Abstract
Objective
Obesity and HIV-infection are associated with an increased incidence of non-infectious co-morbid medical conditions, but the relationship between body mass index (BMI) and the development of non-communicable diseases (NCDs) among individuals on antiretroviral therapy (ART) has not been well-characterized.
Methods
A cohort study of adults initiating ART between 1998 and 2010 at an academic center with systematic laboratory and clinical data collection, including AIDS and NCD diagnoses. The relationship between BMI at ART initiation and the risk of incident cardiovascular, hepatic, renal or oncologic NCDs was assessed using Cox proportional hazard models. BMI was fit using restricted cubic splines and models adjusted for age, sex, race, CD4+ count, protease inhibitor use, year of initiation, and prior AIDS-defining illness.
Results
Among 1089 patients in the analysis cohort, 54% had normal BMI, 28% were overweight, and 18% were obese. Baseline BMI was associated with developing an incident NCD (p=<0.01) but the relationship was non-linear. Compared to a BMI of 25 kg/m2, a BMI of 30 kg/m2 conferred a lower risk of an incident NCD diagnosis (HR 0.59; 95% CI: 0.40, 0.87). This protective effect was attenuated at a BMI of 35 kg/m2 (HR 0.78; 95% CI: 0.49, 1.23). Results were similar in sensitivity analyses incorporating tobacco, alcohol and drug use, statin and antihypertensive exposure, and virologic suppression.
Conclusions
Overweight individuals starting ART have a lower risk of developing NCDs compared to normal BMI individuals, which may reflect a biological effect of adipose tissue versus differences in patient or provider behaviors.
Keywords: HIV, antiretroviral therapy (ART), nutrition, obesity, body mass index (BMI), non-AIDS defining events, non-communicable diseases (NCDs)
Introduction
Overweight and obese individuals constitute an increasing proportion of the HIV-infected population in developed countries, but the relationship between body composition, non-communicable disease events (NCDs; a category also referred to as non-AIDS-defining events [NADEs]), and mortality is not well studied (1, 2). Long-term antiretroviral therapy (ART) is associated with a myriad of cardiovascular and metabolic abnormalities similar to those observed in sedentary, obese, uninfected individuals, suggesting the combination of treated HIV and excess adiposity may compound the risk for the development of co-morbid conditions (3–5).
In the general population, meta-analyses have reported an equivalent or lower risk of all-cause mortality among overweight (BMI 25 to 29.9 kg/m2) individuals compared to normal weight (BMI <25 kg/m2) or obese (BMI >30 kg/m2) individuals (6, 7). However, other large analyses found that incremental increases in BMI above 25 kg/m2 were associated with higher risk of cardiovascular, renal, hepatic, and other cause-specific mortality (8–10). Given the increased prevalence of many NCDs among ART-treated individuals, and the overlapping cardiovascular and metabolic abnormalities observed in both treated HIV and obesity, we hypothesized that the potential benefits of mildly increased adiposity reported in the general population may not pertain to the HIV-infected population, and the risk of many NCDs may rise as patients become progressively heavier. In this analysis, we investigated the risk of incident cardiovascular, hepatic, renal, and oncologic NCDs among HIV-infected individuals initiating ART with a range of BMI values at an academic medical center providing integrated outpatient and inpatient HIV care.
Methods
We conducted a retrospective analysis of HIV-infected adults seen at the Vanderbilt Comprehensive Care Clinic (VCCC) in Nashville, Tennessee between January 1, 1998 and December 31, 2010. Research staff systematically extracts all laboratory and clinical data, including established cardiovascular, hepatic, renal, and oncologic NCD diagnoses and date of onset, on VCCC patients from the electronic medical record (supplementary Table 1). In this analysis, we included only adult VCCC patients known to be HIV treatment-naïve who initiated ART (defined as a combined regimen of ≥3 antiretroviral agents), had a recorded baseline BMI within 180 days prior to or 30 days after starting ART, and were followed in clinic for a minimum of one year. Patients with a documented NCD or diabetes mellitus prior to the start of ART were excluded to reduce potential confounding from pre-existing conditions. Patients with a 12 month or greater gap in care, defined as no clinic visits, were censored at the time of the visit preceding the gap.
