Abstract
Progress in the identification of suitable animal models for human herpesvirus (HHV)-6A and HHV-6B infections has been slow. Recently, new models have been established, mainly for HHV-6A, which reproduce some pathological features seen in humans. Neuroinflammatory signs were observed in infected marmosets and CD46-transgenic mice; although viral replication was not prominent, persistence of viral DNA and specific immunologic responses were detected, suggesting an immune-mediated pathogenic mechanism. Pig-tailed macaques showed robust viral replication concomitant with acute-phase symptoms, and provided a model to study the effects of HHV-6A on AIDS progression. In humanized mice, viral replication was less evident, but infection led to T-cell alterations. Altogether, these recent developments have opened new perspectives for studying the pathogenic role of HHV-6A in humans.
Introduction
HHV-6A and HHV-6B are members of the betaherpesvirus subfamily, and humans are their only known natural host. HHV-6A and HHV-6B share many properties with other herpesviruses, including the establishment of a persistent latent infection characterized by highly restricted viral gene expression, and the ability to reactivate from latency to produce infectious virus. Although originally categorized as two variants, HHV-6A and HHV-6B were recently re-classified as independent viruses based upon differences in epidemiology, tropism, and disease associations [1]. CD46 serves as the main cellular receptor for HHV-6A [2], while CD134 was recently identified as a novel receptor for HHV-6B [3]. In vivo tropism of these viruses includes CD4+ T cells, epithelial cells in salivary glands and liver, endothelial cells, and cells of the central nervous system (CNS) [4]. HHV-6A replicates in neural cells in culture more efficiently than HHV-6B and is thought to be overall more neurotropic [5].
HHV-6B infection is very common in the human population worldwide, with a very high seroprevalence (>90%) by age two [6]. Acute primary HHV-6B infection can result in exanthem subitum [7], a childhood febrile disease accompanied by a rash and, in rare cases, by febrile convulsions. No disease association has been firmly established for HHV-6A, although evidence suggests a role in hematopoietic stem cell and solid organ transplant complications [8], graft-versus-host disease [9], and multiple sclerosis [10, 11]. Disease manifestations by both HHV-6A and HHV-6B are often correlated with host immunosuppression, which may promote viral reactivation from latency. The prevalence of HHV-6A infection is still largely undefined due to a lack of serological assays that can clearly distinguish between HHV-6A and HHV-6B infections.
The lack of animal models that efficiently support HHV-6A or HHV-6B replication has long hindered studies of viral pathogenesis. The focus of this review is on recent work aimed at developing new animal models that sustain HHV-6A and/or HHV-6B replication, which may help to better understand the pathogenic mechanisms of these viruses in humans.
New animal models of HHV-6A- and HHV-6B-induced neuropathology
Marmoset model
Recently, a marmoset (Callithrix jacchus) model was developed to study HHV-6A and HHV-6B infections [12]. Marmosets that received multiple intravenous injections of HHV-6A developed neurological symptoms, including motor weakness and sensory abnormalities, associated with the development of virus-specific antibody responses and with the presence of histopathological lesions in the CNS, primarily microgliosis. Viral DNA was detected in the brain of HHV-6A- and HHV-6B-infected animals, confirming the neurotropism of both viruses. However, while HHV-6A infection led to evident neurological symptoms, infection with HHV-6B remained asymptomatic. Surprisingly, HHV-6A infection through the intranasal route also remained completely asymptomatic and elicited limited, if any, antibody responses despite detectable levels of plasma viremia. The correlation between the development of neurological signs and the elicitation of virus-specific humoral immune responses in this model suggests a possible immune-mediated pathogenic mechanism rather than a direct neuropathic effect of HHV-6A infection. This study provided the first in vivo evidence that HHV-6A infection is able to trigger neurological disease.
