Abstract
Background and Purpose
Patients with Human papillomavirus related (HPV+) head and neck cancers (HNCs) demonstrate improved clinical outcomes compared to traditional HPV negative (HPV−) HNC patients. We have recently shown that HPV+ HNC cells are more sensitive to radiation than HPV− HNC cells. However, roles of HPV oncogenes in regulating the response of DNA damage repair remain unknown.
Material and Methods
Using immortalized normal oral epithelial cell lines, HPV+ HNC derived cell lines, and HPV16 E7-transgenic mice we assessed the repair of DNA damage using γ-H2AX foci, single and split dose clonogenic survival assays, and immunoblot. The ability of E7 to modulate expression of proteins associated with DNA repair pathways was assessed by immunoblot.
Results
HPV16 E7 increased retention of γ-H2AX nuclear foci and significantly decreased sublethal DNA damage repair. While phospho-ATM, phospho-ATR, Ku70, and Ku80 expressions were not altered by E7, Rad51 was induced by E7. Correspondingly, HPV+ HNC cell lines showed retention of Rad51 after γ-radiation.
Conclusions
Our findings provide further understanding as to how HPV16 E7 manipulates cellular DNA damage responses that may underlie its oncogenic potential and influence the altered sensitivity to radiation seen in HPV+ HNC as compared to HPV− HNC.
Keywords: HPV16 E7, DNA damage repair, HPV-positive head and neck cancer
Introduction
Human Papillomaviruses (HPVs) are small non-enveloped DNA viruses that infect squamous cell epithelium, and commonly cause benign lesions. A subgroup of mucosotropic HPVs called high-risk HPVs including HPV type 16, 18, and 31 are causally associated with 5% of human cancers including the majority of anogential cancers and a growing fraction of head and neck cancers [1]. High-risk HPV type 16 (HPV16) is found in the majority of HPV-positive HNCs, which have molecular-genetic alterations indicative of viral oncogene (i.e. E6 and E7) function [2]. E6 and E7 promote degradation of two major tumor suppressor proteins, p53 and retinoblastoma protein (pRb), respectively, resulting in the uncontrolled entry of cells into S-phase, cell proliferation, and malignant transformation [3, 4]. In addition, HPV oncogene expression causes impaired cellular differentiation, increased frequency of spontaneous and mutagen-induced mutations, and chromosomal instability [4]. Due to the function of these two oncogenes, patients with HPV+ HNCs show significant differences from those with HPV− HNCs including improved outcomes [5].
Both E6 and E7 can abrogate normal responses of cells to radiation-induced DNA damage in vitro [6] and in vivo [7]. E7 causes the accumulation of DNA breaks and an increased frequency of cells harboring γ-H2AX nuclear foci, a marker for cellular response to DNA damage [8]. Interestingly, while E6 also can cause an accumulation of DNA breaks, it does not increase the number of γ-H2AX foci [8]. We have previously shown that, in genetically engineered mouse models expressing HPV16 oncogenes in stratified squamous epithelia, HPV16 E7, alone or together with E6, led to an accumulation of epithelial cells harboring γ-H2AX nuclear foci, while E6 alone did not [9–11]. This effect of E7 was enhanced in mice deficient for the Fanconi Anemia DNA repair pathway and this correlated with enhanced susceptibility to cancer [9–11]. Together these results support the hypothesis that E7’s induction of DNA damage contributes to its oncogenic potential.
How E7 modulates the response to DNA damage induced by ionizing radiation and the mechanisms by which E7 deregulates the DNA damage response remains unclear. E7 is not known to possess any known intrinsic enzymatic activity that would cause DNA damage [12, 13]. Consequently, we hypothesized that E7 impedes DNA damage response pathway(s) leading to a delay in the repair of damaged DNA. To test this hypothesis, we utilized immortalized normal cell lines, HNC cell lines, and animal models to investigate the consequence of E7 expression on radiation-induced DNA damage repair. Herein, we demonstrate that E7 expression significantly delays radiation-induced DNA damage repair both in vitro and in vivo. Furthermore, E7 expression increases the level of Rad51, which is associated with homologous recombination repair in response of radiation. Our findings provide further insights into the properties of E7 that contribute to oncogenesis, and may explain why HPV+ HNCs are more susceptible to radiation.
Materials and Methods
Cell lines and culture conditions
Cell lines and culture conditions were obtained from and cultured as per Supplemental Table 1. The identity of all cell lines was confirmed via short tandem repeat testing within 6 months of cell use. Cells were maintained as monolayer cultures at 37°C in a humidified atmosphere of 5% CO2.
