review of the treatment logs revealed that more time during the functional training portion of the treatment sessions was dedicated to gross rather than fine manual dexterity activities. The Pre-Test PPT scores further indicate that four of the five participants presented with fairly significant fine motor impairments, thus trainers selected treatment activities addressing gross manual dexterity, as they were challenging but achievable.
Robotic Outcomes
The reaching task required participants to perform controlled reaching movements with their affected UE. Participants performed the task with smoother movements following the intervention and at Follow-up 3, as seen by reductions in speed maxima counts. Not only were movements smoother, they were straighter and exhibited greater initial accuracy following the intervention and at six months, as captured by decreased path length ratios and decreased initial direction errors, respectively. The aforementioned improvements were not at the expense of speed; although accuracy improved, speed remained similar from Pre- to Post-test and actually increased slightly between Pre-test and Follow-up 3. Overall, the results from the reaching task indicate that participants demonstrate improved control and coordination of reaching movements with their affected UE following the intervention. Of clinical importance is the maintenance of improvements at six months.
The object hit task was used to provide information regarding bimanual coordination and spatial awareness. Perhaps the most clinically relevant outcome from this task pertains to relative use of the affected UE compared to the unaffected UE. As measured by the hit bias, participants demonstrated greater use of their affected UE following intervention, and this improvement was maintained at Follow-up 3. Furthermore, the balls missed were more evenly distributed between the two sides of the screen, providing further evidence for increased use (and possibly improved hitting accuracy) of affected UE. These changes in hit bias and miss bias likely contributed to produce the overall increase in the number of balls hit by participants following the intervention. Observational analysis of participant hand tracings produced during performance of the task also indicated that participants were performing the task with greater control and coordination of their affected UE following intervention.
Limitations
There are limitations specific to this study that must be taken into consideration. First, our dropout rate was high even though several measures were taken to help with adherence (e.g. personal calendars, phone calls, etc.). Second, the follow-up schedule for Participant 1 did not occur at the predetermined two month interval. This participant’s final follow up occurred at five months rather than at six months. A qualitative comparison of the other participants suggests that the additional month would not have dramatically influenced the results. The data for Follow-up 3 of Participant 5 should also be interpreted with caution. This participant completed 14 sessions of a sensory intervention focused solely on UE proprioception between Follow-up 2 and 3. Although this was not a motor intervention, we acknowledge the sensory intervention may have impacted UE function at Follow-up 3. Finally, we used a mixed group of participants with stroke and TBI. While this limits the applicability to one group or the other, the fact that we saw improvements in patients with both stroke and TBI suggests that this intervention holds potential as a broad treatment for individuals with neurological insult. This is of particular clinical importance where inpatient and outpatient rehabilitation units must treat a broad population of neurological patients with heterogeneous brain lesions.
Future Studies
Prior to moving on to the next stage of pilot studies, a development-of-concept trial, it may be appropriate to conduct further consideration-of-concept studies (Dobkin, 2009). Although our study indicates feasibility and potential effectiveness of using tDCS as an adjunct to standard PT, additional investigations need to examine dose-response characteristics, individual differences in response, and sensitivity to electrode placement. Using consideration-of-concept studies to determine these details will allow for better study design of subsequent development-of-concept studies. Once the aforementioned issues have been adequately investigated, the findings of these studies can be incorporated into future development-of-concept studies that include a proper control group, adequate sample, randomization of participants, and blinding of assessors.
Conclusions
In keeping with recommendations on staging of pilot studies, a consideration-of-concept study was conducted to investigate the feasibility and potential effectiveness of a novel intervention strategy combining bihemispheric tDCS with UE therapy in individuals with neurological insult. The addition of tDCS to UE therapy was found to be clinically feasible, as the unit did not hinder participants’ ability to perform treatment activities and stimulation was well-tolerated. The intervention also demonstrated potential effectiveness. Improvements in UE function were seen on both clinical and robotic measures following 24 sessions of UE therapy combined with bihemispheric tDCS. Many of these improvements, though small, were maintained at a six month follow-up. Combining bihemispheric tDCS with UE therapy in individuals with neurological insult warrants further investigation, including additional consideration-of-concept and development of concept studies before moving on to a proof-of-concept MRCT.