We grouped patients into the standard BMI categories of normal (BMI <25 kg/m2), overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥30 kg/m2) to compare clinical and demographic characteristics using Pearson chi-square and Kruskal-Wallis tests as appropriate (11). The multivariable models treated BMI as a continuous variable.
The primary analysis assessed the relationship between BMI and the endpoint of an incident NCD, or the combined endpoint of an incident NCD or death, using Cox proportional hazard models. BMI was fit using restricted cubic splines with four knots to avoid assuming linearity. Models assessing individual NCD categories were adjusted for age at ART initiation, sex, race/ethnicity, and baseline CD4+ cell count. Combined endpoint models also adjusted for PI inclusion in the first ART regimen, year of ART initiation, and a history of an AIDS-defining event (ADE) prior to treatment initiation. The exposure interval for each subject was the time from ART initiation until an NCD event or death occurred, the last recorded activity date (either the last visit dataset or December 31, 2010), or the start of any gap in clinical care lasting more than 12 months. To facilitate interpretation of the model, we calculated the hazard ratio of the outcome (any NCD or NCD/death) at specific BMI values of 20, 30 and 35 kg/m2 versus a reference of BMI 25 kg/m2.
We performed several sensitivity analyses to assess the effect of potential confounders. Data on smoking and alcohol use were available on 52% of our cohort from a separate, clinic-wide survey system instituted in 2010, and drug use data were available on 73%; inclusion of these data required the assumption that a patient’s substance use in 2010 was similar to his/her use in the preceding years. Patients with survey data were more likely to be female (29% versus 23%), had a higher baseline CD4+ T-cell count (238 versus 191 cells/µl), and started ART later (2006 versus 2004, p<0.05 for all), likely reflecting trends in the underlying cohort over the period 1998–2010. Data on virologic suppression, statin and antihypertensive medication use, and cumulative PI exposure were available for all participants.
Analyses were performed using R (version 2.12.1; www.r-project.org). Analysis scripts are posted at biostat.mc.vanderbilt.edu/ArchivedAnalyses. The study protocol was approved by the institutional review board of Vanderbilt University Medical Center.
Results
There were 1274 ART-naïve patients who met inclusion criteria and initiated treatment at the VCCC between January 1, 1998 and December 31, 2010, and 1089 (85%) had a recorded baseline BMI value. Patients missing a baseline BMI measurement were more likely to be male and have a later year of ART initiation (p=0.03 for both) but did not significantly differ by other characteristics. At ART initiation, 590 (54%) had a normal BMI, 303 (28%) were overweight, and 196 (18%) were obese (supplementary table 2).
A total of 111 incident NCD diagnoses were recorded with a median follow-up time of 2.20 years (interquartile range [IQR] 0.75, 4.77); 74 occurred in normal BMI patients (13% of those at risk), 21 in overweight patients (7% of those at risk), and 16 in obese patients (8% of those at risk). By category there were 57 hepatic, 27 renal, 21 oncologic and 16 cardiovascular diagnoses; the total number of individual diagnoses exceeds the number included in the primary analysis as only the first diagnosis was included for participants with more than one event during follow-up. There were a total of 62 deaths recorded: 41 in normal BMI patients (7% of those at risk), 15 in overweight (5% of those at risk), and 6 in obese patients (3% of those at risk).
Baseline BMI was associated with the development of an NCD (p<0.01) and the combined endpoint of NCD/death (p=0.01) in the multivariable models. Because BMI was fit with splines, one hazard ratio does not adequately describe its relationship with the outcome (p<0.01 for test of non-linear relationship between BMI and NCD, and NCD/death). Therefore, the hazards of the outcome for a BMI of 20, 30 and 35 kg/m2 versus a BMI of 25 kg/m2 were reported in the Table. For example, having a BMI of 30 kg/m2 conferred an approximately 41% lower risk of mortality compared to the reference BMI of 25 kg/m2 (HR 0.59; 95% CI: 0.40, 0.87). However, the measure of statistical association was not based on any specific BMI comparison but rather the entire relationship across all BMI levels. The results were similar when missing BMI values were imputed and when BMI was refit using splines with 5 knots (data not shown).