Double Strand Break assessment
DNA double strand breaks were assessed using a dual phosphorylated histone H2AX (γ-H2AX) and p53-binding protein 1 (53BP1) foci assay. Briefly, cells (25,000/well) were plated onto cartridge slides in duplicate, incubated overnight at 37°C, irradiated or mock irradiated with 2Gy and incubated for an additional 24 hours. Cells were washed with PBS, fixed for 15 min using 3% paraformaldehyde and 2% sucrose, and washed again with PBS. Cells were permeabilized for 5 min in PBS with 0.2% Triton X-100, blocked 1 hour in PBS with 3% BSA and incubated for 1 hour with antibody dilution buffer (PBS, 1% BSA, 0.5% Tween-20) with anti-γH2AX (1:500) and anti-53BP1 (1:500) added. Cells were washed 3 times with antibody dilution buffer, incubated with secondary antibodies for 1 hour, sealed with anti-fade/DAPI, and imaged on an Olympus BX51 microscope. Merged images were counted for the number of foci (positive for 53BP1 and γ-H2AX) per cell, normalized to the DNA content of the cell, and the mean number of foci per cell for HPV+ HNC vs. HPV− HNC compared via t-test.
Animal models
Xenografts were established and mice irradiated as previously described [14]. Mice were randomly assigned to either mock radiation or 2Gy and sacrificed 4 and 24 hours after the first dose of radiation. Non-transgenic FVB (NTG) and HPV16 E7 transgenic (K14E7) mice have been previously described [15]. All animals were bred and maintained in a American Association for Accreditation of Laboratory Animal Care-approved Animal care facility and were managed in accordance with an approved animal protocol.
Immunoblot analysis and antibodies
Western blot analysis is previously described [14]. Antibodies and dilutions were as per Supplemental Table 2. Tumors were formalin fixed, paraffin embedded, sectioned, and stained for γ-H2AX by immunohistochemistry. The proportion of cells positive for nuclear γ-H2AX foci was determined by counting 4 high-powered fields.
Three dimensional raft culture
The three dimensional raft culture is previously described [16].
Measuring the repair rate of radiation-induced DNA damage in cells and animal tissues
For the single dose irradiation studies, cells and mice were exposed to 2Gy γ-radiation from a 137Cs source. The exposed cells were fixed in 4% para-formalin for 15 minutes at these times, 0, 0.5, 1, 2, 4, and 8 hour following the radiation. NTG and K14E7 mice were sacrificed at 0, 1, 2, 4, and 8 hours and the dorsal animal skin was harvested and fixed in 4% para-formalin for 24 hours. One-sided Wilcoxon rank sum test was used to determine the significance of differences in the repair rate of radiation-induced DNA in cells and animals.
Assessment of Sub-lethal DNA damage repair (SLDR) ability
To assess sublethal damage repair capacity, cells were plated at low density (100–500 cells/well) into 6-well cell culture plates in triplicate. 24 hours after seeding, all plates were irradiated with a 2Gy dose of radiation. One plate received a second dose of radiation immediately following the first. For all other plates the second radiation dose was delivered at the indicated time-point following the first dose. Plates were maintained, colonies stained, and plating efficiency (PE) calculated as previously described [14]. The ratio of the PE between single (t=0) and split dose was calculated and graphed. Each cell line was examined in triplicate in three separate experiments.
Clonogenic survival assays
Cells were trypsinized to create single cell suspensions, seeded into 6 well plates at defined densities, incubated overnight to ensure log-phase of growth and irradiated with single doses of radiation using a JL Shepherd 137Cs irradiator delivering a dose rate of approximately 400 cGy/minute. After 10 to 15 days, colonies containing more than 50 cells were counted, the surviving fraction calculated and clonogenic survival curves fit to a linear-quadratic model as previously described [17]. Clonogenic survival curves were compared using the extra sum-of-squares F test in GraphPad Prism. Each point represents the mean surviving fraction calculated from 3 independent experiments performed in triplicate.
Rad51 foci assay
Each cell lines was plated in duplicate onto an 8-well chamber slide. After overnight incubation, cells were treated with radiation (4 Gy) or mock therapy. Cells were fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 4 and 24 hours after treatment. Fixed cells were probed with anti-Rad51 antibody and anti-γH2AX antibody and secondarily labeled by Alexa Fluor 488- and Alexa Fluor 546-conjugated secondary antibodies, respectively. Slides were mounted in Prolong antifade with DAPI (Molecular Probes, Eugene, OR). Fluorescent foci were then imaged on an Olympus BX51 microscope. One hundred randomly chosen intact nuclei were analyzed per treatment group for foci counting.
