Abstract
The entorhinal cortex (EC) is one of the first brain areas to display neuropathology in Alzheimer’s disease (AD). A mouse model which simulates amyloid-β (Aβ) neuropathology, the Tg2576 mouse, was used to address these early changes. Here we show EC abnormalities occur in 2–4 month-old Tg2576 mice, an age prior to β-amyloid deposition and where previous studies suggest that there are few behavioral impairments. First we show, using sandwich ELISA, that soluble human Aβ40 and Aβ42 are detectable in the EC of 2-month-old Tg2576 mice prior to β-amyloid deposition. We then demonstrate that 2–4 month-old Tg2576 mice are impaired at object placement, an EC-dependent cognitive task. Next we show that defects in NeuN expression and myelin uptake occur in the superficial layers of the EC in 2–4-month-old Tg2576 mice. In slices from Tg2576 mice that contained the EC, there were repetitive field potentials evoked by a single stimulus to the underlying white matter, and a greater response to reduced extracellular magnesium ([Mg2+]o), suggesting increased excitability. However, deep layer neurons in Tg2576 mice had longer latencies to antidromic activation than wild type mice. The results show changes in the EC at early ages, and suggest that altered excitability occurs before extensive plaque pathology.
Keywords: Entorhinal cortex, retrosplenial cortex, prefrontal cortex, object placement, NeuN, excitability
1. INTRODUCTION
Alzheimer’s disease (AD), the primary cause of dementia, is characterized by two types of neuropathology: the accumulation of β-amyloid plaque, caused by overproduction of amyloid-β (Aβ), and neurofibrillary tangles, caused by hyperphosphorylation of tau. Progressive memory impairment and neurodegeneration are also hallmarks of AD.
The hippocampal-entorhinal circuitry appears to be particularly vulnerable in AD (Braak and Braak, 1996; Morrison and Hof, 2002), and several studies suggest that the entorhinal cortex (EC) is one of the structures that is affected early in AD (Bobinski, et al., 1999; Braak and Braak, 1991; Braak and Braak, 1996; deToledo-Morrell, et al., 2007; Hof, 1997; Scharfman and Chao, 2013). The first neurons in the EC to deteriorate appear to be the stellate cells in layer II (Braak and Braak, 1985; Braak and Braak, 1991; Kordower, et al., 2001; Stranahan and Mattson, 2010), which form the perforant path projection to the dentate gyrus (DG). Based on experiments in mice, it has been suggested that Aβ and tau pathology originates in the EC and subsequently spreads to the hippocampus by a trans-synaptic mechanism (de Calignon, et al., 2012; Harris, et al., 2010; Lazarov, et al., 2002; Liu, et al., 2012).
These findings are important because they suggest that prevention of EC neuropathology could prevent or delay AD. However, it is unclear why the EC is vulnerable and why it is susceptible early in AD. To gain insight into these questions, we used the Tg2576 mouse model of AD neuropathology. The Tg2576 mouse uses the hamster prion promoter to overexpress human amyloid precursor protein (hAPP) 695 with the mutation of a Swedish family with AD (K670N/M671L), and progressive Aβ pathology occurs after 6 months of age (Hsiao, et al., 1996; Irizarry, et al., 1997; Kawarabayashi, et al., 2001; Lee and Han, 2013; Westerman, et al., 2002).
Notably, one study showed with immunohistochemistry that Aβ is detectable in the olfactory bulb by 3–4 months of age (Wesson, et al., 2010), suggesting that Aβ neuropathology develops earlier than 6 months of age. In fact, there is some evidence that structural and plasticity defects in the hippocampus occur in Tg2576 mice prior to 6 months of age: there was a decrease in spine density of granule cells (GCs), the principal cell of the DG, in 4-month-old mice (Jacobsen et al., 2006). There also was a deficit in long-term potentiation of the perforant path projection to the GCs (Jacobsen, et al., 2006). Also, in 3-month-old Tg2576 mice, there was a decrease in synaptic contacts and increase in synaptic length in stratum lacunosum-moleculare of CA1 (Balietti, et al., 2013). These changes could originate in the EC, because the perforant path projection to the DG was affected and there were defects in the area where the perforant path projects to CA1.