Acknowledgements
The authors would like to thank the Doctorate of Physical Therapy students who assisted with delivery of the intervention and our lab coordinators Angela Ross, Amanda Estep, and Kristen Hadley who handled the scheduling of participants and trainers. We would also like to thank the participants who contributed their time and effort. Addie Middleton, PT, DPT is funded by a NIH T32 pre-doctoral research training grant via the National Institute of General Medical Sciences.
Footnotes
Declaration of Interest
The authors have no commercial interest relevant to the subject of the manuscript, nor any other conflicts of interest to report.
REFERENCES
- Ang KK, Guan C, Phua KS, Wang C, Teh I, Chen CW, Chew E. Transcranial direct current stimulation and EEG-based motor imagery BCI for upper limb stroke rehabilitation. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4128–4131. doi: 10.1109/EMBC.2012.6346875. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Colombo R, Sterpi I, Sanguineti V, Burdet E. Robotic assessment of upper limb motor function after stroke. Am J Phys Med Rehabil. 2012;91(11 Suppl 3):S255–S269. doi: 10.1097/PHM.0b013e31826bcdc1. [DOI] [PubMed] [Google Scholar]
- Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, Fregni F. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25(9):819–829. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Tung WL, Wu WL, Huang MH, Su FC. Effects of robot-aided bilateral force-induced isokinetic arm training combined with conventional rehabilitation on arm motor function in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88(10):1332–1338. doi: 10.1016/j.apmr.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23(5):435–440. doi: 10.1177/1545968308331146. [DOI] [PubMed] [Google Scholar]
- Coderre AM, Zeid AA, Dukelow SP, Demmer MJ, Moore KD, Demers MJ, Scott SH. Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabil Neural Repair. 2010;24(6):528–541. doi: 10.1177/1545968309356091. [DOI] [PubMed] [Google Scholar]
- Debert CT, Herter TM, Scott SH, Dukelow S. Robotic assessment of sensorimotor deficits after traumatic brain injury. J Neurol Phys Ther. 2012;36(2):58–67. doi: 10.1097/NPT.0b013e318254bd4f. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17(5):217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Hocherman S, Pillar T, Shaham R. Stroke rehabilitation. Three exercise therapy approaches. Phys Ther. 1986;66(8):1233–1238. doi: 10.1093/ptj/66.8.1233. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Progressive Staging of Pilot Studies to Improve Phase III Trials for Motor Interventions. Neurorehabil Neural Repair. 2009;23(3):197–206. doi: 10.1177/1545968309331863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J. Stroke Impact Scale-16: A brief assessment of physical function. Neurology. 2003;60(2):291–296. doi: 10.1212/01.wnl.0000041493.65665.d6. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63(10):1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- Einav O, Geva D, Yoeli D, Kerzhner M, Mauritz KH. Development and validation of the first robotic scale for the clinical assessment of upper extremity motor impairments in stroke patients. Top Stroke Rehabil. 2011;18(Suppl 1):587–598. doi: 10.1310/tsr18s01-587. [DOI] [PubMed] [Google Scholar]
- Feng WW, Bowden MG, Kautz S. Review of transcranial direct current stimulation in poststroke recovery. Top Stroke Rehabil. 2013;20(1):68–77. doi: 10.1310/tsr2001-68. [DOI] [PubMed] [Google Scholar]
- Fulk GD, Ludwig M, Dunning K, Golden S, Boyne P, West T. How much change in the stroke impact scale-16 is important to people who have experienced a stroke? Top Stroke Rehabil. 2010;17(6):477–483. doi: 10.1310/tsr1706-477. [DOI] [PubMed] [Google Scholar]
- Fusco A, De Angelis D, Morone G, Maglione L, Paolucci T, Bragoni M, Venturiero V. The ABC of tDCS: Effects of Anodal, Bilateral and Cathodal Montages of Transcranial Direct Current Stimulation in Patients with Stroke-A Pilot Study. Stroke Res Treat. 2013;2013:837595. doi: 10.1155/2013/837595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe V, Krebs HI, Volpe BT, Pascual-Leone A, Rykman A, Zeierati G, Edwards DJ. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: The dimension of timing. NeuroRehabilitation. 2013 doi: 10.3233/NRE-130927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987;66(4):376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Shawky OA, El-Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, Tohamy AM. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):592–601. doi: 10.1177/1545968313484808. [DOI] [PubMed] [Google Scholar]