CD46 transgenic mouse model
Since monkey experiments are often limited by ethical constraints and elevated costs, efforts were made in last few years to develop mouse models of HHV-6-associated neuropathology. The human transmembrane complement regulatory protein CD46 was identified as the receptor for HHV-6A entry into host cells [2], opening novel possibilities to develop humanized murine models of HHV-6A infection. Recently, it has been demonstrated that intracranial and intraperitoneal infection of CD46 transgenic mice with HHV-6A results in long-term persistence of viral DNA in the brains of infected animals, followed by lymphocyte infiltration and upregulation of the chemokine CCL5/RANTES, in the absence of clinically apparent signs of disease [13]. In the presence of HHV-6A-infected human lymphocytes, transgenic murine primary brain cultures were shown to produce viral proteins and develop syncytia (Figure 1); however, viral RNA and proteins have not been detected in vivo in mice. Infection with HHV-6B did not yield any signs of viral replication in transgenic murine CD46 transgenic cells either in vitro or in vivo, probably due to the main utilization of another recently identified entry receptor, CD134 [3]. The secretion of a panel of chemokines was increased after HHV-6A infection of transgenic primary murine brain glial cultures and the induced chemokine expression was inhibited when TLR9 signaling was blocked. These results described the first murine model for HHV-6A-induced brain infection and highlighted the potential importance of the TLR9 pathway in HHV-6A-initiated neuroinflammation, opening novel perspectives for the study of virus-associated neuropathology.
Figure 1. HHV-6A infection of primary murine glial brain culture from CD46 trangenic mice.
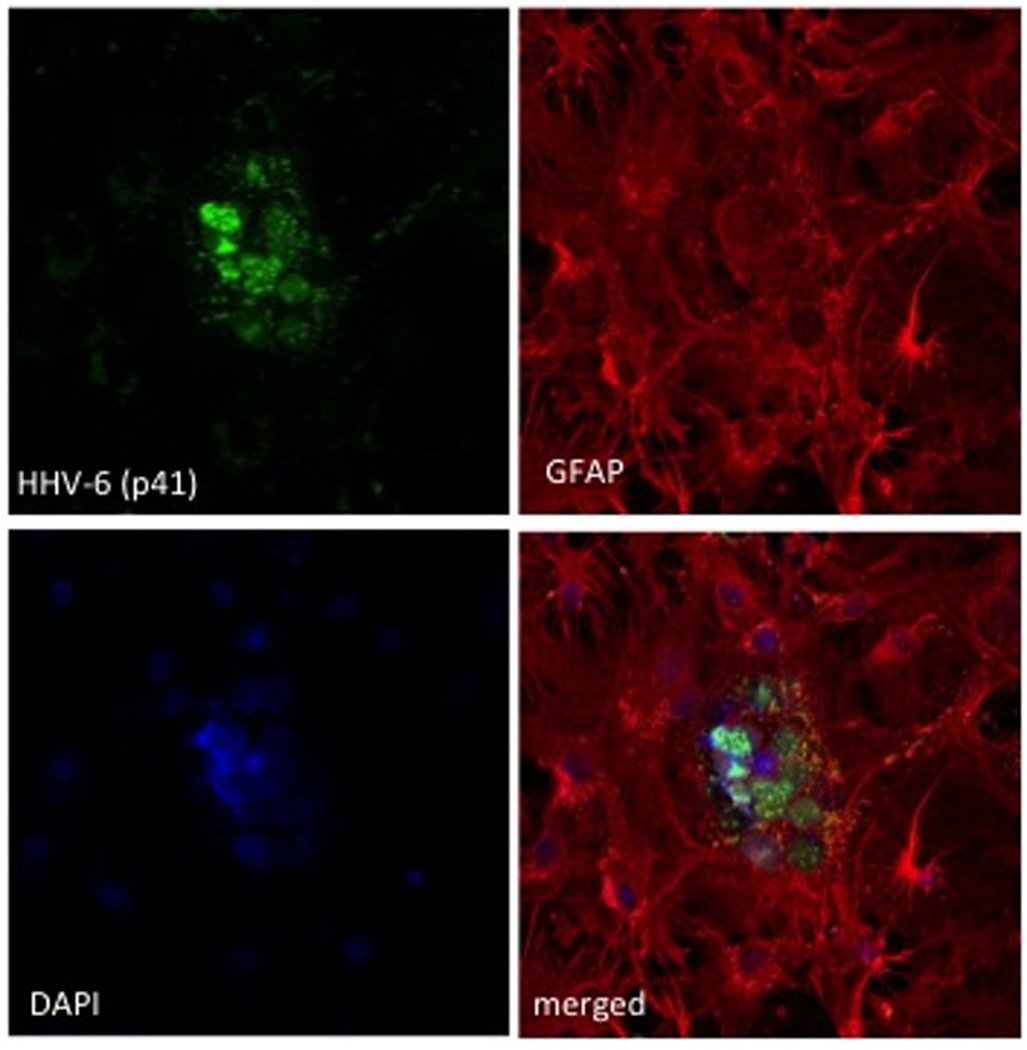
Primary murine brain glial cells generated from CD46-transgenic mice were co-cultured with HHV-6A-infected HSB2 cells as described previously [13]. Seven days after the establishment of co-culture, supernatants and non-adherent cells were removed, and adherent cells were fixed and analyzed for the presence of viral antigens by confocal microscopy. Cells were stained with antibodies against HHV-6 proteins (green) p41 (A–C) or gp116 (D), and glial fibrillary acidic protein (GFAP) antibody (red) and cell nuclei were stained with DAPI (blue).
