Abstract
Background
Venous thromboembolism (VTE) has been recently recognized as a complication of sickle cell disease (SCD); however, the incidence of VTE in SCD is unknown.
Objectives
The primary objective of this study was to determine the incidence of first VTE, including pulmonary embolism (PE) and deep venous thrombosis (DVT), among SCD patients age ≥15 years. We also evaluated genotypic differences in VTE risk and determined the relationship between VTE and mortality.
Patients/Methods
In this retrospective cohort study, we used data from the Cooperative Study of Sickle Cell Disease to calculate incidence rates for first VTE. We used Cox proportional hazard models to estimate hazard ratios (HR) for time to VTE by genotype and time to death by VTE status.
Results
We included 1,523 SCD patients age ≥15 years with 8,862 years of follow-up in this analysis. The incidence rate for first VTE was 5.2 events/1000 person-years (95% confidence interval (CI) 3.8, 6.9) with a cumulative incidence of 11.3% (CI 8.3, 15.3) by age 40. Individuals with SS/Sβ0-thalassemia genotype had the highest rate of VTE (7.6 events/1000 person-years [CI 5.3, 10.6)]. The incidence of PE exceeded that of isolated DVT (3.6 [CI 2.5, 5.1] events/1000 person-years versus 1.6 [CI 0.9–2.7] events/1000 person-years), although this difference was not statistically significant. SCD patients with VTE had a higher mortality (adjusted HR 2.32 [CI 1.20, 4.46]) than those without VTE.
Conclusions
Patients with SCD are at substantial risk for VTE, and individuals with VTE are at higher risk of death than those without VTE.
Keywords: mortality, pulmonary embolism, sickle cell disease, venous thromboembolism, venous thrombosis
Introduction
Sickle cell disease (SCD) is a genetic disorder resulting in the production of abnormal hemoglobin (Hb S) that polymerizes in a concentration-dependent manner under conditions of stress and hypoxia.[1] Although the pathophysiology of clinical sequelae in SCD is multi-factorial, hypercoagulability and vasculopathy are being increasingly recognized as major contributors to well-defined complications of SCD, including childhood stroke, acute chest syndrome, and pulmonary hypertension.[2–5] Hemostatic abnormalities such as coagulation activation, endothelial dysfunction, and vaso-occlusion are all known to occur in SCD,[6–8] yet the risk of venous thromboembolism (VTE) in SCD has not been well-established. Few studies have shown a high overall prevalence of VTE among adult SCD patients and an increased risk of VTE among hospitalized SCD patients compared to controls, with a seemingly higher pulmonary embolism (PE) risk compared to deep vein thrombosis (DVT).[8–12] A similar paradoxical phenomenon in PE versus DVT risk has also been observed in sickle trait using a case-control design.[13]
The Cooperative Study of Sickle Cell Disease (CSSCD) was a multicenter observational study conducted with the primary goal of determining factors associated with morbidity and early mortality in SCD patients.[14] Significant insights into complications of SCD have been gained using the CSSCD data;[15–17] however, the risk of VTE using this database has not previously been explored.
The primary objective of this study was to determine the overall incidence rate of VTE, including PE and DVT, among individuals with SCD aged 15 and older in the CSSCD cohort. We also aimed to compare the hazard rate of VTE by SCD genotype and to determine the relationship of VTE and mortality among SCD patients.
Materials and methods
Setting
SCD patients from 15 clinical centers (23 hospitals) throughout the United States were recruited to participate in the CSSCD study from 1978–1982 and were followed until 1988, as has been previously described.[14] The study was designed as an observational cohort and did not incorporate any interventions into its protocol. CSSCD data is publically available from the National Heart, Lung, and Blood Institute (NHLBI) and has been de-identified according to NHLBI standards. This present research study using the CSSCD database was approved by the Johns Hopkins Institutional Review Board (IRB) and was deemed exempt from review.
Study population
Patients in the CSSCD were enrolled into one of 4 cohorts depending on their age at entry. We used only the subset of patients who were enrolled at age 15 or older, because the number of VTE events was negligible among younger patients and because this cut-off allows for comparison of incidence rates with other hereditary thrombophilias.[18, 19] Demographic information was collected on all patients upon enrollment. Genotype analysis was performed by the Centers for Disease Control and Prevention (CDC) using hemoglobin electrophoresis and quantitative chromatography. The majority of patients with hemoglobin SS also underwent alpha gene mapping to determine the presence of an alpha thalassemia mutation. Genotypic assignments were made by the CSSCD laboratory committee based on these results. Hematologic parameters including hemoglobin, reticulocyte count, and white blood cell (WBC) count were obtained during an initial baseline visit.