- Kidgell DJ, Goodwill AM, Frazer AK, Daly RM. Induction of cortical plasticity and improved motor performance following unilateral and bilateral transcranial direct current stimulation of the primary motor cortex. BMC Neurosci. 2013;14:64. doi: 10.1186/1471-2202-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley TJ, Samargia S, Moore LG, Shakya JK, Lang CE. Comparison of amounts and types of practice during rehabilitation for traumatic brain injury and stroke. J Rehabil Res Dev. 2010;47(9):851–862. doi: 10.1682/jrrd.2010.02.0019. [DOI] [PubMed] [Google Scholar]
- Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther. 2007;31(1):3–10. doi: 10.1097/01.npt.0000260568.31746.34. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y. Dual-tDCS Enhances Online Motor Skill Learning and Long-Term Retention in Chronic Stroke Patients. Front Hum Neurosci. 2012;6:343. doi: 10.3389/fnhum.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Thonnard JL, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single Session of Dual-tDCS Transiently Improves Precision Grip and Dexterity of the Paretic Hand After Stroke. Neurorehabil Neural Repair. 2013 doi: 10.1177/1545968313478485. [DOI] [PubMed] [Google Scholar]
- Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, Hsieh CL. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- Lin KC, Chuang LL, Wu CY, Hsieh YW, Chang WY. Responsiveness and validity of three dexterous function measures in stroke rehabilitation. J Rehabil Res Dev. 2010;47(6):563–571. doi: 10.1682/jrrd.2009.09.0155. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29(6):411–420. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23(7):641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Boudrias MH, Stagg CJ, Bachtiar V, Kischka U, Blicher JU, Johansen-Berg H. Predicting behavioural response to TDCS in chronic motor stroke. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi M, Saeki S, Oda T, Matsushima Y, Hachisuka K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. J Rehabil Med. 2013;45(2):137–140. doi: 10.2340/16501977-1099. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21(1):99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- Pellicciari MC, Brignani D, Miniussi C. Excitability modulation of the motor system induced by transcranial direct current stimulation: A multimodal approach. Neuroimage. 2013;83C:569–580. doi: 10.1016/j.neuroimage.2013.06.076. [DOI] [PubMed] [Google Scholar]
- Russo R, Wallace D, Fitzgerald PB, Cooper NR. Perception of Comfort During Active and Sham Transcranial Direct Current Stimulation: A Double Blind Study. Brain Stimul. 2013 doi: 10.1016/j.brs.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Scott SH, Dukelow SP. Potential of robots as next-generation technology for clinical assessment of neurological disorders and upper-limb therapy. J Rehabil Res Dev. 2011;48(4):335–353. doi: 10.1682/jrrd.2010.04.0057. [DOI] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Tyryshkin K, Coderre AM, Glasgow JI, Herter TM, Bagg SD, Dukelow SP, Scott SH. A robotic object hitting task to quantify sensorimotor impairments in participants with stroke. J Neuroeng Rehabil. 2014;11(1):47. doi: 10.1186/1743-0003-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao JL, Zhu QX, Li J, Meng PP. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. J Rehabil Med. 2011;43(7):619–625. doi: 10.2340/16501977-0819. [DOI] [PubMed] [Google Scholar]
- Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil. 2013;94(1):1–8. doi: 10.1016/j.apmr.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Zollo L, Gallotta E, Guglielmelli E, Sterzi S. Robotic technologies and rehabilitation: new tools for upper-limb therapy and assessment in chronic stroke. Eur J Phys Rehabil Med. 2011;47(2):223–236. [PubMed] [Google Scholar]