New animal models of HHV-6A and HHV-6B immunomodulation and immunodeficiency
Pig-tailed macaque model
Various nonhuman primate species have been studied in the past for their susceptibility to HHV-6A, HHV-6B and HHV-7 infections with limited success [14] and (Lusso et al., unpublished), reflecting the inefficient in vitro replication of these viruses in primary lymphocytes from the same animals [15]. However, the pig-tailed macaque (Macaca nemestrina) was singled out for its ability to sustain HHV-6A replication with human-like efficiency both in vitro [16] and, more recently, in vivo [17]. Intravenous inoculation of HHV-6A into naïve pig-tailed macaques resulted in a rapid appearance of plasma viremia and viral RNA transcription in lymph nodes, associated with transient clinical manifestations such as fever, lymphadenopathy and, in one animal, cutaneous rash; IgG antibody seroconversion ensued after approximately 3 weeks of inoculation [17]. After the acute phase, HHV-6A infection entered a clinically latent state, analogous to observations in children, and no long-term clinical or immunological alterations were detected, except for the occasional detection of transient, low-level plasma viremia. These results reproduced the main virological, immunological and clinical features of acute HHV-6B infection in humans (to date, no definitive data are available for acute HHV-6A infection), suggesting that pig-tailed macaques may represent a reliable experimental model for these viruses.
The pig-tailed macaque model was also instrumental for investigating the in vivo interactions between HHV-6A and simian immunodeficiency virus (SIV), the monkey homologue of human immunodeficiency virus (HIV). Animals co-infected with HHV-6A and SIV showed a dramatic acceleration of SIV-disease progression towards full-blown AIDS, associated with early depletion of both CD4+ and CD8+ T cells and increased SIV expression in lymph nodes (Figure 2); interestingly, as seen in immunodeficient humans, frequent HHV-6A plasma viremia was observed in co-infected animals, concomitant with a progressive deterioration of the host immunologic defenses [17]. These results provided the first in vivo evidence for an accelerating effect of HHV-6A on AIDS progression.
Figure 2. Enhanced replication of SIV in lymph nodes from HHV-6A-co-infected pig-tailed macaques.