Body mass index at antiretroviral therapy initiation and the risk of new non-communicable disease (NCD) diagnosis or the combined endpoint of NCD/death (n=1089)
| Risk of new NCD diagnosis** | Risk of new NCD diagnosis or death† | ||||||
|---|---|---|---|---|---|---|---|
| Adjusted Hazard Ratio |
95% confidence interval |
p-value | Adjusted Hazard Ratio |
95% confidence interval |
p-value | ||
| Baseline body mass index (BMI)* | <0.01 | 0.01 | |||||
| 20 kg/m2 | 1.25 | (0.87, 1.80) | 1.30 | (0.97, 1.73) | |||
| 25 (reference) | 1.00 | 1.00 | |||||
| 30 | 0.59 | (0.40, 0.87) | 0.67 | (0.49, 0.93) | |||
| 35 | 0.78 | (0.49, 1.23) | 0.83 | (0.56, 1.24) | |||
| Baseline CD4+count†† | 0.95 | (0.85, 1.05) | 0.31 | 0.91 | (0.83, 0.99) | 0.04 | |
| Baseline age (per 1 year increase) | 1.03 | (1.01, 1.05) | <0.01 | 1.03 | (1.01, 1.05) | <0.01 | |
| Female sex | 0.87 | (0.51, 1.49) | 0.61 | 1.13 | (0.74, 1.72) | 0.58 | |
| Nonwhite race | 0.83 | (0.55, 1.24) | 0.36 | 0.96 | (0.68, 1.35) | 0.81 | |
| Protease inhibitor in first ART regimen | 1.52 | (1.01, 2.30) | 0.04 | 1.21 | (0.85, 1.71) | 0.29 | |
| Year of ART initiation (per year) | 0.94 | (0.88, 1.00) | 0.05 | 0.92 | (0.87, 0.97) | <0.01 | |
| History of AIDS-defining event prior to starting ART | 1.31 | (0.79, 2.18) | 0.29 | 1.53 | (1.01, 2.31) | 0.04 | |
The adjusted hazard ratios represent a comparison of the point estimates of a BMI of 20, 30 and 35 kg/m2 versus a BMI of 25 kg/m2 extracted from the model.
Includes incident cardiovascular, hepatic, renal, and oncologic disease diagnoses; 111 events over a median follow-up of 2.20 years (interquartile range 0.75, 4.77); 74, 21, 16 for normal, overweight, and obese, respectively.
154 deaths or new NCD diagnoses; 100, 34, 20 events for normal, overweight, and obese respectively. The number of events is lower than the sum of all NCDs and deaths as only the first event was counted among those patients who died at the same time as the NCD event or after.
CD4+ T-cell count is square root transformed
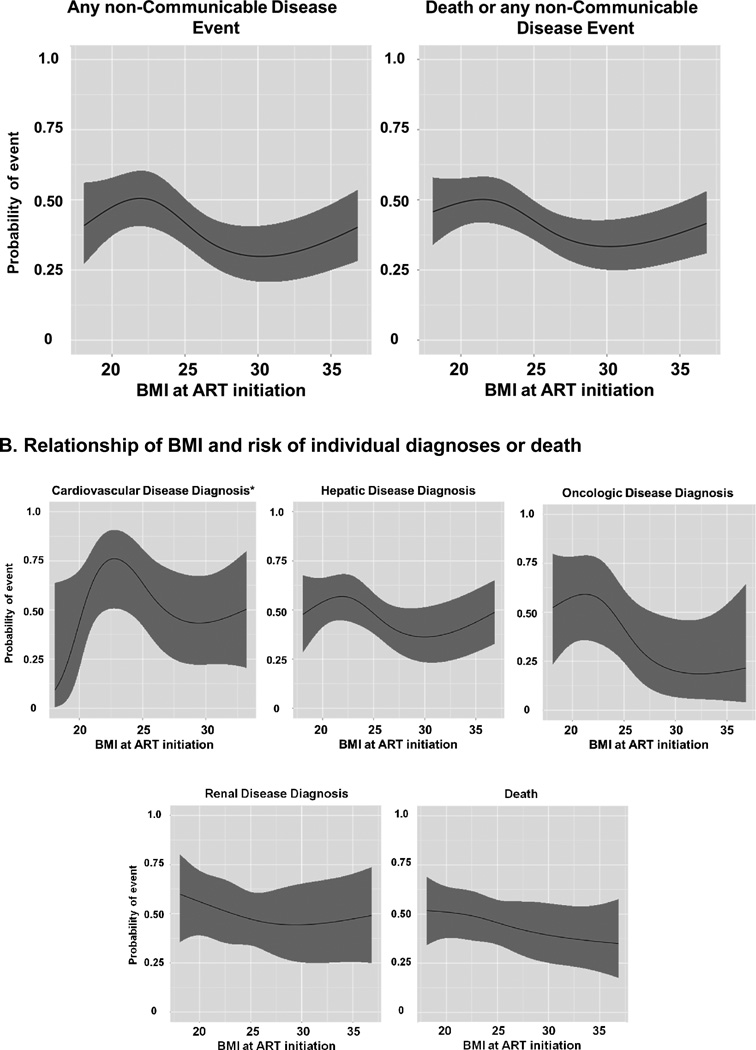
The Figure shows the relationship between BMI at ART initiation and the predicted probability of developing any NCD, the combined endpoint of NCD/death, or each individual NCD diagnosis from the multivariable models. Among the individual NCDs, only hepatic events were statistically associated with BMI. However, the ratio of events to model covariates in all of the individual diagnosis models is small and these models would be considered ‘overfit’ according to standard statistical conventions, so these graphs are only provided for illustrative purposes (12).
