Abstract
BACKGROUND
Previous reports of WNV RNA persistence in blood compartments have raised concerns around the remaining risk of WNV transfusion-transmission. This study characterized the dynamics of WNV viremia in blood compartments in a longitudinal cohort of 54 WNV-infected blood donors.
STUDY DESIGN AND METHODS
Blood samples were collected throughout the year after WNV RNA+ blood donation (index) and characterized for anti-WNV IgM and IgG antibodies and for WNV RNA by real-time reverse-transcription polymerase chain reaction. WNV viral loads were compared in plasma and whole blood samples and correlated with blood groups and clinical outcomes.
RESULTS
WNV RNA persisted in the red blood cell (RBC) compartment up to three months post-index in 42% of the donors. Donors with the highest WNV RNA levels in plasma at index maintained the highest WNV RNA levels in whole blood over the three months post-index. Blood group A donors maintained higher post-index WNV viral load in whole blood than blood group O individuals (P=0.027). Despite a trend for WNV RNA to persist longer in whole blood from symptomatic subjects, no significant association was found between WNV RNA levels in whole blood and disease outcome.
CONCLUSION
This study confirmed that WNV RNA persists in the RBC fraction in whole blood and further suggested that the level of persistence in whole blood may be a reflection of initial viral burden in plasma. The association with blood groups suggests that WNV adherence to RBCs may be mediated by molecules overrepresented at the surface of blood group A RBCs.
Keywords: West Nile Virus, Viral persistence, Blood screening, Blood donors, Longitudinal cohort, Plasma, Whole blood, Red Blood Cells
INTRODUCTION
West Nile (WNV), a mosquito-borne arbovirus and transfusion-transmitted flavivirus, was introduced into the United States (US) in 1999 and has since been responsible for more than 38,000 reported clinical cases, of which 16,453 presented with neuroinvasive disease, 20,876 with West Nile fever, and 1,579 had a fatal outcome.1 However WNV infection is asymptomatic in >80% of cases2 and a recent study projected that over 3 million persons have been infected with WNV in the US from 1999 to 2010, resulting in about 780,000 illnesses.3,4 With no treatment and no vaccine to prevent the development of symptomatic infections in humans,5 it is essential to prevent transmission.
The demonstration of WNV transfusion-transmission in 2002 alerted the blood banking community to the blood safety implications of large-scale arbovirus epidemics, and to the need for expanded hemovigilance and focused research to proactively protect recipients from this class of blood-borne agents.6 In late 2002, the US Food and Drug Administration (FDA), US blood collection organizations, and test manufacturers began an accelerated program to develop and implement WNV nucleic acid amplification technology (NAT) assays to screen donors for WNV before the 2003 season. Assays were developed for use in a mini-pool (MP)-NAT format (plasma from 6–16 donations is pooled and tested), and by July 2003 essentially all blood donations in the US were being tested for WNV using MP-NAT assays developed by Roche and Gen-Probe. Although MP-NAT testing of blood donations prevented hundreds of cases of WNV infection in 2003, it failed to detect units with a low level of viremia, some of which were antibody-negative and infectious.7 Documentation of MP-NAT “breakthrough” infections suggested that a significant rate of low-level viremic donations were missed by MP-NAT. Consequently relatively cost-effective strategies for targeted NAT testing of individual donations (ID-NAT) in high-prevalence regions was developed and implemented successfully in 2004 with subsequent refinements over the past decade.8
Capitalizing on blood bank resources allowed for unparalleled access to WNV RNA+ donors. Samples were collected from these otherwise inaccessible individuals in the a/pre-symptomatic stage of acute WNV infection and systematic follow-up studies of viremic donors were conducted which contributed to a better understanding of the natural history of viremia and immune responses.9–14 Those studies provided insights into the dynamics of viremia and immune responses; the rates, determinants and pathogenesis of symptomatic WNV disease; and to refinement of screening strategies and deferral policies which have essentially eliminated the risk of WNV transfusion-transmission in the US. However, some questions remain unanswered and require further study, including the risk for transfusion-transmission by units collected in the tail-end of WNV viremia.9,10,15
WNV RNA compartmentalization (i.e., the association of viral nucleic acids and infectious virus with blood cells) was first reported in a cross-sectional cohort by Rios et al.16 and was recently further characterized by Lai et al.17 in a longitudinal cohort of 10 WNV-infected blood donors from Blood Systems. Notably, WNV RNA concentrations were consistently greater in whole blood than in plasma after seroconversion, and viral RNA was detected in whole blood up to 200 days after initial detection.17 Further work was needed to more precisely characterize the dynamics of acute viremia in blood compartments and the association with WNV clinical disease. The present study reports these findings based on laboratory and clinical characterization of a longitudinal cohort of 54 additional Blood Systems WNV-infected blood donors with symptom data collected around the time of initial donation and samples collected throughout the year post-donation. This study also explores the association between WNV RNA persistence in whole blood and blood groups, with potentially informative insights into WNV pathogenesis.
Methods
Study population
WNV infected donors were enrolled by Blood Systems Research Institute (BSRI) between 2009 and 2011. Demographics such as age and gender were collected from all donors. All donors who tested positive for WNV RNA by routine donation screening (index) (Procleix WNV transcription-mediated amplification [TMA] assay [Gen-Probe]) at United Blood Services collection facilities were eligible for enrollment. Infection was confirmed when index donation was repeat reactive by TMA and follow-up samples showed seroconversion to anti-WNV IgM. Confirmed infected donors were enrolled after signing an informed consent approved by the UCSF Committee on Human Research. Samples were successfully collected from 54 WNV+ donors. Symptom questionnaires covering 12 possible WNV-related symptoms were administered at study enrollment and two weeks later for all donors.11 As previously described,11,13,18 a cutoff of four symptoms was used to categorized infected donors as asymptomatic (AS, number of reported symptoms < 4, n=26) or symptomatic (S, number of reported symptoms ≥ 4, n=28). The average age was 51 years old for the WNV+ cohort, 48 years for asymptomatic and 53 years for symptomatic WNV+ donors (P = 0.19).