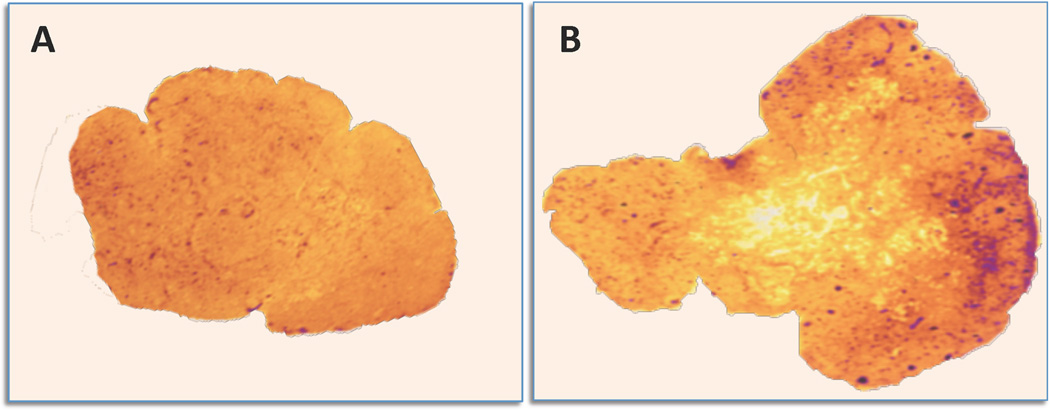
In situ hybridization in lymph node tissues from macaques singly infected with SIVsmE660 (A) or dually infected with SIV and HHV-6A (B). In tissue from the animal singly infected with SIV, the overall architecture is conserved and low levels of SIV RNA (purple signal) are visible throughout the parenchyma, with little, if any, specific signal within reactive germinal centers. In tissue from the dually-infected animal, a florid follicular hyperplasia is visible with an intense SIV RNA signal. Co-infection with HHV-6A induced a dramatic acceleration of disease progression toward full-blown AIDS.
RAG-hu mouse model
Humanized mice are an attractive model for the study of human viral pathogens because they produce human target cells and can generate human anti-viral immune responses. In the humanized Rag2−/−γc−/− mouse (RAG-hu) model, human CD34+ hematopoietic stem cells are extracted from cord blood or fetal liver and injected into neonatal immunodeficient mice. Engrafted animals produce a variety of human lymphoid and myeloid cells, including CD4+ T cells, which are major target cells for HHV-6A.
Recent data show that RAG-hu mice can be infected with HHV-6A following intraperitoneal injection of either cell-free or cell-associated virus, with persistence of viral DNA in blood and lymphoid organs [18]. Viral DNA was detected only sporadically in plasma and blood cells, possibly due to inefficient replication and establishment of latent infection. The bone marrow was positive for viral DNA in all animals tested at 1 week post-infection. Brain infection has not yet been examined, although human immune cells have been detected in the brain of humanized mice, accompanied by HIV-1 penetration, after peripheral HIV-1 inoculation [19]. Human thymocyte populations were modified after peritoneal inoculation of HHV-6A, indicating cytopathic effects in that organ. The CD3+CD4− and CD3−CD4+CD8− populations were depleted in infected animals (Figure 3). Interestingly, depletion of the CD3−CD4+CD8− thymocyte population had previously been observed in a SCID-hu thy/liv humanized mouse model where HHV-6A or HHV-6B was injected directly into the thymic organoid [20]. A possible contributing mechanism is CD3 downregulation, which has previously been reported in peripheral blood T cells [21] and in cells extracted from lymphoid tissues [22] and is likely mediated by the viral U24 protein [23]. An unusual T-cell population detected in infected RAG-hu mice was an elevated proportion of CD3+CD4+CD8+ T cells in blood, as compared to mock-infected animals. While the origin of these cells is still unclear, HHV-6A infection can promote expression of CD4 on cells that do not normally express it [24]. Thus, it is possible that these cells were either infected with HHV-6A, or triggered to exit the thymus prematurely (most CD4+CD8+ cells reside in thymus).
Figure 3. Alteration of thymocyte populations in humanized mice after HHV-6A infection.