After enrollment, patients were followed annually and interim complications were recorded. Additionally, during hospitalizations for vaso-occlusive crisis, acute chest syndrome, pregnancy, or surgery, CSSCD investigators collected comprehensive information including radiographic results and discharge diagnoses.
Venous thromboembolism and genotype definitions
VTE events were not explicitly collected in the CSSCD except when associated with pregnancy. However, because ICD-9 diagnosis codes for interim events were collected during each annual visit and codes for discharge diagnoses were recorded for each acute event hospitalization, we were able to identify VTE events occurring during the study period.
The following ICD-9 diagnosis codes were used to define VTE: PE and infarction (415.1), thrombophlebitis of the lower extremities (451.0, 451.11, 451.19, 451.2, 451.81), thrombophlebitis of the upper extremities (451.82, 451.83, 451.84, 451.89), and thrombophlebitis of the unspecified veins (451.9, 453.79, 453.8, 453.9). In the setting of acute chest syndrome, we used additional criteria to identify cases of PE. Acute chest syndrome in the CSSCD was defined as: (1) a new pulmonary infiltrate on chest X-ray or other imaging and (2) pleuritic chest pain in the absence of a pulmonary infiltrate. In the latter case, a perfusion scan was required to demonstrate lung involvement. The ICD-9 code 415.1 was assigned to indicate a final diagnosis of pulmonary infarction or PE, if applicable. Because the term pulmonary infarction can be used to describe changes associated with acute chest,[20] we considered only patients without a lung infiltrate, but who by definition had a perfusion defect on lung scan, as having a VTE event. The date of VTE was assigned as the date of the annual visit for any interim VTE or as the date of discharge for any VTE that occurred during a hospitalization. Patients with a self-reported prior history of DVT on initial assessment were excluded. We categorized genotypes according to phenotypic similarity. The first category included genotypes SS and Sβ0-thalassemia, the second included patients with SS-α-thalassemia, and the third included patients with SC and Sβ+-thalassemia. Patients with rare or unknown genotypes were not included in our analysis.
Statistical analysis
The primary outcome measure was incidence of first VTE. Incidence rates were calculated for the entire cohort as well as by SCD genotype category and VTE type (PE versus DVT). Confidence intervals for incidence rates were calculated using a Poisson distribution assumption. To compare the risk of VTE between genotype categories, we used Cox proportional hazard models to estimate hazard ratios (HR) for time to VTE using the SS/Sβ0 patients as the comparison subgroup and adjusting for sex. We used age as our primary timescale for analysis since age represents a clinically-relevant time metric and allows for comparison to estimated incidence rates in other populations. Because we used age as the timescale, baseline age was not included in the adjusted hazard models. The proportional hazards assumption was tested by visual inspection of overlay of unadjusted predictions based on Cox regression and Kaplan-Meier survival curves, visual inspection of complementary log-log plots, and the Schoenfeld residuals test for proportionality. Time to death was analyzed using a time-dependent model assigning person-time according to VTE status. The HR for death comparing those with and without VTE was adjusted for sex and genotype. Kaplan-Meier curves for VTE risk by genotype and mortality risk by VTE status were generated. All significance tests were performed as 2-sided, and a p value of <0.05 was considered significant. All calculations were performed using STATA version 12.[21]
Results
Baseline characteristics
After excluding 108 patients with a recorded history of DVT, a total of 1,523 SCD patients age ≥ 15 with 8,862 person-years of follow-up were included for analysis. Among this cohort, 832 (54.6%) patients had a genotype of SS/Sβ0-thalassemia, 301 (19.8%) had SS-α-thalassemia, and 390 (25.6%) had SC/Sβ+-thalassemia. Baseline characteristics for the entire included cohort and by genotype category are shown in Table 1. The mean age at enrollment for the entire cohort was 25.9 years and 55.7% of participants were female. The mean laboratory values for the cohort were a hemoglobin of 9.5 g/dL, absolute reticulocyte count of 281.1 109/L, and WBC of 10.9 109/L. For the subgroup of patients with SC/Sβ+-thalassemia, hemoglobin levels were higher than the rest of the cohort with a mean value of 11.9 g/dL, and both mean absolute reticulocyte and WBC counts were lower with mean values of 173.2 109/L and 8.0 109/L, respectively. No participants had missing data for the demographic variables included in subsequent analyses; however, 156 individuals did have missing data on one or more of the baseline laboratory values reported in Table 1.