Sample preparation
Whole blood, peripheral blood mononuclear cell (PBMC), and plasma samples were prepared from anticoagulated blood specimens collected in ethylenediaminetetraacetate (EDTA) tubes. Blood was centrifuged at 872 x g for 10 minutes before plasma was removed and aliquoted for long term storage. The remaining WBCs, RBCs and small volume plasma, referred to here as “whole blood”, were also aliquoted into cryovials for long term storage at −80°C. PBMCs were isolated on a Ficoll-Paque PLUS density gradient (GE Healthcare Life Sciences). Aliquots of 10 × 106 cells were frozen in medium containing 90% FBS (HyClone) and 10% DMSO (Fisher BioReagents) and stored in liquid nitrogen.
WNV real-time RT-PCR assay
The WNV real-time RT-PCR assay in this study was used as previously described.17 Briefly, RNA was extracted from undiluted thawed plasma and whole blood samples, and washed PBMCs (to remove any trace of DMSO) using the Qiagen Viral RNA kit (Qiagen) with procedures slightly modified from the package insert. Viral RNA was extracted from 100 μl of plasma or whole blood samples and from 10 × 106 PBMCs (thawed, washed with 500 μL of Phosphate-buffered saline (PBS) and resuspended in 100 μL of PBS). Real time RT-PCR used primers and probes that targeted highly conserved sequences within the capsid region or the NS1/NS2 region of the WNV genome. 19 After amplification, the mean cycle threshold (Ct) values from two replicate tests were determined for whole blood and plasma-derived samples processed in parallel. WNV RNA–positive plasma with a known concentration, originally sourced from an FDA stock of WNV isolate (NY99) culture supernatant, was obtained from CBER/FDA and spiked into plasma as well as whole blood which were then used as the standards for viral load extrapolation as previously described.17
Anti-WNV IgM and IgG antibody assay
Serological testing of plasma for WNV IgM/IgG was performed using ELISA kits (Focus Diagnostics) in accordance with the manufacturer’s instructions and as previously described.20
Statistical analysis
The excel student’s t-test was used to compare the age of asymptomatic and symptomatic WNV+ donors. The Graph Pad Prism software was used to compare differences in viral load between blood group A and blood group O WNV+ donors and between asymptomatic and symptomatic WNV+ donors by the non-parametric Mann-Whitney test. The non-parametric Wilcoxon signed rank test for matched pairs was used to compare viral load levels in plasma, whole blood, and PBMCs samples from the same 10 donors at a given time point. The non-parametric Mann-Whitney test was used to compare viral loads at index time-points between groups of WNV+ donors maintaining high versus low viral loads in whole blood at 60 days post-index. The method of generalized estimating equations (GEE) was used to examine the difference between blood groups A and O over the time post-index and between asymptomatic and symptomatic WNV+ blood donors in association with WNV viral load mean quantities per mL of whole blood. Statistical significance was determined at P < 0.05.
Results
WNV RNA is maintained in whole blood at higher levels than in plasma for up to three months post-index
The 54 WNV+ blood donors with available plasma and whole blood samples included in this study were enrolled between 2009 and 2011 as part of an intensive follow-up study that allowed for the collection of pedigreed biospecimens characterized for immune markers (Fig. 1A) and WNV viral load in plasma and whole blood (Fig. 1B). Frozen follow-up plasma and whole blood samples were available from these donors at one week, two weeks, three weeks, four weeks, six weeks, two months, three months and six months post-initial blood donation (index). Specimens were thawed and characterized for WNV viral load by real-time RT-PCR (Figs. 1 and 2).
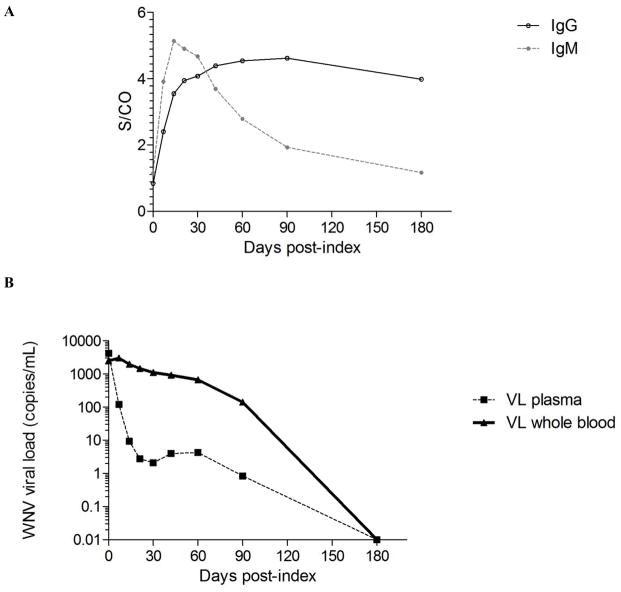
Fig 1. Viral and immune parameters of WNV infection over the six months post-index donation.
(A) Mean anti-WNV IgM and IgG titers are shown for 54 WNV+ donors over the 180 days after index donation are expressed as fold increase from cut-off (signal to cut-off, S/CO). (B) Mean WNV viral load measured by real time RT-PCR in plasma (dash line) and whole blood samples (solid line) from the same 54 WNV+ donors over the same period are expressed in copies per mL.
Fig 2. WNV viral load in plasma and whole blood samples from 54 WNV+ blood donors over the year post-index donation.