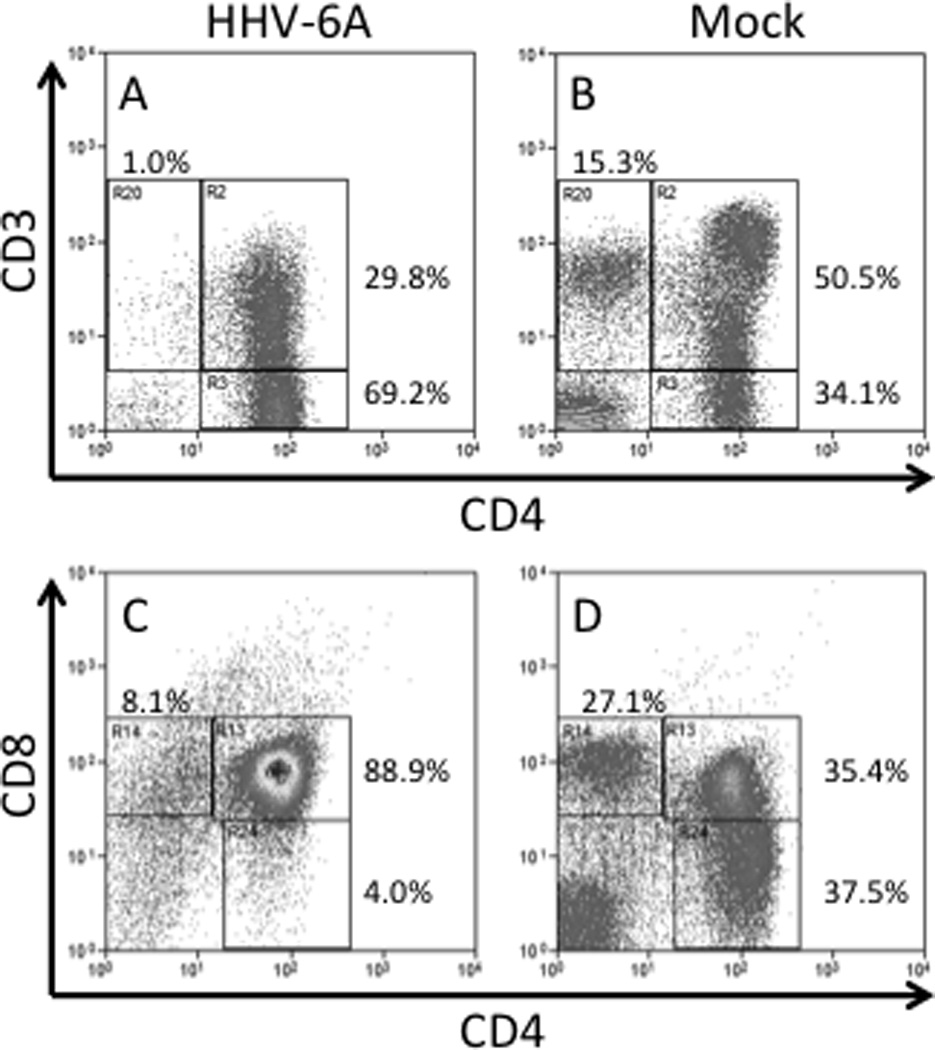
RAG-hu mice were infected with HHV-6A (or mock infected) and thymocytes were collected at 7.5wpi and analyzed by flow cytometry. The CD3+CD4− population is depleted in HHV-6A infected animals (A) but not in mock infected animals (B). Gating for panels A/B was on lymphocytes, and data were normalized to the sum of gates R2/R3/R20. The CD4+CD8− population is depleted in HHV-6A infected animals (C) but not in mock infected animals (D). Data in panels C/D were not gated and normalized to the sum of gates R13/R14/R24. Although changes in the CD8+CD4− and CD4–CD8− populations can be seen in this representative case, these findings were not statistically significant for the entire group of 6animals.
Taken together, the findings in infected RAG-hu mice suggested that HHV-6A has a natural tropism for the human thymus and bone marrow and that infection leads to alteration of T lymphocyte subpopulations. Depletion and/or alteration of specific thymocyte subsets may play an important role in HHV-6A-induced immunomodulation and the ability of this virus to persist in the host.
Conclusions and future perspectives
The predominantly latent nature of human roseolovirus infections and our ignorance of the mechanisms that control such viruses in vivo have made studies in animal models particularly challenging. Nevertheless, new animal models to study HHV-6A- and HHV-6B-induced pathology have been developed during the last decade (Table 1). Although they do not mimic entirely all aspects of the human infections, these models have provided some important insights into the neurological and immunological disorders associated with these viruses. Work in the marmoset model showed neurological symptoms in association with HHV-6A infection. A correlation between HHV-6A infection and multiple sclerosis (MS) has been noted for some time, specifically with increased detection of viral nucleic acids and anti-viral antibody responses in MS patients [25, 26]. Further work in the marmoset model may yield additional insights into the role of HHV-6A in this disease whose etiology remains poorly understood. The CD46 transgenic mouse model has further illustrated the potential for neurologic disease associated with HHV-6A in humans by demonstrating that various proinflammatory chemokines are upregulated after infection both in vivo and in vitro and that immune cells respond to HHV-6A infection in the brain. The TLR9 pathway was identified as a pathway responsible for chemokine upregulation and has been implicated in a different animal model of MS [27], thus providing additional evidence that HHV-6A may be linked to the development of MS.