Results
Altered DNA repair capacity in HPV positive head and neck cancer cell lines
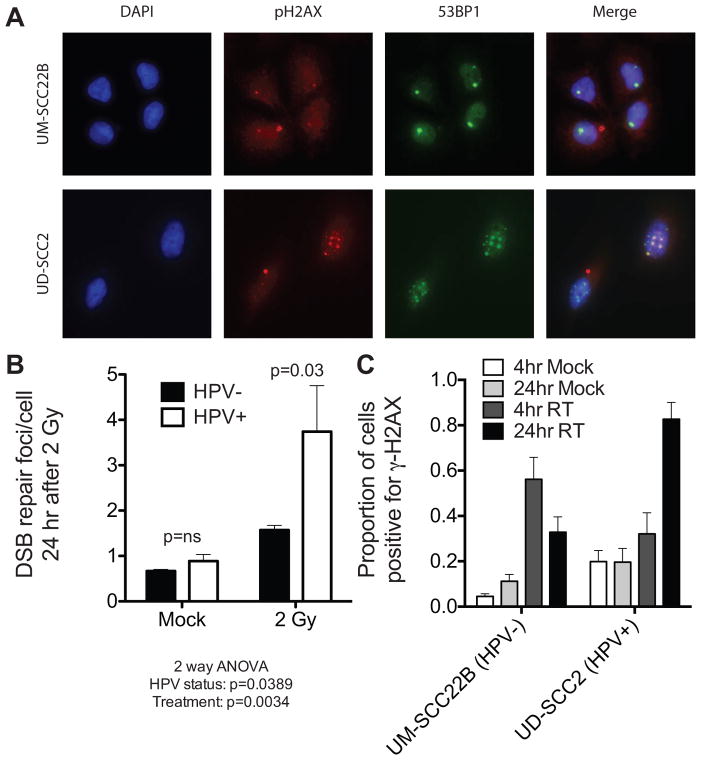
We examined DNA repair capacity using a dual nuclear foci assay 24 hours after a single 2Gy dose of radiation in which both γ-H2AX and 53BP1 were simultaneously assessed. These markers, activated at sites of DNA damage, function to coordinate the repair activity necessary to resolve double strand breaks (DSBs) [18, 19]. We quantified cells harboring γ-H2AX/53BP1 double nuclear foci after radiation in a panel of 4 HPV+ and 4 HPV− HNC cell lines (Figure 1A/B, Supplemental Table 3). All HPV+ cell lines contain HPV type 16 [20]. Overall, HPV+ cell lines had significantly more foci 24 hours after radiation than did HPV− cell lines. To assess whether similar responses were seen using an in vivo system, we utilized cell line xenografts in which established human HNC cells were implanted into the flank of nude mice and given with a single 2Gy radiation dose. Radiation increased the proportion of cells positive for γ-H2AX foci, 4 hours after radiation, regardless of HPV status. Consistent with our in vitro results, 24 hours after radiation, significantly more γ-H2AX were present in the HPV+, UD-SCC2 cell-derived xenograft, than in the HPV− UM-SCC22B cell-derived xenograft (P=0.0002 in Figure 1C). Taken together, these results suggest that the repair of radiation-induced DNA damage is significantly delayed by HPV in HNC cell lines, both in tissue culture and in xenografts.
Figure 1. Prolonged activation of DNA damage repair signals in HPV+ HNC cell lines.
A, immunofluorescence staining was performed for anti-γ-H2AX (red) and anti-53BP1 (green) in HPV+ and HPV− HNC cells 24 hours after a 2 Gy dose of radiation. Nuclei counterstained with DAPI (blue). B, Assessment of dual γ-H2AX/53BP1 nuclear foci 24 hours following radiation (2Gy) shows significant radiation induced DNA damage foci in aggregate within HPV+ HNC cells (P=0.03), but not HPV− HNC cells (P>0.05). C, Xenografts of UD-SCC2 (HPV+) and UM-SCC22B (HPV−) HNC cells confirm a failure to resolve γ-H2AX nuclear foci in vivo after a single 2Gy dose of radiation.
HPV16 E7 expression caused an increase in γ-H2AX expression and induced the formation of γ-H2AX foci
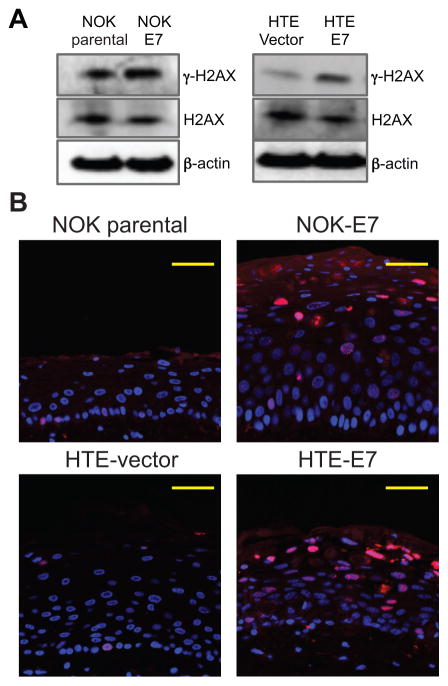
We have previously shown that HPV16 E7, but not E6, increased the number of γ-H2AX nuclear foci positive cells in the cervix and head/neck regions using our transgenic animal models [9–11]. To investigate the effect of E7 on the repair of radiation-induced DNA damage, we utilized two different immortalized oral epithelial cell lines; NOK (Normal Oral Keratinocytes) [21] and HTE (Human Tonsilar Epithelial) [22]. NOK and HTE cells were transduced by HPV16 E7 oncogene (NOK-E7 and HTE-E7 cells) as previously described [21, 22]. No significant difference in cell cycle distribution was seen between HPV-16 E7 and vector transduced cell lines (Supplemental Figure 1). Western-blot analysis demonstrated increased expression of γ-H2AX, but not total H2AX, in HPV16 E7 expressing NOK and HTE cells (Figure 2A). Using a three-dimensional organotypic raft culture system, we also identified an increased proportion of γ-H2AX foci in E7 positive cells. The majority of γ-H2AX foci were localized in cells in the suprabasal epithelial layer (Figure 2B). These findings indicate that HPV16 E7 significantly deregulates levels of γ-H2AX and induces the formation of γ-H2AX foci.