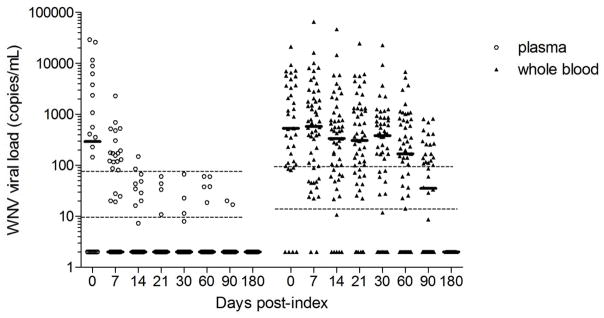
WNV viral load was measured by real time RT-PCR in plasma and whole blood samples from 54 WNV+ donors over the three months post-index donation and expressed in copies per mL. Bars indicate median viral loads. Dashed lines indicate the 50% and 95% limit of detection of the assay, as previously characterized,17 respectively for plasma and whole blood.
At the time of index RNA+ donations, when only 6 of 37 (16%) WNV+ donors with viral load and antibody data had seroconverted to anti-WNV IgM (Table 1), there was no significant difference in the level of WNV viral load between plasma (4123 copies/mL) and whole blood (2488 copies/mL) (P=0.36) (Fig. 1B). At follow-up visits, as an increasing number of WNV+ donors seroconverted to anti-WNV IgM (at day 7, 47 out of 54 WNV+ donors were IgM+ and by day 14 all had seroconverted) (Table 1), viral loads were significantly higher in whole blood than in corresponding plasma samples (25-fold difference at day 7, P<0.0001). Viral loads persisted at higher levels in whole blood than in plasma throughout the first three months post-index (fold difference at day 14=210, at day 21=525, at day 30=532, at day 60=157, at day 90=167 times higher in whole blood than in plasma; P<0.0001) (Fig. 2). The average WNV viral load was >1000 copies/mL over the first month (median >300 copies/mL), then decreased over the second (mean=666 copies/mL, median=169 copies/mL) and third months (mean=139 copies/mL, median=35 copies/mL) post-index. While 100% of the WNV+ donors had WNV RNA levels below the 95% upper limit of detection (LOD) of the assay in plasma (73.7 copies/mL) by the third week post-index, 42% of them maintained WNV RNA levels above the 95% LOD of the assay in whole blood (89 copies/mL) up to three months post-index (Fig. 2).
Table 1.
Availability of samples and data over first three follow-up visits
| Index | 1st Follow-up visit | 2nd Follow-up visit | 3rd Follow-up visit | ||
|---|---|---|---|---|---|
| WNV+ donors with TMA and IgM/IgG data | n | 44 | 54 | 54 | 54 |
| TMA+a,b | 44 (100) | 45 (83) | 35 (65) | 24 (44) | |
| IgM+a | 10 (23) | 45 (83) | 54 (100) | 54 (100) | |
| IgG+a | 8 (18) | 32 (59) | 47 (87) | 53 (98) | |
| Days PIc | 0 | 8 [2–35] | 16 [9–48] | 23 [16–56] | |
| WNV+ donors with viral load data | n | 37 | 54 | 54 | 54 |
| TMA+ | 37 (100) | 45 (83) | 35 (65) | 24 (44) | |
| IgM+ | 6 (16) | 45 (83) | 54 (100) | 54 (100) | |
| IgG+ | 4 (11) | 32 (59) | 47 (87) | 53 (98) | |
| Days PI | 0 | 8 [2–35] | 16 [9–48] | 23 [16–56] |
TMA and IgM/IgG reactivity are listed as n (%);
Samples were considered TMA-reactive (+) if at least one of the 2 or 3 replicates were reactive;
Days post-index (PI) are listed as mean [range];
WNV RNA is associated with the red blood cell rather than the white blood cell compartment
To investigate whether the persistent, high levels of WNV RNA detected in whole blood relative to plasma is due to associations with the white and/or red blood cell compartments of the whole blood, samples from the 10 WNV+ donors previously characterized by Lai et al.17 for WNV viral loads in plasma and whole blood were further characterized for WNV viral load in plasma, PBMCs and whole blood by real time RT-PCR (Fig. 3).While WNV RNA was confirmed to be significantly higher in whole blood than in plasma at day 7 (P=0.002), day 14 (P=0.004), day 21 (P=0.002), and day 30 (P=0.008) it was also significantly higher in whole blood than in the corresponding concentrated PBMC preparations at day 14 (P=0.0039), day 21 (P=0.002), and day 60 (P=0.015). WNV RNA levels were slightly higher in PBMCs than in plasma but the difference was only significant at day 21 (P=0.03). Overall we concluded that WNV RNA was associated with the whole blood but not the PBMC compartment, and therefore we inferred that the WNV RNA was more likely associated with long lived red blood cells (RBCs) than with short lived granulocytes and platelets.
Fig 3. WNV viral loads in plasma, PBMC, and whole blood samples from 10 WNV+ blood donors over the six months post-index donation.
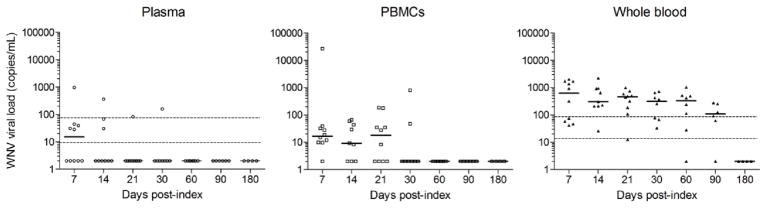
Relative distribution of WNV RNA was investigated in plasma, PBMCs, and whole blood samples from a longitudinal cohort of 10 WNV+ donors enrolled in 2008.17 WNV viral loads were measured in plasma, PBMCs, and whole blood samples. WNV RNA concentrations are expressed in copies per mL. Bars are for medians. Dashed lines indicate the 50% and 95% limit of detection of the assay, as previously characterized,17 respectively for plasma and whole blood.