Table 1.
Characteristics of Sickle Cell Disease (SCD) patients, by Genotype
| Characteristic | Genotype |
|||
|---|---|---|---|---|
| All genotypes (n = 1523) |
SS/Sβ0- thalassemia (n = 832) |
SS-α-thalassemia (n = 301) |
SC/Sβ+- thalassemia (n = 390) |
|
| Age at baseline, Years (range) | 25.9 (15–66) | 25.0 (15–60) | 25.9 (15–61) | 27.7 (15–66) |
| Sex, No (%) | ||||
| Females | 848 (55.7) | 456 (54.8) | 164 (54.5) | 228 (58.5) |
| Males | 675 (44.3) | 376 (45.2) | 137 (45.5) | 162 (41.5) |
| Baseline laboratory values | ||||
| Hemoglobin, Mean (SD), g/dL | 9.5 (2.1) | 8.6 (1.5) | 8.9 (1.6) | 11.9 (1.6) |
| Reticulocyte percent, Mean (SD), % | 9.8 (6.9) | 12.5 (6.9) | 9.8 (5.6) | 4.1 (3.1) |
| Absolute retic count, Mean (SD), 109/L | 281.1 (17.4) | 327.8 (17.7) | 291.2 (15.1) | 173.2 (13.3) |
| WBC, Mean (SD), 109/L | 10.9 (3.9) | 12.2 (3.8) | 10.9 (3.2) | 8.0 (2.8) |
SD = standard deviation, WBC = white blood cell count
Venous thromboembolism incidence
After a mean of 5.9 years of follow-up, 46 first VTE events were identified during the study period, of which 35 occurred in patients with SS/Sβ0-thalassemia, 8 in patients with SS-α-thalassemia, and 3 in patients with SC/Sβ+-thalassemia. The mean age at VTE for the three genotypes was 27.8, 27.5 and 39.4 years, respectively.
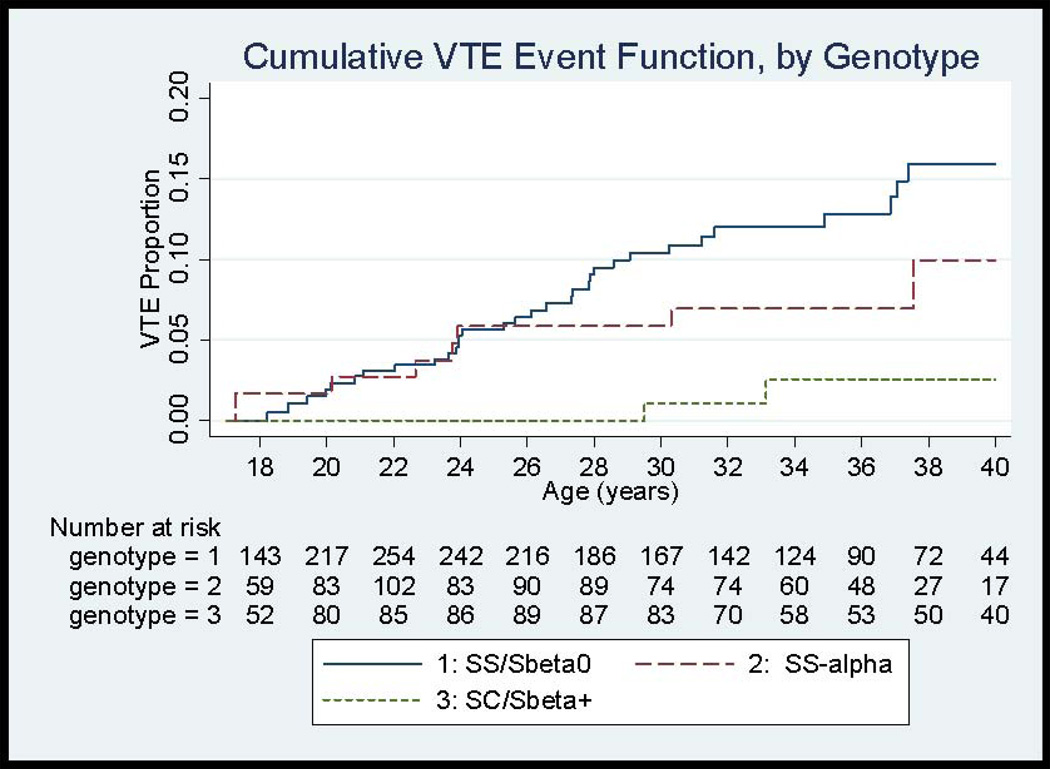
The incidence of first VTE among all genotypes was 5.2 events/1000 person-years (CI 3.8, 6.9) with a cumulative incidence of 7.4% (CI 5.2, 10.3) by age 30 and 11.3% (CI 8.3, 15.3) by age 40 (Figure 1, Table 2). The incidence rate for VTE from age 15–30 years in this study was 6.7 events/1000 person–years (CI 4.7, 9.2). However, the incidence rate for VTE did differ considerably by genotype. The highest incidence for VTE was observed for patients with SS/Sβ0-thalassemia, with