Figure 2. HPV16 E7 expression led to an induced γ-H2AX expression and an increased γ-H2AX foci formation.
A, E7-expressing NOK and HTE cells showed more γ-H2AX level compared with their no E7 expressing cells in total cellular lysate. The expression of total H2AX was not altered by E7. β-actin was used as a loading control (30μg). B, The amount of γ-H2AX nuclear foci (red) positive cells was increased by E7 in our three-dimensional organotypic raft culture of NOK and HTE cells. DAPI staining (blue) was utilized to detect nuclei.
HPV16 E7 caused the retention of radiation-induced γ-H2AX nuclear foci in both in vitro and in vivo systems
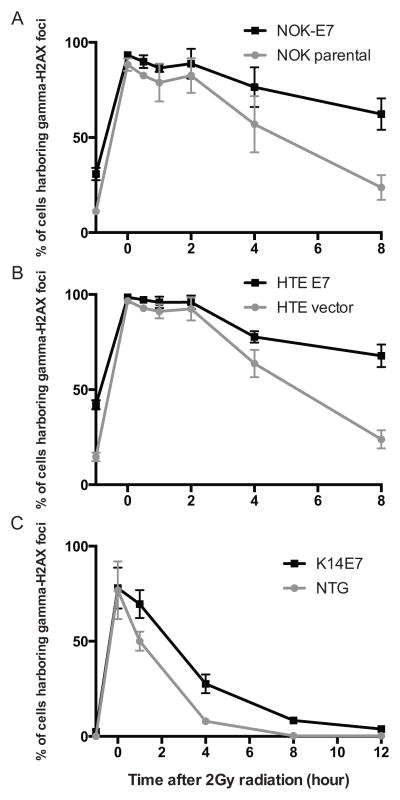
To understand further the mechanism by which E7 causes an increased expression of γ-H2AX and the formation of γ-H2AX foci, we sought to determine the effect of E7 on the resolution of γ-H2AX foci following induction of strand breaks. NOK and HTE cells were irradiated with a single 2Gy dose of ionizing radiation in the absence or presence of E7 expression. Following radiation, cells were fixed and immunofluorescence-staining used to quantitate the frequency of cells with γ-H2AX-positive nuclear foci at a series of time points after radiation (i.e. 0, 0.5, 1, 2, 4, and 8 hours after radiation). At least one hundred cells were scored per condition and per cell line for nuclear γ-H2AX foci. The slope of the resulting curve between 0 and 8hours was determined and used to compare the kinetics of repair on the basis of E7 expression. In both NOK and HTE cells, a negative slope was identified, consistent with resolution of γ-H2AX-positive nuclear foci. The presence of HPV16 E7 resulted in significantly slower resolution of γ-H2AX foci in both NOK (NOK-E7; −3.883 ± 0.906 vs. parental NOK; −8.004 ± 0.383, P=0.025 in Figure 3A) and HTE (HTE-E7; −3.844 ± 0.914 vs. parental HTE; −9.088 ± 0.530, P=0.025 in Figure 3B) cell lines. To confirm these findings in an in vivo system we utilized NTG and K14E7 [15]. The dorsal skin of mice was irradiated by a single 2Gy dose and cells with γ-H2AX-positive nuclear foci were detected by immunofluorescence-staining at a series of time points following radiation (i.e. 0, 1, 2, 4, and 8 hours). Significantly more γ-H2AX-positive nuclear foci were present in K14E7 mice than in NTG mice 1 hour after radiation (P=0.049 in Figure 3C). Eight hours after radiation, the frequency of γ-H2AX-positive nuclear foci had returned to basal levels in NTG mice (−1 hour vs. 8 hour in NTG mice, P>0.05 in Figure 3C). However, K14E7 mice had significantly more residual γ-H2AX foci positive cells than baseline, untreated mice at this time point (−1 hour vs. 8 hour in K14E7 mice, P<0.05 in Figure 3C). Taken together, these results suggest that E7 expression results in retention of γ-H2AX nuclear foci following radiation induced DNA damage.
Figure 3. Retention of γ-H2AX nuclear foci positive cells in E7-expressing NOK/HTE cell lines and animal models.
A and B, Immortalized human oral epithelial cells were treated with a single 2Gy dose of radiation and scored for γ-H2AX nuclear foci at indicated time points. Both NOK (A) and HTE (B) cells expressing HPV16 E7 demonstrated a significant delay in radiation-induced DNA-damage repair (P<0.05). ‘−1 hour’ indicates no radiation treatment and the basal level of γ-H2AX nuclear foci positive cells. C, γ-H2AX nuclear foci positive cells were measured in the animal dorsal skin of non-transgenic (NTG) and HPV16 E7-transgenic (K14E7) mice. K14E7 mice show a significantly higher number of γ-H2AX foci positive cells compared with unirradiated K14E7 mice (−1 hour). All assays were performed three independent times.