Correlation between the level of WNV RNA in whole blood and the level of WNV RNA in plasma at index donation
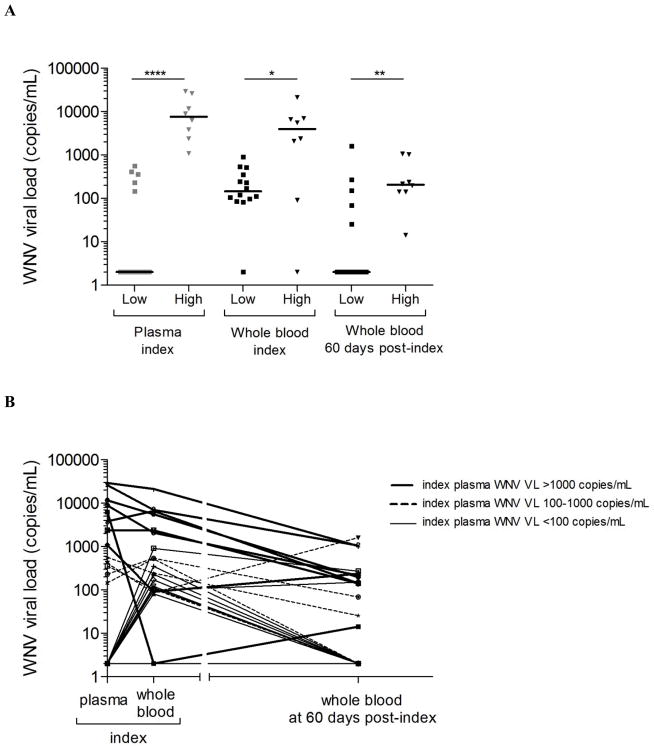
WNV viral load data in plasma and whole blood were only available for a limited number of index donations (n=22). WNV+ donors with the highest levels of WNV RNA in plasma (classified as “high” when index WNV RNA was >1,000 copies/mL, compared to those classified as “low” when index WNV RNA was <1,000 copies/mL) exhibited higher levels of WNV RNA in whole blood at index (P =0.034) and also maintained higher levels of WNV RNA in whole blood at 60 days post-index compared to those who exhibited lower levels of WNV RNA in plasma at index (P=0.01) (Fig. 4A). Eleven of the twelve donors with WNV RNA in plasma > 100 copies/mL had detectable WNV RNA in whole blood, except for one donor who had a negative whole blood sample result but later had detectable WNV RNA in whole blood at 60 days post-index. All 8 donors with the highest levels (>1000copies/mL) of WNV RNA in plasma at index had WNV RNA in whole blood at 60 days post-index, whereas 60% (3/5) of those with WNV RNA in plasma between 100 and 1000 copies/mL maintained detectable WNV RNA levels in whole blood at 60 days post-index and 8/9 donors with WNV RNA in plasma <100 copies/mL at index had undetectable WNV RNA levels in whole blood at 60 days post-index (Fig. 4B).
Fig 4. Plasma WNV RNA levels in the first two weeks post-index predict persistent WNV RNA in whole blood at two months post-index.
WNV viral load was measured by real time RT-PCR in plasma and whole blood samples from 54 WNV+ donors over the three months post-index donation and expressed in copies per mL for each individual donor over the three weeks post-index in plasma and over the three months post-index in whole blood. (A) WNV+ donors with WNV viral load <1,000 copies per mL in plasma at index were classified as having “low” plasma WNV viral load and those with WNV viral load >1,000 copies per mL in plasma at index were classified as having “high” plasma WNV viral load. WNV viral load in whole blood for those classified as “high” or “low” WNV viral load in plasma at index are shown at index and at 60 days post-index. Middle bars as for medians. * is for P<0.05, ** is for P<0.01 and **** is for P<0.0001 by Mann Whitney test. (B) Individual WNV viral loads are shown for the 22 WNV+ donors with available WNV RNA viral load data in plasma at index and for corresponding whole blood samples from the same donors at index and at 60 days post-index. Thicker lines are for WNV+ donors with index plasma viral load >1,000 copies per mL. Dash lines are for those with index plasma viral load between 100 and 1,000 copies per mL. Thinner lines are for those with index plasma viral load <100 copies per mL.
Overall these data suggest that WNV RNA was already present in the whole blood fraction at index (even though 84% of the WNV+ donors had not yet seroconverted to anti-WNV IgM at that time), and donors with the highest WNV RNA levels in plasma during acute viremia were also those with the highest persistent levels of WNV RNA in whole blood.