Table 1.
New animal models recently established to study HHV-6A and HHV-6B pathogenesis
| Model [reference] |
Virus(es) studied |
Route of infection |
Major pathologic findings |
Disadvantages |
|---|---|---|---|---|
| Pig-tailed macaques [17] | HHV-6A | Intravenous | Acute-phase symptoms, robust viral replication, antibody responses, accelerated AIDS progression | Costly, ethical constraints |
| Marmosets [12] | HHV-6A and HHV-6B | Intravenous | CNS pathology (HHV-6A only), antibody responses | Low viral replication, costly, ethical constraints |
| Humanized Rag2−/−γc−/− mice [18] | HHV-6A | Intraperitoneal | Viral DNA persistence in blood, antibody responses, alteration of human thymocyte and T cell populations | Low viral replication |
| huCD46-transgenic mice [13] | HHV-6A | Intracranial + intraperitoneal | Long-term viral DNA persistence in CNS, antibody responses, CNS production of pro-inflammatory cytokines | Low viral replication |
The recent findings in RAG-hu mice have provided in vivo evidence to support a role for HHV-6A in immunosuppression associated with alterations of thymocyte populations. Since the thymus is responsible for T cell development, this may represent a novel mechanism for viral persistence by manipulating T cells before they become functional. The ramifications of thymocyte depletion are currently unclear, but could promote generalized immunosuppression. In addition, the macaque studies have provided in vivo evidence to support the hypothesis that HHV-6A co-infection leads to more rapid AIDS progression in HIV-infected individuals. Further studies are required to firmly establish a role for HHV-6A in human immunosuppression in vivo; moreover, if a role for this virus in AIDS progression is confirmed, HHV-6A may represent an important new drug target for AIDS treatment.
More animal models have been described for HHV-6A than for HHV-6B infection, possibly reflecting the conservation and ubiquitous distribution of the main HHV-6A receptor, CD46. The recent identification of the immunoregulatory molecule CD134 (OX40), which is expressed predominantly on activated human T cells, as a novel receptor for HHV-6B [3] will certainly lead to the development of additional models for this virus, including transgenic mice. The absence of CD134 expression on CNS cells may explain the apparently lower neurotropism of HHV-6B, compared to HHV-6A; whether and to what extent at least some strains of HHV-6B can also utilize CD46 as a receptor, as previously reported [2], remains uncertain. The availability of suitable animal models, especially murine models for which a wide array of experimental tools are available, should facilitate further studies of virus-host interactions and pathogenesis and open novel perspectives for devising effective therapeutic and preventive approaches for HHV-6A and HHV-6B.
Highlights.