HPV16 E7 expression decreased sub-lethal DNA damage repair
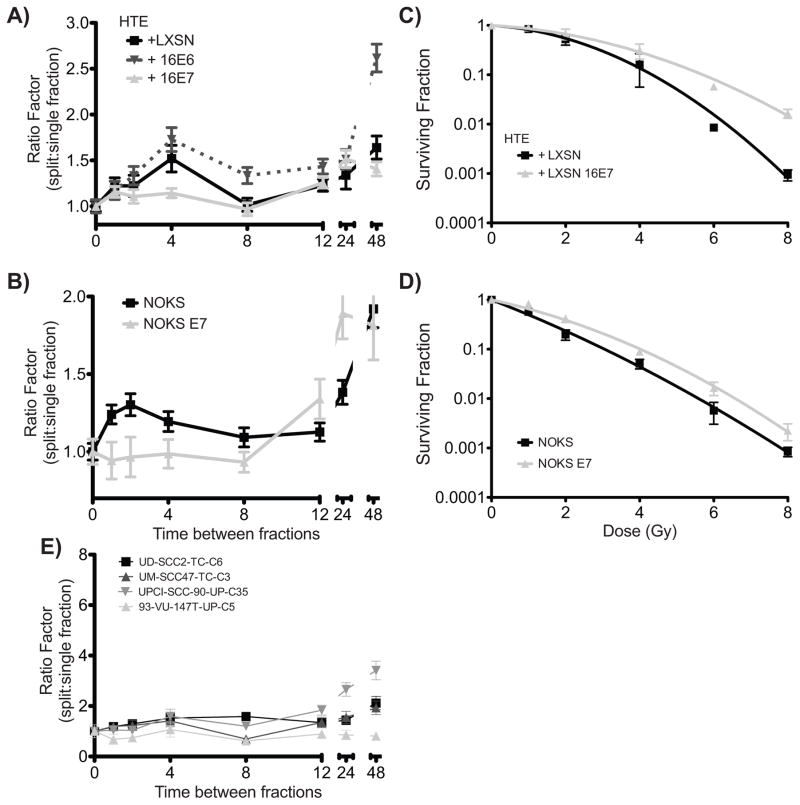
To assess the functional effect of delayed γ-H2AX foci resolution in HPV16 E7 cells, we used a sublethal DNA damage repair assay [23, 24]. Briefly, colony formation was determined after two identical radiation doses (2Gy) delivered either consecutively (no sublethal damage repair) or with a range of time intervals between first and second doses of radiation. The surviving fraction of each time interval was compared to that when the two doses were delivered consecutively to calculate the ratio of colony formation for split vs. single dose delivery (Ratio factor or RF). In both parental HTE and NOK cells (Figure 4A/B), an initial increase in the RF is identified consistent with repair of sublethal DNA damage. The subsequent decrease in RF is thought to be due to redistribution of cells into relatively radiation sensitive phases of the cell cycle, while the final increase in the RF is classically due to cellular proliferation or repopulation. In both HTE-E7 and NOK-E7 cells, little sublethal damage recovery was seen as identified by a relatively stable RF at the early time points and a subsequent increase in RF later (Figure 4A/B). In HTE cells expressing both HPV-16 E6 and E7 split dose experiments demonstrated modestly delayed DNA repair compared to E6 alone or E7 alone (data not shown).
Figure 4. Delayed repair of radiation-induced DNA damage in E7-expressing NOK/HTE cells via sublethal DNA damage repair assay (SLDR).
A and B, SLDR was assessed by delivering two identical radiation doses separated by indicated timepoints. The plating efficiency with no interval between doses (i.e. single dose) was set as 1 and the ratio between this plating efficiency and that of each indicated time point (ratio factor) determined by colony formation assay. No initial repair of sublethal damage was seen in E7 expressing cells while vector alone and E6 expressing cells demonstrated the expected increase in plating efficiency between 1 and 4 hours after radiation. C and D, Clonogenic survival of HTE and NOK cells expressing vector alone of HPV-16 E7 demonstrate increased colony formation across a range of radiation doses of HPV-16 E7 expressing cells. E, SLDR assay of HPV+ cell lines demonstrates minimal repair of sublethal damage.
When similar experiments were performed using HPV+ cell lines confirmed to express HPV16 E7 [14], a similar pattern was seen consistent with impaired DNA damage repair (Figure 4E). Likewise, in HPV− cell lines, relatively normal DNA damage recovery was seen (Supplemental Figure 1A). These findings suggest that HPV16 E7 not only results in delayed resolution of γ-H2AX foci, but also results in delayed DNA damage repair.
To determine the effect of HPV16 E7 on radiation sensitivity, we performed standard clonogenic survival assays with a range of single fraction radiation doses. Unexpectedly, in both NOK and HTE cells, expression of HPV16 E7 resulted in resistance to radiation (Figure 4C/D, P<0.0001 for both) across a range of radiation doses.