Blood group A donors maintained higher WNV viral load in whole blood over the three months post-index than blood group O donors
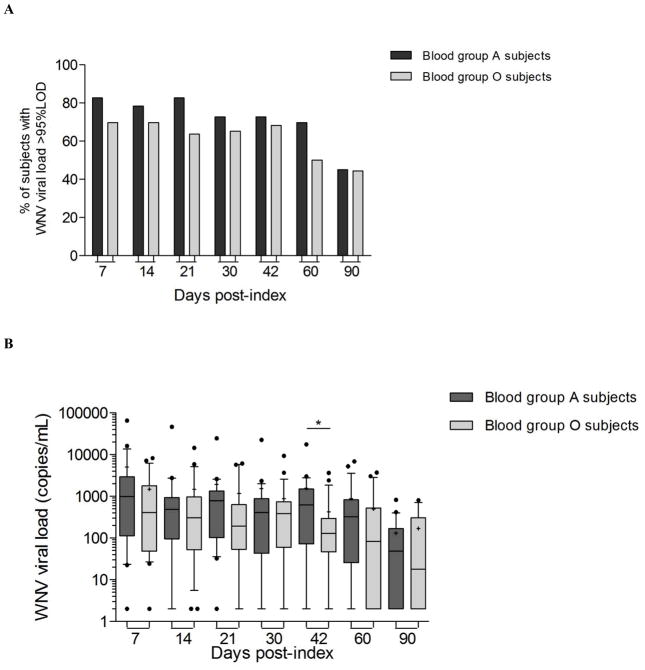
Within the 54 WNV+ donors included in this analysis, there were 23 blood group O, 23 blood group A, 6 blood group B, and 2 blood group AB individuals. Looking at the WNV viral load dynamics over time post-index in blood group O (n=23) and blood group A (n=23) (excluding blood group B and AB individuals), it seemed that a slightly higher percentage of blood group A (70%) than blood group O donors (50%) maintained WNV RNA levels above the 95% limit of detection of the assay in whole blood at two months post index (Fig. 5A). It appeared that blood group A donors retained higher median WNV viral loads in whole blood than others during the three months following index donation (Fig. 5B). At 42 days post-index blood group A donors had higher median viral loads than blood group O donors (medians A=624 and O=128 copies/mL, P=0.028). Comparing the mean WNV viral load over time in whole blood from blood group O and A WNV+ donors, it appeared that blood group A WNV+ donors maintained higher mean viral loads over the three months post-index period than blood group O WNV+ donors(GEE P=0.027) (Fig.5B).
Fig 5. Dynamics of WNV viral load in whole blood samples from WNV+ blood donors with different blood groups.
(A) The histograms represent the percentage of donors within blood group O (n=23) and blood group A (n=23) WNV+ donors with WNV viral load in whole blood above the 95% limit of detection of the WNV real time RT-PCR assay at different time-points post-index. (B) Box and whiskers for 10–90 percentiles represents the distribution of WNV viral load in whole blood samples from blood group A and blood group O WNV+ donors with middle bars for medians and + symbol for means. * is for P-value < 0.05 by Mann Whitney test to compare blood group A to blood group O WNV+ donors at day 42 post-index.
WNV RNA persistence is not significantly associated with WNV disease outcome
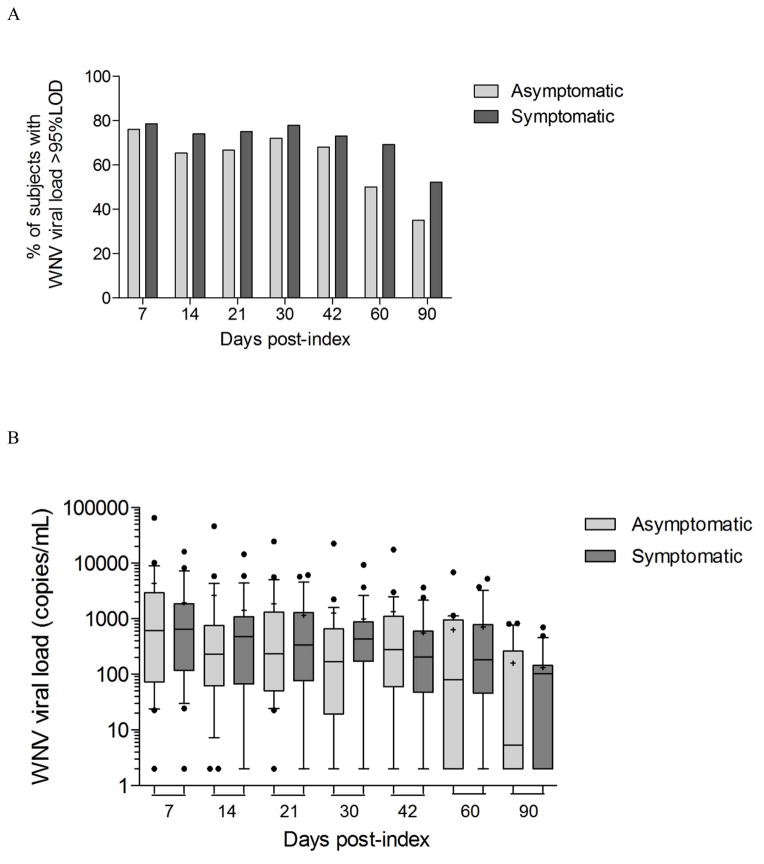
The association between WNV persistence in whole blood and pathogenesis was investigated by comparing WNV RNA levels in asymptomatic (n=26) and symptomatic WNV+ donors (n=28) over the three months period following index donation (Fig. 6). While at day 7 post-index 79% of symptomatic and 76% of the asymptomatic WNV+ donors had WNV RNA levels above the 95% limit of detection (LOD) of the assay in whole blood, 52% of the symptomatic versus 35% of the asymptomatic WNV+ donors with available samples had still detectable WNV RNA in whole blood at 90 days post-index (Fig. 6A), suggesting that asymptomatic WNV+ donors may clear WNV RNA from whole blood faster than symptomatic WNV+ donors. Despite a trend for the median viral load in whole blood to be 2.3 and 23 times higher in symptomatic than in asymptomatic WNV+ donors at 2 months and 3 months post-index respectively, there was no significant difference between symptomatic and asymptomatic WNV+ donors when mean or median WNV viral loads in whole blood were compared (Fig. 6B). Therefore even though WNV RNA seemed to persist longer in symptomatic WNV+ donors than in asymptomatic WNV+ donors, there was no significant association between WNV RNA persistence in whole blood and WNV disease outcome.
Fig 6. Dynamics of WNV viral load in whole blood samples from asymptomatic and symptomatic WNV+ donors over the three months post-index donation.