an incidence rate of 7.6 events/1000 person-years (CI 5.3, 10.6) and cumulative incidence of 15.9% (CI 11.2, 22.3) by age 40. Individuals with concomitant α-thalassemia mutations (SS-α-thalassemia) had a lower incidence rate of VTE of 4.0 (CI 1.7–7.8). The lowest incidence was observed among patients with SC/Sβ+-thalassemia with an incidence rate of 1.3 (CI 0.3, 3.9) events/1000 person-years. The HR for first VTE, adjusted for sex, was lower for SC/Sβ+-thalassemia compared to SS/Sβ0-thalassemia patients [HR 0.18 (CI 0.05–0.58, p=0.004)] as was the adjusted HR for SS-α-thalassemia compared to SS/Sβ0-thalassemia [HR 0.52 (0.24–1.13), p=0.098)].
Figure 1.
Cumulative Event Functions for First Venous Thromboembolism (VTE) in SCD patients, by Genotype
Table 2.
Incidence Rates and Cumulative Incidence for First VTE, by Genotype
| Characteristic | Genotype |
|||
|---|---|---|---|---|
| All genotypes (n = 1523) |
SS/Sβ0- thalassemia (n = 832) |
SS-α-thalassemia (n = 301) |
SC/Sβ+- thalassemia (n = 390) |
|
| Duration of follow-up | ||||
| Mean (SD), years | 5.9 (2.2) | 5.6 (2.4) | 6.8 (1.4) | 5.8 (2.2) |
| Age at first VTE | ||||
| Mean (SD), years | 28.6 (8.4) | 27.8 (7.3) | 27.5 (9.3) | 39.4 (14.0) |
| Age range at first VTE, years | 17–55 | 18–47 | 17–45 | 30–55 |
| Incidence Rate, per 1000 person-years (CI) | ||||
| Total VTE events | 5.2 (3.8–6.9) | 7.6 (5.3–10.6) | 4.0 (1.7–7.8) | 1.3 (0.3–3.9) |
| PE events | 3.6 (2.5–5.1) | 5.4 (3.5–8.0) | 2.5 (0.8–6.0) | 0.9 (0.1–3.2) |
| DVT events | 1.9 (1.1–3.1) | 2.4 (1.2–4.3) | 2.0 (0.5–5.1) | 0.9 (0.1–3.2) |
| Cumulative Incidence (%, CI) | ||||
| by age 30 | 7.4 (5.2–10.3) | 10.4 (7.1–15.0) | 5.8 (2.4–13.7) | 1.1 (0.2–7.4) |
| by age 40 | 11.3 (8.3–15.3) | 15.9 (11.2–22.3) | 10.0 (4.5–21.4) | 2.5 (0.6–9.9) |
| Hazard Ratio (CI)* | -- | 1.00 | 0.52 (0.24–1.13) | 0.18 (0.05–0.58) |