Figure. Body mass index at antiretroviral therapy initiation and the probability of incident non-communicable disease diagnoses (cardiovascular, hepatic, oncologic, and renal) or death.
Distribution of individual NCD diagnoses and death by BMI category: 57 hepatic diagnoses (34 normal BMI, 13 overweight, 10 obese,); 21 oncologic diagnoses (18 normal BMI, 2 overweight, one obese); 27 renal diagnoses (19 normal BMI, 4 overweight, and 4 obese); 16 cardiovascular diagnoses (12 normal BMI, 2 overweight, and 2 obese); and 62 deaths (41 normal BMI, 15 overweight, and 6 obese).
* Multivariable model for cardiovascular events only includes men in the cohort since all events occurred in males.
In sensitivity analyses, achieving a plasma HIV-1 RNA of <400 copies/ml in the first 12 months of ART (observed in 80% of the cohort) was associated with a lower hazard of an incident NCD diagnosis (AHR 0.38, p<0.01), but there was minimal change in the hazard of an incident NCD at the selected BMI point estimates and the overall relationship of BMI and the outcome remained statistically significant (p<0.01).
Smoking and alcohol use data were available on 52% of participants and drug use data on 73% of participants. Tobacco and marijuana smoking prevalence decreased as BMI increased, while heavy alcohol (>7 drinks/week) and other illicit drug use were similar across BMI categories (supplementary Table 2). Smoking was associated with a higher risk of an NCD in the subset with these data, though the effect was not statistically significant (AHR 1.40, p=0.30). The model was relatively insensitive to the inclusion of smoking status; the hazard of an incident NCD changed from 1.25 to 1.33 for the BMI 20 kg/m2 point estimate compared to the BMI 25 kg/m2 reference, from 0.59 to 0.68 for BMI 30 kg/m2, and from 0.78 to 0.60 for BMI 35 kg/m2. The overall relationship of BMI and incident NCDs was not statistically significant when the cohort was limited to those with substance use data (both with and without inclusion of a term for smoking status in the model), likely reflecting the smaller sample size. Heavy alcohol use, marijuana use, and other illicit drug use were not associated with incident NCDs, and the relationship of BMI and incident NCDs was insensitive to the inclusion of these terms in the model.
Statin and antihypertensive use at baseline was more prevalent at higher BMI levels (supplementary table 2). Neither medication was significantly associated with the outcome, and the relationship of BMI and incident NCDs was insensitive to the inclusion of these terms in the model (p-value remained <0.01). Lastly, a greater proportion of total treatment time (i.e., prior to censoring) spent on a PI-containing regimen conferred an increased, but not statistically significant, risk of an incident NCD diagnosis (AHR 1.36, p=0.21), but the relationship of BMI and incident NCDs was again insensitive to the inclusion of this variable in the model (p-value remained <0.01). However, the sensitivity analyses incorporating virologic suppression and duration of treatment on a PI should be interpreted with caution as these terms are longitudinal while other covariates were collected at baseline.
Discussion
In a longitudinal cohort of HIV-infected adults, the relationship between BMI and systematically validated incident NCD diagnoses was non-linear, and patients in the overweight to mildly obese range at the time of ART initiation were observed to have a lower risk of developing an incident NCD when compared to persons having lower and higher BMI values. While a similar spectrum of metabolic disorders and heightened inflammatory biomarkers are observed in treated HIV and excess adiposity, the combination of these conditions may not confer increased risk of incident NCDs until BMI is definitively in the obese range (3, 13, 14).