The expression level of Rad51 in homologous recombination DNA repair system was induced by HPV16 E7 expression
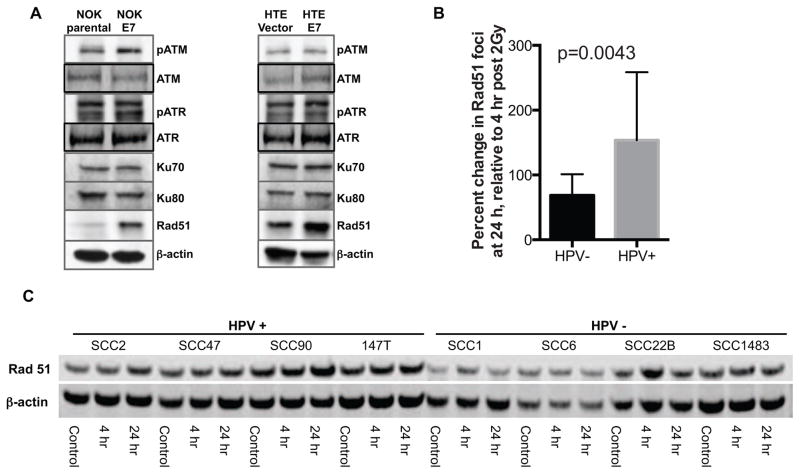
To determine how HPV16 E7 modulates the DNA damage repair system, we examined alterations in several proteins involved in the two major DNA repair pathway: homologous recombination (HR) and non-homologous end joining (NHEJ). We first looked at proteins involved in activation of checkpoint control: ATM and ATR [25]. While HPV16 E7 resulted in increased phospho-ATM in NOK cells, no similar pattern was identified in HTE cells (Figure 5A). No difference in phospho-ATR levels was seen on the basis of E7 expression. Likewise, no alterations in Ku70 and Ku80, two well-known markers for NHEJ [26, 27], were identified (Figure 5A). Interestingly, in both NOK and HTE cells, Rad51, a essential element of the HR pathway [28], was highly up-regulated by E7.
Figure 5. Rad51 expression is modulated by E7 expression.
A, western blot analysis was performed for several cellular proteins associated with DNA damage repair systems using total cell lysate. Among the selected proteins, Rad51 is up-regulated by E7 in NOK as well as HTE cells. B, The proportion of cells with 5 or more Rad51 foci were counted at baseline, 4 and 24 hours after a 2 Gy dose of radiation. Increased Rad51 foci was seen in all cells 4 hours after radiation. In HPV− cells the number of foci-positive cells returned towards baseline by 24 hours while in HPV+ cells, the proportion increased. C, To monitor Rad51 expression following single 2Gy dose radiation, western blot analysis was performed in the above time points in HPV+ and HPV− HNC cell lines. HPV+ HNC cells showed higher levels of Rad51 at baseline and following radiation. Rad51 expression stayed elevated from 4 to 24 hours after radiation in HPV+ cell lines, but returned towards baseline in HPV− cell lines.
We further investigated expression level of Rad51 in our HNC cell lines after a single 2Gy dose of radiation. At baseline, no difference in the number of Rad51 foci was seen (data not shown, p=0.61). Four hours after a single dose of radiation, both HPV+ and HPV− HNC cell lines demonstrated increased Rad51. While Rad51 returned towards baseline 24 hours after radiation in HPV− HNC cells, in HPV+ HNC cells Rad51 remained elevated compared to 4 hrs (Figure 5B/C). Our findings suggest that HPV16 E7 alters Rad51 regulating resulting in impaired DNA repair.
Discussion
Herein we provide further evidence that HPV oncoproteins play critical roles in the response of HPV-positive head and neck cancers to ionizing (i.e. therapeutic) radiation. While we have previously described a role for E6 in modulating p53-mediated cell cycle arrest and apoptosis [14], here we provide further evidence for the role of E7 in modulating DNA damage repair. Our findings are consistent with those of Rieckman et al who first described impaired DNA repair in a panel of HPV+ HNC cell lines [29]. We have extended this work by identifying E7 as the cause of impaired DNA damage repair. While the full function of E7 in HPV+ HNC remains a subject of ongoing investigation, E7 appears to delay the resolution of γ-H2AX foci and increase Rad51.
Several studies may provide insight into how HPV16 E7 modulates H2AX activation. For example, E7 increases expression of the deacetylase, Sirt1 [30] which interacts with the histone acetyl-transferase, Tip60 and negatively regulates Tip60-mediated acetylation of γ-H2AX, as well as p53 [31, 32]. The release of γ-H2AX from damaged DNA and the subsequent repair of that damage require its acetylation and subsequent ubiquitination [32]. Thus, E7 may delay the rate of H2AX turnover at sites of DNA damage by altering the Sirt1/Tip60 complex and inhibiting endogenous DNA repair. Interestingly, although HPV16 E6 expression is also reported to destabilize Tip60 both in vivo and in vitro, E6 has not been shown to alter the frequency of γ-H2AX foci positive cells [8, 9, 11]. Our data further suggest that E7 not only delays resolution of γ-H2AX foci, but also results in impaired kinetics of DNA damage repair. These properties of E7 could contribute to the accumulation of damaged DNA in E7-expressing cells [8].