Adjusted for sex. SD = standard deviation, CI = 95% confidence interval, DVT = deep venous thrombosis, PE= pulmonary embolism, VTE = venous thromboembolism
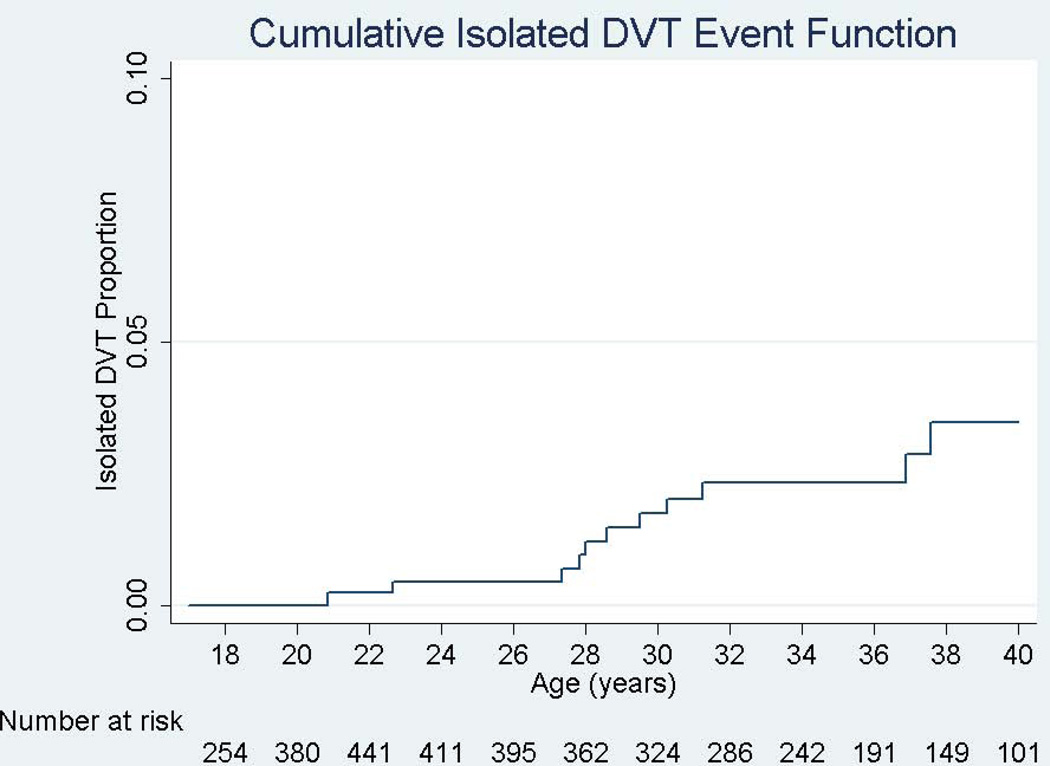
Incidence rates also differed by VTE type. The incidence for PE was higher than that of DVT for all genotypes, with an overall incidence rate for PE of 3.6 (CI 2.5–5.1) events/1000 person-years compared to 1.6 (CI 0.9–2.7) events/1000 person-years for isolated DVT (Figure 2), although the confidence intervals did overlap.
Figure 2.
Cumulative Event Functions for Pulmonary Embolism (PE) and Isolated Deep Venous Thrombosis (DVT) in SCD patients
Mortality
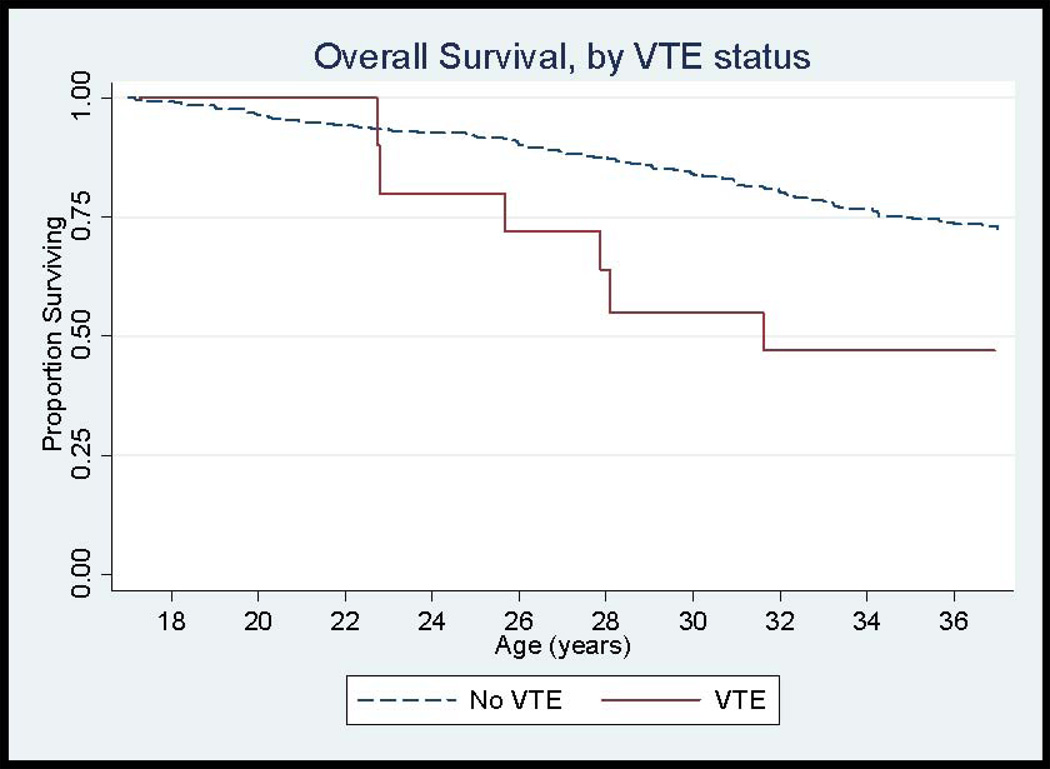
One hundred seventy-nine (11.8%) patients died during the follow-up period, 10 deaths of which were among SCD patients with VTE and the remaining 169 of which were in the non-VTE group. The overall survival curve by VTE status is shown in Figure 3. The mortality rate for individuals with a history of VTE was 6.4%/year compared to 1.9%/year for those without VTE (p=0.002). In patients with VTE who died during the follow-up period, the mean time to death was 3.3 years (range 0–6.8 years). Adjusting for sex and genotype, the HR for death was 2.32 (CI 1.20, 4.46) times higher for SCD patients with VTE compared to those without VTE.
Figure 3.
Overall Survival Curves in SCD patients, by Venous Thromboembolism Status
Discussion
SCD is associated with underlying hypercoagulability, yet VTE has only recently been recognized as a common complication of SCD. Using a longitudinal cohort study of SCD patients, with over 1,500 participants ≥ 15 years of age, we found that the incidence rate of VTE was 5.2 events/1000 person-years (CI 3.8, 6.9), with a rate of 6.7 events/1000 person-years (CI 4.7, 9.2) from ages 15–30 years. This incidence rate from ages 15–30 is nearly 4 times higher than reported rates in Factor V Leiden carriers (1.8–2.5/1000 person-years) in the same age stratum.