WNV viral load was measured by real time RT-PCR in whole blood samples from asymptomatic (n=26) and symptomatic (n=28) WNV+ donors over the three months post-index donation. (A) The histograms represent the percentage of donors within asymptomatic and symptomatic WNV+ donors with WNV viral load in whole blood above the 95% limit of detection of the WNV real time RT-PCR assay at different time-points post-index. (B) Box and whiskers for 10–90 percentile represents the distribution of WNV viral load in whole blood samples from asymptomatic and symptomatic donors at 7, 14, 21, 30, 42, 60, and 90 days post-index with middle bars for medians and + symbols for means.
Discussion
The present study was designed to measure WNV RNA levels in plasma and whole blood from a cohort of 54 WNV-infected blood donors enrolled from 2009 to 2011 who had demographic and symptom data collected around the time of donation and samples collected throughout the year post initial detection. Longitudinal samples were characterized for levels of anti-WNV IgM and IgG antibodies and WNV RNA. The phenomenon of WNV blood compartmentalization17 was confirmed: while 100% of the 54 donors had cleared WNV RNA from their plasma within the first three weeks post-index, 42% of the donors maintained substantial levels of WNV RNA in whole blood over the first two months post-index and in some cases up to three months post-index. As previously reported by others,16 WNV RNA was found associated with whole blood and not the PBMC fraction of whole blood. Interestingly those maintaining the highest levels of WNV RNA in whole blood were those with the highest levels of WNV RNA in plasma at index, suggesting that WNV RNA in whole blood could be the consequence of initial viral burden as reflected in the levels of acute viremia in plasma. Furthermore, WNV RNA persisted in whole blood from blood group A donors at higher levels than in whole blood from blood group O donors, suggesting binding to RBC may be linked to blood group glycoproteins or mediated by molecules present at greater frequency on blood group A RBCs. Additionally, despite a trend for WNV RNA to persist longer in whole blood from symptomatic WNV+ donors than in whole blood from asymptomatic WNV+ donors, no strong association was found between between WNV burden in whole blood and WNV disease course.
The persistence of WNV RNA in whole blood does not necessarily mean that infectious WNV is present. The persistence of infectious WNV in host tissues long after plasma clearance was demonstrated after investigating clusters of WNV organ-transplant transmissions.21 Indeed WNV was transmitted by organs collected from a donor without plasma viremia and with high titers of anti-WNV antibodies.21 This suggests that low-level persistent viral particles are able to transmit WNV infection even after the development of humoral immune responses and clearance of detectable plasma WNV RNA. This is a concern as infectious diseases present particular risk to recipients of organ transplantations, who are often immunosuppressed and hence at greater risk of serious disease sequelae. From a transfusion-transmission perspective, all cases of WNV transfusion-transmissions have been linked to donations that tested WNV plasma RNA-positive.22–26 Even though, a group at the FDA reported that infectious WNV could be amplified by co-cultivating whole blood collected months after index donation with Vero cells and monocyte-derived cells despite the presence of anti-WNV antibodies,16,27,28 only one out of thousands units collected in the seropositive convalescent stage of WNV infection.9,29 has ever been linked to a breakthrough infection Therefore it is probably reasonable to assume that WNV particles persisting at low levels bound to RBC in whole blood are neutralized after the development of anti-WNV antibodies. However, the recent exception is a case of WNV transfusion-transmission that occurred in 2012 and had a fatal outcome despite donor seroconversion to anti-WNV IgM antibodies;30 This patient, who had non-Hodgkin's lymphoma, chemotherapy and an autologous stem cell transplant, received allogenic, leukoreduced, irradiated blood products collected from multiple donors including a donor who had tested non-reactive for WNV RNA by TMA and had seroconverted to anti-WNV IgM antibodies.30 Therefore, with several cases of transplantation-transmission21 and isolated cases of transfusion-transmission despite universal blood screening, WNV remains a concern for the blood bank and organ transplantation communities.31
The findings in the present study could have implications for enhanced sensitivity screening of blood collected from donors in the seropositive phase of WNV infection in that a higher level of sensitivity could be achieved by screening whole blood instead of plasma. Improvement of the sensitivity for WNV detection assays might help in reducing WNV transfusion-transmission from low-level viremia units which may be particularly important for bone marrow and organ transplant recipients who are a more vulnerable population.21 With better sensitivity, MP-NAT screening might be extended to reduce the period for ID-NAT screening, which could in turn decrease the cost for blood screening. Donors who tested positive for WNV RNA in plasma are deferred for a period of 120 days. This deferral period was defined based on evidence of WNV clearance in plasma after few weeks post-index donation; however, WNV RNA can now be identified in whole blood for several months post index. Compromising between WNV MP or ID-NAT testing and adjusting the deferral period during which blood donors who tested reactive for WNV are excluded from donation are key parameters to ensure high quality, affordability, and availability of blood in the US. The present study suggests that an increased sensitivity could be reached by screening whole blood. However, the current screening platforms from Roche and Gen-Probe/Hologic, Inc. are optimized for plasma and it will require adaptation to be able to process whole blood.
The molecular basis underlying the persistence of WNV RNA in the red blood cell fraction of the whole blood, including whether the virus sticks to the red blood cell membrane or penetrates into the RBCs, remains unclear. The findings in the present study corroborate the association of WNV with the RBC fraction of the whole blood previously reported by Rios et al., who suggested that WNV adheres to RBCs.16 Based on the finding that WNV RNA was associated with the whole blood but not the PBMC compartment, we inferred that WNV RNA was more likely associated with long lived RBCs than with short lived granulocytes and platelets. However it would be interesting to further investigate the potential adherence to platelets, and if detected the infectivity of platelet-associated WNV, as most of those are transfused to severely immunocompromised recipients who are at risk for the development of symptomatic WNV disease outcome.