-
●
New animal models recently established in mice and non-human primates
-
●
Pathological changes seen in CNS of marmosets and CD46-transgenic mice
-
■
Acute-phase symptoms with robust viral replication seen in macaques
-
■
Various human thymocyte subpopulations altered in humanized mice
Acknowledgments
This work was supported in part by the Intramural Research Program of the NIAID, NIH (PL), the HHV-6 Foundation (BKB) and the INSERM and ARSEP, France (BH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ICTV. ICTV Master Species List 2011. [Accessed on July 17, 2014];International Committee on Taxonomy of Viruses. 2011 Available from: http://talk.ictvonline.org/files/ictv_documents/m/msl/4090.aspx. [Google Scholar]
- 2.Santoro F, et al. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9096–9099. doi: 10.1073/pnas.1305187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Mirandola P, Menotti L. Human herpesvirus 6: An emerging pathogen. Emerg Infect Dis. 1999;5:353–366. doi: 10.3201/eid0503.990306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bolle L, et al. Quantitative analysis of human herpesvirus 6 cell tropism. J Med Virol. 2005;75:76–85. doi: 10.1002/jmv.20240. [DOI] [PubMed] [Google Scholar]
- 6.Okuno T, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989;27:651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanishi K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 8.Le J, Gantt S A.I.D.C.o. Practice. Human herpesvirus 6, 7 and 8 in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):128–137. doi: 10.1111/ajt.12106. [DOI] [PubMed] [Google Scholar]
- 9.Agut H. Deciphering the clinical impact of acute human herpesvirus 6 (HHV-6) infections. J Clin Virol. 2011;52(164–171) doi: 10.1016/j.jcv.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Behzad-Behbahani A, et al. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J. Microbiol. Immunol. Infect. 2011;44:247–251. doi: 10.1016/j.jmii.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Virtanen JO, et al. Evidence for human herpesvirus 6 variant A antibodies in multiple sclerosis: diagnostic and therapeutic implications. J. Neurovirol. 2007;13:347–352. doi: 10.1080/13550280701381332. [DOI] [PubMed] [Google Scholar]
- 12. Leibovitch E, et al. Novel marmoset (Callithrix jacchus) model of human Herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization. PLoS Pathog. 2013;9:e1003138. doi: 10.1371/journal.ppat.1003138. This paper described the new marmoset model of HHV-6A-induced neuropathology.
- 13. Reynaud JM, et al. Human Herpesvirus 6A Infection in CD46 Transgenic Mice: Viral Persistence in the Brain and Increased Production of Proinflammatory Chemokines via Toll-Like Receptor 9. J Virol. 2014;88:5421–5436. doi: 10.1128/JVI.03763-13. This paper described the new transgenic mouse model of HHV-6A-induced neuropathology.
- 14.Yalcin S, et al. Experimental infection of cynomolgus and African green monkeys with human herpesvirus 6. J Gen Virol. 1992;73:1673–1677. doi: 10.1099/0022-1317-73-7-1673. [DOI] [PubMed] [Google Scholar]
- 15.Lusso P, et al. In vitro susceptibility of T lymphocytes from chimpanzees (Pan troglodytes) to human herpesvirus 6 (HHV-6): a potential animal model to study the interaction between HHV-6 and human immunodeficiency virus type 1 in vivo. J Virol. 1990;64:2751–2758. doi: 10.1128/jvi.64.6.2751-2758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusso P, Secchiero P, Crowley RW. In vitro susceptibility of Macaca nemestrina to human herpesvirus 6: a potential animal model of coinfection with primate immunodeficiency viruses. AIDS Res Hum Retroviruses. 1994;10:181–187. doi: 10.1089/aid.1994.10.181. [DOI] [PubMed] [Google Scholar]
- 17. Lusso P, et al. Human herpesvirus 6A accelerates AIDS progression in macaques. Proc Natl Acad Sci U S A. 2007;104:5067–5072. doi: 10.1073/pnas.0700929104. This paper described the new macaque model of HHV-6A acute infection and acceleration of SIV immunodeficiency.
- 18. Tanner A, et al. Human herpesvirus 6A infection and immunopathogenesis in humanized Rag2−/−γc−/− Mice. J Virol. 2013;87:12020–12028. doi: 10.1128/JVI.01556-13. This paper described the new mouse model of HHV-6A-induced immune modulation/deficiency.
- 19.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobbi A, et al. Human herpesvirus 6 (HHV-6) causes severe thymocyte depletion in SCID-hu Thy/Liv mice. J Exp Med. 1999;189:1953–1960. doi: 10.1084/jem.189.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusso P, et al. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J Immun. 1991;147:685–691. [PubMed] [Google Scholar]
- 22.Grivel JC, et al. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J Virol. 2003;77:8280–8289. doi: 10.1128/JVI.77.15.8280-8289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan BM, Coscoy L. Downregulation of the T-cell receptor complex and impairment of T-cell activation by human herpesvirus 6 u24 protein. J Virol. 2008;82:602–608. doi: 10.1128/JVI.01571-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusso P, et al. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991;349:533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- 25.Goodman AD, et al. Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis. 2003;187:1365–1376. doi: 10.1086/368172. [DOI] [PubMed] [Google Scholar]
- 26.Soldan SS, et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 27.Prinz M, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]