[19, 22, 23] Although our study design differs from these thrombophilia studies since it relies on diagnosis codes for identification of VTE, the high incidence rate underscores the thrombotic risk starting at an early age in individuals with SCD.
We also demonstrate that the risk of VTE appears to be associated with genotype, a relationship that has been well-described in other complications of SCD such as vaso-occlusive crisis, leg ulcers, and stroke. This phenotypic variation in SCD is thought to be related to a complex interplay between hemolysis, vaso-occlusion, endothelial dysfunction, and hyperviscosity.[24] The highest incidence rate for VTE was observed among patients with SS/Sβ0-thalassemia, followed by SS-α-thalassemia, and lastly by SC/Sβ+-thalassemia. The absolute number of VTE events in each genotypic category, however, was small and longer follow-up is needed to fully define this relationship. Although percent hemoglobin S was not specifically recorded in this study, the observed genotypic variation may suggest a dose-dependent relationship between sickle hemoglobin and the risk of VTE and also suggests that underlying hemolysis and vasculopathy may play an important role in VTE risk in SCD. The findings from this study contrast from a previous report that demonstrated an increased prevalence of VTE among patients with SC/Sβ+-thalassemia compared to SS/Sβ0-thalassemia genotypes.[12] The difference in results may reflect age-related and mechanistic differences in VTE risk, as our current study represented a young cohort and the number of events in SC/Sβ+-thalassemia patients was low.