Intriguingly, while WNV RNA is retrieved in the RBC fraction of all blood groups, blood group A donors seemed to maintain higher WNV RNA levels in whole blood than blood group O donors. This observation raises the possibility that the molecules mediating WNV attachment to the red blood cell membrane may be present at a higher concentration on the surface of RBCs from blood group A individuals than at the surface of RBCs from blood group O individuals. Similar mechanisms described for other pathogens such as P. falciparum could be at play during WNV infection. The utilization of blood group A antigen as a receptor or co-receptor for P. falciparum to enter the RBCs has been documented32 and severe malaria outcomes are more frequent in blood group A individuals.33 The longer persistence of the parasite in blood group A than in blood group O individuals was also explained by lower macrophage avidity for blood group A than for blood group O-infected erythrocytes.34 Our finding of higher persistence of WNV RNA in the RBC compartment of blood group A individuals warrants further investigation as it could reveal mechanisms of viral entry and propagation, and potential genetic susceptibility that would have to be addressed in larger cohorts and ex vivo through spiking experiments investigating possible direct viral or antibody-mediated mechanisms. Characterizing the molecular basis for WNV adherence to RBCs is also important to understand whether WNV binds to the membrane at the surface of RBCs or penetrates into the cytoplasm of RBCs, and whether the virus can be washed away during deglycerolyzation of frozen RBCs. Although not statistically significant, the association between WNV RNA persistence and clinical disease outcome also suggests a link with pathogenesis. Indeed more symptomatic than asymptomatic WNV+ donors exhibited persistence of WNV RNA up to three months post-index. This means WNV infectious particles or WNV antigens may be persisting longer in symptomatic than in asymptomatic WNV+ donors despite the development of the humoral and cellular immune response. Red blood cells have a life span of 120 days and represent potent vehicles to carry viruses and other pathogens throughout the body and the tissues, including the central nervous system, until they are eliminated. This may allow for infectious particles to reach otherwise inaccessible organs and tissues in a timely manner. This also poses a threat to organ recipients who are immunosuppressed and at risk for the development of symptomatic WNV disease after receiving transplants with remaining blood potentially carrying RBC-associated WNV. The persistence of WNV RNA in blood during the approximate life-span the RBCs in the circulation may be responsible for sustained immune responses that could translate into sustained tissue inflammation and immunopathogenesis. Further work in animal models may be required to address the correlation between levels of WNV RNA persisting in whole blood and initial viral burden in plasma as well as the potential association with pathogenesis in groups with different clinical disease outcomes.
In conclusion, the present study confirmed the phenomenon of WNV RNA persistence for extended periods of time in whole blood compared to plasma in a cohort of 54 WNV-infected donors. The mechanisms underlying the persistence of WNV RNA in the RBC fraction remains unclear, but the association between the persistence of higher WNV RNA levels in whole blood from blood group A donors is intriguing and requires further investigation as it could lead to new insights into the molecular basis of WNV attachment to host cells. This study examined the association between WNV RNA persistence and disease outcome with suggestive but not conclusive findings. Further studies will be required to determine whether longer WNV RNA persistence in whole blood from symptomatic WNV+ donors is the cause or the consequence of immunopathogenesis. Finally, further studies addressing the persistence of infectivity in whole blood are needed to ascertain whether current screening strategies are sufficient for ensuring the highest quality of blood in the US.
Acknowledgments
We thank Nelly Gefter, Lubov Pitina and Karla Murcia for repository management, and Daniel Hindes and Brian Custer for symptom database management.
Funding support: This work was supported by a grant from the National Heart Lung and Blood Institute RC2HL101.
Footnotes
Conflict of Interest: The authors have reported no conflict of interest.
References
- 1.CDC. 2012 Available at http://www.cdc.gov/ncidod/dvbid/westnile/ [monograph on the internet]
- 2.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–4. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 3.Carson PJ, Borchardt SM, Custer B, Prince HE, Dunn-Williams J, Winkelman V, Tobler L, Biggerstaff BJ, Lanciotti R, Petersen LR, Busch MP. Neuroinvasive disease and west nile virus infection, north dakota, USA, 1999–2008. Emerg Infect Dis. 2012;18:684–6. doi: 10.3201/eid1804.111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2012:1–5. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond MS. Progress on the development of therapeutics against West Nile virus. Antiviral Res. 2009;83:214–27. doi: 10.1016/j.antiviral.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98:495–503. doi: 10.1111/j.1423-0410.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Update: West Nile virus screening of blood donations and transfusion-associated transmission-- United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:281–4. [PubMed] [Google Scholar]
- 8.Vamvakas EC, Kleinman S, Hume H, Sher GD. The development of West Nile virus safety policies by Canadian blood services: guiding principles and a comparison between Canada and the United States. Transfus Med Rev. 2006;20:97–109. doi: 10.1016/j.tmrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, Matud JL, Prince HE, Lanciotti RS, Wright DJ, Linnen JM, Caglioti S. Virus and Antibody Dynamics in Acute West Nile Virus Infection. J Infect Dis. 2008 doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 10.Busch MP, Tobler LH, Saldanha J, Caglioti S, Shyamala V, Linnen JM, Gallarda J, Phelps B, Smith RI, Drebot M, Kleinman SH. Analytical and clinical sensitivity of West Nile virus RNA screening and supplemental assays available in 2003. Transfusion. 2005;45:492–9. doi: 10.1111/j.0041-1132.2005.04382.x. [DOI] [PubMed] [Google Scholar]