Interestingly, when radiation was delivered as a single fraction, we failed to observe increased sensitivity to radiation in cells expressing HPV16 E7 alone. This lies in stark contrast to data we have previously reported with HPV16 E6 alone [14]. Others and we have also demonstrated increased sensitivity to radiation in cell lines expressing both E6 and E7 [14, 29, 33]. This may represent a situation whereby the results of single dose assays do not perfectly correlate with those seen with the delivery of multiple radiation doses. DNA damage that is unrepaired at the time of a second dose of radiation may convert from sublethal to lethal due to the quantity of damage present.
Rad51 is an essential factor for homologous recombination and the repair of DNA double strand breaks, helping cells resist the damaging effects of ionizing radiation, chemotherapeutic agents, and the spontaneous breaks and aberrations that occur during DNA replication. Rad51 expression is increased in p53-negative cells, and since p53 is often mutated in tumor cells, there is a tendency for Rad51 to be overexpressed in tumor cells, leading to increased resistance to DNA damage and drugs used in chemotherapies [34]. Expression of HPV16 E6 results in degradation of p53, which may contribute to increased Rad51 expression. However, in the studies presented here, HPV16 E7 expression alone is sufficient to increase Rad51 levels. This is consistent with a prior study showing expression of other oncogenes led to an induction of Rad51 through ATM/ATR related pathways [35]. For example, the BCR/Abl oncogene increases Rad51 expression, which mediates resistance to cisplatin and mitomycin C [36]. While we did not see a significant alteration in phospho-ATM or phospho-ATR expression, Rad51 expression is also cell cycle-dependent: highest in S/G2 phase and lowest in non-growing cells. HPV16 E7 is well known to disrupt cell cycle regulation and result in an uncontrolled S phase entry. In addition, radiation induces a prolonged G2 cell cycle checkpoint arrest in HPV+ HNC cells, which may help explain increased Rad51 levels [14, 29].
Rad51 appears to play divergent roles depending on the setting and expression levels. For example, it has been suggested to contribute to drug resistance in prostate cancer [37]. p16INK4A, a protein overexpressed in HPV+ cancers due to the effects of E7 has recently been shown to impair the recruitment of Rad51 to sights of DNA damage [38]. Thus elevated levels of Rad51 may not correlate directly with DNA repair capacity. Consistent with this, high levels of Rad51 have also been reported to increase genomic instability through the formation of DNA-RNA hybrids which ultimately lead to damaged DNA [39]. Clearly, further work is needed to determine whether overexpression of proteins involved in homologous recombination may play a role in the decreased sensitivity to radiation seen in single dose assays. These findings emphasize the importance of careful regulation of Rad51 expression and activity; a balance that may be altered by HPV-16 E7.
In conclusion, we have demonstrated the role of HPV16 E7 in altering the cellular response to DNA damage. Our findings indicate that the E7 expression significantly delays radiation-induced DNA damage repair in head and neck epithelial cells and head and neck cancer cell lines both in vitro and in vivo. This delay results in a slowing of the normal kinetics of DNA damage repair, a finding that may have important clinical implications regarding the role of twice daily radiation in HPV+ HNC. Unlike what we have previously shown for E6, E7 may exert its effects in a more time and dose dependent manner by priming the cell for increased DNA DNA damage. E7 appears to work by altering expression of Rad51, one of the essential components of the homologous recombination pathway. These findings provide new insight into how E7 contributes to oncogenesis, and raises interesting questions as to why HPV+ HNCs are more sensitive to radiotherapy.
Supplementary Material
A, SLDR assay was performed to measure the DNA repair rate in HPV− HNC cell lines.