As with similar studies of health outcomes in HIV cohorts and the general population, variability in patient behaviors, primary prevention, and disease screening may confound the relationship between adiposity and health outcomes. The potential role of behavior is particularly complex. While obesity is more prevalent among those with less education and lower socioeconomic status, two factors associated with lower access to health care and worse health outcomes, obese patients in both our cohort and the Women’s Interagency HIV Study (WIHS) were less likely to smoke or engage in moderate-heavy alcohol intake or illicit drug use (15, 16). Secondly, cardiovascular and other chronic disease risk factors are often not at goal levels in HIV-infected individuals, and if provider behavior regarding primary prevention and disease screening varies according to a patient’s body composition this difference may be reflected in health outcomes (17, 18). In our cohort, obese patients were over three times as likely to be on an anti-hypertensive medication than those of normal weight, potentially indicating a bias in primary prevention.
In summary, we observed a lower risk of incident NCDs among overweight HIV-infected individuals initiating ART compared to those in the normal BMI range. While adiposity affects inflammation and cardiovascular risk factors in HIV-infected individuals, further studies are needed to understand how biological and behavioral (both patient and provider) factors interact to produce the observed variability in health outcomes (19, 20). As HIV-infected individuals survive longer on effective ART, the prevention and management of NCDs will remain a challenge for health providers.
Supplementary Material
Acknowledgements
This work was supported by NIAID [grant numbers K23 100700 and K24 AI65298], the Vanderbilt Meharry Center for AIDS Research [grant number AI54999], and the Vanderbilt Clinical and Translational Science award from NCRR/NIH [grant number UL1 RR024975-01]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
JK, CJ, BS, and TS designed the study, CJ and BS created the statistical models, MT, SB, and CW collected and validated the clinical data and assisted with statistical analyses, and all authors contributed to writing the manuscript and approved the final version.
No authors report a conflict of interest.
References
- 1.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–561. [PubMed] [Google Scholar]
- 3.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009;17:53–59. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 4.Wand H, Calmy A, Carey DL, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21:2445–2453. doi: 10.1097/QAD.0b013e3282efad32. [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGee DL Diverse Populations C. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prospective Studies C, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365:901–908. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Heart Lung and Blood Institute (NHLBI) The Evidence Report: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: 1998. NIH Publication No. 98-4083. [Google Scholar]
- 12.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 13.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and hypoadiponectinemia. Diabetes Care. 2007;30:113–119. doi: 10.2337/dc06-1075. [corrected] [DOI] [PubMed] [Google Scholar]
- 14.Koethe JR, Dee K, Bian A, et al. Circulating Interleukin-6, Soluble CD14, and Other Inflammation Biomarker Levels Differ Between Obese and Nonobese HIV-Infected Adults on Antiretroviral Therapy. AIDS Res Hum Retroviruses. 2013;29 doi: 10.1089/aid.2013.0016. 1091-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boodram B, Plankey MW, Cox C, et al. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women's Interagency HIV Study. AIDS Patient Care STDS. 2009;23:1009–1016. doi: 10.1089/apc.2009.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CY, Hogan JW, Snyder B, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37(Suppl 2):S69–S80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 17.Reinsch N, Neuhaus K, Esser S, et al. Are HIV patients undertreated? Cardiovascular risk factors in HIV: results of the HIV-HEART study. Eur J Prev Cardiol. 2012;19:267–274. doi: 10.1177/1741826711398431. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein KA, Armon C, Buchacz K, et al. Provider compliance with guidelines for management of cardiovascular risk in HIV-infected patients. Prev Chronic Dis. 2013;10:E10. doi: 10.5888/pcd10.120083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koethe JR, Hulgan T, Niswender K. Adipose Tissue and Immune Function: A Review of Evidence Relevant to HIV Infection. J Infect Dis. 2013;208:1194–1201. doi: 10.1093/infdis/jit324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan K, Harris DR, Monte D, et al. Obesity and dyslipidemia in behaviorally HIV-infected young women: Adolescent Trials Network study 021. Clin Infect Dis. 2010;50:106–114. doi: 10.1086/648728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.