- 11.Custer B, Kamel H, Kiely NE, Murphy EL, Busch MP. Associations between WNV infection and symptoms reported by blood donors identified through nucleic acid test screening. Transfusion. 2009;49:278–88. doi: 10.1111/j.1537-2995.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 12.Lanteri MC, Heitman JW, Owen RE, Busch T, Gefter N, Kiely N, Kamel HT, Tobler LH, Busch MP, Norris PJ. Comprehensive analysis of west nile virus-specific T cell responses in humans. J Infect Dis. 2008;197:1296–306. doi: 10.1086/586898. [DOI] [PubMed] [Google Scholar]
- 13.Lanteri MC, O'Brien KM, Purtha WE, Cameron MJ, Lund JM, Owen RE, Heitman JW, Custer B, Hirschkorn DF, Tobler LH, Kiely N, Prince HE, Ndhlovu LC, Nixon DF, Kamel HT, Kelvin DJ, Busch MP, Rudensky AY, Diamond MS, Norris PJ. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest. 2009;119:3266–77. doi: 10.1172/JCI39387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobler LH, Cameron MJ, Lanteri MC, Prince HE, Danesh A, Persad D, Lanciotti RS, Norris PJ, Kelvin DJ, Busch MP. Interferon and interferon-induced chemokine expression is associated with control of acute viremia in West Nile virus-infected blood donors. J Infect Dis. 2008;198:979–83. doi: 10.1086/591466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JS. Insights on donor screening for West Nile virus. Transfusion. 2005;45:460–2. doi: 10.1111/j.0041-1132.2005.45041.x. [DOI] [PubMed] [Google Scholar]
- 16.Rios M, Daniel S, Chancey C, Hewlett IK, Stramer SL. West Nile virus adheres to human red blood cells in whole blood. Clin Infect Dis. 2007;45:181–6. doi: 10.1086/518850. [DOI] [PubMed] [Google Scholar]
- 17.Lai L, Lee TH, Tobler L, Wen L, Shi P, Alexander J, Ewing H, Busch M. Relative distribution of West Nile virus RNA in blood compartments: implications for blood donor nucleic acid amplification technology screening. Transfusion. 2012;52:447–54. doi: 10.1111/j.1537-2995.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 18.Lanteri MC, Kaidarova Z, Peterson T, Cate S, Custer B, Wu S, Agapova M, Law JP, Bielawny T, Plummer F, Tobler LH, Loeb M, Busch MP, Bramson J, Luo M, Norris PJ. Association between HLA class I and class II alleles and the outcome of West Nile virus infection: an exploratory study. PLoS ONE. 2011;6:e22948. doi: 10.1371/journal.pone.0022948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyamala V. Identification of oligonucleotides for the capture, detection and quantitation of West Nile Virus. US: Novartis Vaccines and Diagnostics, Inc; Emeryville, Calif. (US): 2011. [Google Scholar]
- 20.Prince HE, Tobler LH, Lape-Nixon M, Foster GA, Stramer SL, Busch MP. Development and persistence of West Nile virus-specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316–20. doi: 10.1128/JCM.43.9.4316-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nett RJ, Kuehnert MJ, Ison MG, Orlowski JP, Fischer M, Staples JE. Current practices and evaluation of screening solid organ donors for West Nile virus. Transpl Infect Dis. 2012;14:268–77. doi: 10.1111/j.1399-3062.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 22.Busch MP, Caglioti S, Robertson EF, McAuley JD, Tobler LH, Kamel H, Linnen JM, Shyamala V, Tomasulo P, Kleinman SH. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353:460–7. doi: 10.1056/NEJMoa044029. [DOI] [PubMed] [Google Scholar]
- 23.Kleinman SH, Williams JD, Robertson G, Caglioti S, Williams RC, Spizman R, Morgan L, Tomasulo P, Busch MP. West Nile virus testing experience in 2007: evaluation of different criteria for triggering individual-donation nucleic acid testing. Transfusion. 2009;49:1160–70. doi: 10.1111/j.1537-2995.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 24.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–7. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 25.Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, Goodman JL, Chamberland ME. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–45. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 26.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–9. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 27.Rios M, Daniel S, Dayton AI, Wood O, Hewlett IK, Epstein JS, Caglioti S, Stramer SL. In vitro evaluation of the protective role of human antibodies to West Nile virus (WNV) produced during natural WNV infection. J Infect Dis. 2008;198:1300–8. doi: 10.1086/592277. [DOI] [PubMed] [Google Scholar]
- 28.Rios M, Zhang MJ, Grinev A, Srinivasan K, Daniel S, Wood O, Hewlett IK, Dayton AI. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion. 2006;46:659–67. doi: 10.1111/j.1537-2995.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 29.Carson PJ, Prince HE, Biggerstaff BJ, Lanciotti R, Tobler LH, Busch M. Characteristics of antibody responses in West Nile virus-seropositive blood donors. J Clin Microbiol. 2014;52:57–60. doi: 10.1128/JCM.01932-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatal West Nile virus infection after probable transfusion-associated transmission--Colorado, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:622–4. [PMC free article] [PubMed] [Google Scholar]
- 31.Brubaker SA, Robert Rigney P. West Nile Virus workshop: scientific considerations for tissue donors. Cell Tissue Bank. 2012;13:499–511. doi: 10.1007/s10561-011-9282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barragan A, Kremsner PG, Wahlgren M, Carlson J. Blood group A antigen is a coreceptor in Plasmodium falciparum rosetting. Infect Immun. 2000;68:2971–5. doi: 10.1128/iai.68.5.2971-2975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cserti CM, Dzik WH. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110:2250–8. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- 34.Wolofsky KT, Ayi K, Branch DR, Hult AK, Olsson ML, Liles WC, Cserti-Gazdewich CM, Kain KC. ABO blood groups influence macrophage-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes. PLoS Pathog. 2012;8:e1002942. doi: 10.1371/journal.ppat.1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]