Interestingly, our study demonstrates a 2-fold higher incidence of PE compared to DVT in SCD patients. This increased risk of PE compared to DVT has been noted in previous reports of both SCD and sickle cell trait.[9, 13] While the difference in incidence rates was not statistically significant in this cohort, large longitudinal studies have observed the opposite phenomenon, with a nearly 2 fold higher incidence of DVT compared to PE in the general population.[25] Although the mechanism for an increased risk of PE in sickle hemoglobinopathies is not known, possibilities include an increased risk of embolization, increased symptoms with PE in SCD, or in situ pulmonary thrombosis formation.[11]
In addition, our study shows that the mortality rate for SCD patients with a history of VTE is significantly higher than for patients without VTE. We have described a similar relationship in cross-sectional study at our institution.[12] Sudden death accounted for a large number of deaths in the CSSCD, as has been previously reported.[26] The association of VTE with mortality, therefore, could represent a pathologic link between death and thrombosis in SCD. Alternatively, VTE may be a surrogate for severity of disease in SCD patients. Future studies will be required to elucidate the mechanism underlying this relationship.
A considerable strength of our current study is our ability to describe VTE incidence in a large prospective cohort of SCD patients. In addition, because the CSSCD cohort was designed for the collection of SCD-specific data, we were able to investigate the effect of genotype on VTE, which would not have been possible with an administrative database. Furthermore, the prospective design of the CSSCD allowed us to determine overall and PE and DVT-specific incidence rates, which have not previously been characterized, and will prove useful for the design of future prospective studies about VTE in SCD.
The major limitation of this study is we relied on ICD-9 codes to identify cases of VTE. We may have under-estimated PE incidence in our study by only including cases that were both coded with the PE ICD-9 code and were not associated with a pulmonary infiltrate. The diagnosis of PE in SCD is clinically challenging since pulmonary symptoms are common and are often attributed as manifestations of vaso-occlusive crisis or acute chest syndrome. In one study, pulmonary thrombosis of the central and segmental arteries diagnosed by computed tomography was demonstrated to occur in 17% of patients with acute chest syndrome with a pulmonary infiltrate. [5] Our definition of PE in this study, therefore, may have been conservative. However, the annual incidence rate for PE using this definition in our study was similar to the rate previously observed using a state-specific administrative database.[10] Other weaknesses include that VTE may not have been recorded in some cases because VTE was not explicitly collected during the study and that details about VTE and SCD-specific treatment at the time of VTE were not known. In addition, we did not have access to the outpatient or hospital records for VTE cases identified by ICD-9 code and, therefore, we could not directly verify the presence or anatomic location of the events or determine triggering factors for VTE. We would, however, expect recorded cases to be reliable given the events were being collected as part of a clinical study rather than for discharge billing.
In summary, our study indicates that the incidence rate of VTE among patients aged ≥ 15 years with SCD is higher than other common hereditary thrombophilias such as Factor V Leiden and that the incidence of PE is higher in SCD than that for DVT. Patients with SS/Sβ0-thalassemia appear to be at the highest risk of VTE. Mortality rates also appear to be higher among SCD patients with a history of VTE. Future studies are needed to better characterize the risk factors for VTE among patients with SCD and to determine which patients would most benefit from prophylactic anticoagulation.
Acknowledgments
Funding
R.P. Naik was supported by award 2K12HL087169-06. C. Haywood Jr. was supported by award 1K01HL108832-01 from the NHLBI. This manuscript was prepared using CSSCD Research Materials obtained from the NHLBI via BioLINCC (https://biolincc.nhlbi.nih.gov/).
Footnotes
Addendum
R. P. Naik and S. Lanzkron designed the study and interpreted the results. R. P. Naik analyzed the results. R. P. Naik, M. B. Streiff, C. Haywood Jr., J. B. Segal, and S. Lanzkron wrote and critically revised the manuscript.