Acknowledgments
Financial support: Supported by R00 CA160639 (RJK), P01 CA022443 (PFL) and U01 CA141583 (PFL).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.McDougall JK. Immortalization and transformation of human cells by human papillomavirus. Curr Top Microbiol Immunol. 1994;186:101–19. doi: 10.1007/978-3-642-78487-3_6. [DOI] [PubMed] [Google Scholar]
- 3.Duensing S, Munger K. Centrosome abnormalities and genomic instability induced by human papillomavirus oncoproteins. Prog Cell Cycle Res. 2003;5:383–91. [PubMed] [Google Scholar]
- 4.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–28. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–54. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Slebos RJ, Lee MH, Plunkett BS, Kessis TD, Williams BO, Jacks T, et al. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994;91:5320–4. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song S, Gulliver GA, Lambert PF. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95:2290–5. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duensing S, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62:7075–82. [PubMed] [Google Scholar]
- 9.Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–68. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JW, Shin MK, Lambert PF. High incidence of female reproductive tract cancers in FA-deficient HPV16-transgenic mice correlates with E7’s induction of DNA damage response, an activity mediated by E7’s inactivation of pocket proteins. Oncogene. 2013 doi: 10.1038/onc.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JW, Shin MK, Pitot HC, Lambert PF. High Incidence of HPV-Associated Head and Neck Cancers in FA Deficient Mice Is Associated with E7’s Induction of DNA Damage through Its Inactivation of Pocket Proteins. PLoS One. 2013;8:e75056. doi: 10.1371/journal.pone.0075056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384:335–44. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. The Journal of biological chemistry. 2006;281:578–86. doi: 10.1074/jbc.M508455200. [DOI] [PubMed] [Google Scholar]
- 14.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed]
- 15.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. Journal of virology. 1996;70:1873–81. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores ER, Allen-Hoffmann BL, Lee D, Lambert PF. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. Journal of virology. 2000;74:6622–31. doi: 10.1128/jvi.74.14.6622-6631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimple RJ, Vaseva AV, Cox AD, Baerman KM, Calvo BF, Tepper JE, et al. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16:912–23. doi: 10.1158/1078-0432.CCR-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S. gammaH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutation research. 2013 doi: 10.1016/j.mrrev.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, Pedersen RS, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243–53. doi: 10.1038/ncb2201. [DOI] [PubMed] [Google Scholar]
- 20.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piboonniyom SO, Duensing S, Swilling NW, Hasskarl J, Hinds PW, Munger K. Abrogation of the retinoblastoma tumor suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res. 2003;63:476–83. [PubMed] [Google Scholar]
- 22.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–8. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 23.Deschavanne PJ, Guichard M, Malaise EP. Repair of sublethal and potentially lethal damage in lung cells using an in vitro colony method. The British journal of radiology. 1981;54:973–7. doi: 10.1259/0007-1285-54-647-973. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz JL, Giovanazzi S, Weichselbaum RR. Recovery from sublethal and potentially lethal damage in an X-ray-sensitive CHO cell. Radiation research. 1987;111:58–67. [PubMed] [Google Scholar]
- 25.Helt CE, Wang W, Keng PC, Bambara RA. Evidence that DNA damage detection machinery participates in DNA repair. Cell Cycle. 2005;4:529–32. doi: 10.4161/cc.4.4.1598. [DOI] [PubMed] [Google Scholar]
- 26.Kotnis A, Du L, Liu C, Popov SW, Pan-Hammarstrom Q. Non-homologous end joining in class switch recombination: the beginning of the end. Philos Trans R Soc Lond B Biol Sci. 2009;364:653–65. doi: 10.1098/rstb.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Featherstone C, Jackson SP. Ku, a DNA repair protein with multiple cellular functions? Mutation research. 1999;434:3–15. doi: 10.1016/s0921-8777(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 28.Sung P, Robberson DL. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell. 1995;82:453–61. doi: 10.1016/0092-8674(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 29.Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Allison SJ, Jiang M, Milner J. Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity protein in human cervical cancer cells. Aging (Albany NY) 2009;1:316–27. doi: 10.18632/aging.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. The Journal of biological chemistry. 2010;285:11458–64. doi: 10.1074/jbc.M109.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamagata K, Kitabayashi I. Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX. Biochem Biophys Res Commun. 2009;390:1355–60. doi: 10.1016/j.bbrc.2009.10.156. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen BS, Busk M, Olthof N, Speel EJ, Horsman MR, Alsner J, et al. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother Oncol. 2013;108:500–5. doi: 10.1016/j.radonc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA repair. 2008;7:686–93. doi: 10.1016/j.dnarep.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauklin S, Kristjuhan A, Maimets T, Jaks V. ARF and ATM/ATR cooperate in p53-mediated apoptosis upon oncogenic stress. Biochem Biophys Res Commun. 2005;334:386–94. doi: 10.1016/j.bbrc.2005.06.097. [DOI] [PubMed] [Google Scholar]
- 36.Slupianek A, Schmutte C, Tombline G, Nieborowska-Skorska M, Hoser G, Nowicki MO, et al. BCR/ABL regulates mammalian RecA homologs, resulting in drug resistance. Mol Cell. 2001;8:795–806. doi: 10.1016/s1097-2765(01)00357-4. [DOI] [PubMed] [Google Scholar]
- 37.Fan R, Kumaravel TS, Jalali F, Marrano P, Squire JA, Bristow RG. Defective DNA strand break repair after DNA damage in prostate cancer cells: implications for genetic instability and prostate cancer progression. Cancer Res. 2004;64:8526–33. doi: 10.1158/0008-5472.CAN-04-1601. [DOI] [PubMed] [Google Scholar]
- 38.Dok R, Kalev P, Van Limbergen EJ, Asbagh LA, Vazquez I, Hauben E, et al. p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res. 2014;74:1739–51. doi: 10.1158/0008-5472.CAN-13-2479. [DOI] [PubMed] [Google Scholar]
- 39.Wahba L, Gore SK, Koshland D. The homologous recombination machinery modulates the formation of RNA-DNA hybrids and associated chromosome instability. Elife. 2013;2:e00505. doi: 10.7554/eLife.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, SLDR assay was performed to measure the DNA repair rate in HPV− HNC cell lines.