Conflicts of Interest
B. Streiff reports personal fees from Boehringer-Inglheim, Daiichi-Sankyo, Janssen Healthcare, Sanofi-Aventis and Pfizer; and grants from Portola, outside the submitted work.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Tam DA. Protein C and protein S activity in sickle cell disease and stroke. J Child Neurol. 1997;12:19–21. doi: 10.1177/088307389701200103. [DOI] [PubMed] [Google Scholar]
- 3.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, Sohier C, Hinderliter A, Parise LV, Orringer EP. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93:20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 4.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekontso Dessap A, Deux JF, Abidi N, Lavenu-Bombled C, Melica G, Renaud B, Godeau B, Adnot S, Brochard L, Brun-Buisson C, Galacteros F, Rahmouni A, Habibi A, Maitre B. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2011;184:1022–1029. doi: 10.1164/rccm.201105-0783OC. [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Key NS. Hypercoagulability in sickle cell disease: New approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007:91–96. doi: 10.1182/asheducation-2007.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Lim MY, Ataga KI, Key NS. Hemostatic abnormalities in sickle cell disease. Curr Opin Hematol. 2013 Sep;20:472–477. doi: 10.1097/MOH.0b013e328363442f. [DOI] [PubMed] [Google Scholar]
- 8.Naik RP, Streiff MB, Lanzkron S. Sickle cell disease and venous thromboembolism: What the anticoagulation expert needs to know. J Thromb Thrombolysis. 2013;35:352–358. doi: 10.1007/s11239-013-0895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med. 2006;119:897, e7–e11. doi: 10.1016/j.amjmed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Novelli EM, Huynh C, Gladwin MT, Moore CG, Ragni MV. Pulmonary embolism in sickle cell disease: A case-control study. J Thromb Haemost. 2012;10:760–766. doi: 10.1111/j.1538-7836.2012.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Langevelde K, Flinterman LE, van Hylckama Vlieg A, Rosendaal FR, Cannegieter SC. Broadening the factor V leiden paradox: Pulmonary embolism and deep-vein thrombosis as 2 sides of the spectrum. Blood. 2012;120:933–946. doi: 10.1182/blood-2012-02-407551. [DOI] [PubMed] [Google Scholar]
- 12.Naik RP, Streiff MB, Haywood C, Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: A serious and under-recognized complication. Am J Med. 2013;126:443–449. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C, Hooper WC. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110:908–912. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 14.Gaston M, Rosse WF. The cooperative study of sickle cell disease: Review of study design and objectives. Am J Pediatr Hematol Oncol. 1982;4:197–201. [PubMed] [Google Scholar]
- 15.Smith JA, Espeland M, Bellevue R, Bonds D, Brown AK, Koshy M. Pregnancy in sickle cell disease: Experience of the cooperative study of sickle cell disease. Obstet Gynecol. 1996;87:199–204. doi: 10.1016/0029-7844(95)00367-3. [DOI] [PubMed] [Google Scholar]
- 16.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. the cooperative study of sickle cell disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- 17.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: Clinical presentation and course. cooperative study of sickle cell disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- 18.Lijfering WM, Brouwer JL, Veeger NJ, Bank I, Coppens M, Middeldorp S, Hamulyak K, Prins MH, Buller HR, van der Meer J. Selective testing for thrombophilia in patients with first venous thrombosis: Results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood. 2009;113:5314–5322. doi: 10.1182/blood-2008-10-184879. [DOI] [PubMed] [Google Scholar]
- 19.Heit JA, Sobell JL, Li H, Sommer SS. The incidence of venous thromboembolism among factor V leiden carriers: A community-based cohort study. J Thromb Haemost. 2005;3:305–311. doi: 10.1111/j.1538-7836.2004.01117.x. [DOI] [PubMed] [Google Scholar]
- 20.Charache S, Scott JC, Charache P. "Acute chest syndrome" in adults with sickle cell anemia. microbiology, treatment, and prevention. Arch Intern Med. 1979;139:67–69. [PubMed] [Google Scholar]
- 21.Abdul-Rauf A, Gauderer M, Chiarucci K, Berman B. Long-term central venous access in patients with sickle cell disease. incidence of thrombotic and infectious complications. J Pediatr Hematol Oncol. 1995;17(4):342–345. doi: 10.1097/00043426-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Middeldorp S, Meinardi JR, Koopman MM, van Pampus EC, Hamulyak K, van Der Meer J, Prins MH, Buller HR. A prospective study of asymptomatic carriers of the factor V leiden mutation to determine the incidence of venous thromboembolism. Ann Intern Med. 2001;135:322–327. doi: 10.7326/0003-4819-135-5-200109040-00008. [DOI] [PubMed] [Google Scholar]
- 23.Simioni P, Sanson BJ, Prandoni P, Tormene D, Friederich PW, Girolami B, Gavasso S, Huisman MV, Buller HR, Wouter ten Cate J, Girolami A, Prins MH. Incidence of venous thromboembolism in families with inherited thrombophilia. Thromb Haemost. 1999;81:198–202. [PubMed] [Google Scholar]
- 24.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 26.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]