To address early changes in the EC in Tg2576 mice, we used mice that were younger than 4-months-old, timing most of the analyses for an age after puberty (2-months-old) to prevent puberty-associated changes from complicating the analysis. We also chose assays that are uncommon – but ones that we thought would be ideal to probe the EC – to determine if atypical approaches would better detect defects in the EC of young mice. For example, rather than using whole brain homogenate, we microdissected the EC and other regions and performed a sandwich enzyme linked immunosorbent assay (ELISA) to detect soluble human Aβ40 and Aβ42. In addition, instead of probing behavior with the Morris water maze, the object placement (OP) task was used to examine behavior because the EC is known to be involved in this task (Parron and Save, 2004; Steffenach, et al., 2005; Witter and Moser, 2006). Immunohistochemistry using an antibody to a neuronal nuclear antigen (NeuN), was also used because NeuN expression decreases with neurotrophin deficits in the EC (Duffy, et al., 2011). Other methods were also used, such as examining the uptake of myelin using histochemistry.
The behavioral and anatomical data suggested that there were impairments in the EC of Tg2576 mice at <4 months of age, so electrophysiological recordings from the EC were made in slices to gain more insight. We found evidence of increased excitability, supporting previous suggestions that epileptiform activity is a characteristic of mouse models of AD where familial mutations of hAPP are overexpressed (Noebels, 2011; Palop and Mucke, 2009). Furthermore, the data support the idea that defects that are relevant to AD occur in the EC (Chin, et al., 2007; Francis, et al., 2012). They suggest that impairments occur very early in life but they may require assays other than those that are commonly used to be detected.
2. METHODS
2.1 Animals
The experimental procedures were carried out in accordance with NIH guidelines, and were approved by the Institutional Animal Care and Use Committee at The Nathan Kline Institute. Tg2576 mice (Hsiao, et al., 1996) and wild type (WT) littermates were bred on a mixed C57BL/6 and SJLF1/J background (Wesson, et al., 2011; Wesson and Wilson, 2011). Mice were housed 3–4/cage, in standard mouse cages with corncob bedding and a 12-hr light-dark cycle (lights on, 7:00 a.m.). Food (Purina 5001 chow; W.F. Fisher, Somerville, N.J., U.S.A.) and water were available ad libitum. Different cohorts of mice were used for behavior, anatomy and electrophysiology. Male and diestrous female mice were pooled because differences were not detected (see Supplemental Material). However, for ELISA, only males were used.
2.2 ELISA
2.2.1 Microdissection
Following deep anesthesia by isoflurane inhalation (Aerrane; Baxter Healthcare Corporation, Deerfield, IL, U.S.A.), mice were rapidly decapitated and the brain was excised. The brain was placed ventral surface down, on a cold metal block covered with aluminum foil. Regions of the brain were isolated by microdissection using a razor blade and rapidly frozen at −80°C. In addition to the EC and hippocampus, the frontal area was isolated because of the relevance of this region to AD. The brainstem and cerebellum were included because they are areas that appear to develop β-amyloid plaque pathology late in the APP/presenilin 1 mouse models of AD neuropathology (Holcomb, et al., 1998; Wadghiri, et al., 2013) and in human AD (Serrano-Pozo, et al., 2011; Thal, et al., 2002). Therefore, we hypothesized that they might serve as negative controls.
The regions were microdissected with methods similar to those previously described (Chakraborty, et al., 2012). A coronal cut was made at the middle cerebral artery to isolate the frontal area. To isolate brainstem and cerebellum, coronal cuts were made immediately anterior and posterior to the cerebellum. Then a cut was made in the horizontal plane to separate the cerebellum from the underlying brainstem. The brainstem is defined here as the region underneath the cerebellum. The EC was isolated from the rest of the forebrain by sectioning the posterior part of the forebrain at a slight angle relative to the coronal plane. To remove the hippocampus, a curved spatula was inserted between the ventricular wall and the alveus and the hippocampus was “rolled out” from the overlying cortex.
It should be noted that this procedure did not isolate the EC and hippocampus perfectly. For example, some of the posterior hippocampus was sometimes included in the EC sample and parts of the cortical regions adjacent to the EC were sometimes included as well. Also, the sample called the “EC” did not include the most medial and ventral EC.
2.2.2 Tissue homogenization and diethylamine (DEA) extraction
The EC regions from 2 animals were pooled and weighed and the same was then done for the other regions. Ten percent (w/v) homogenates were prepared (in 250 mM sucrose, 20 mM Tris base, 1 mM EDTA, 1 mM EGTA and protease inhibitors) as previously described (Morales-Corraliza, et al., 2009; Schmidt, et al., 2012b). All chemicals were from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.) unless otherwise stated. A DEA solution (0.4% DEA, 100 mM NaCl) was used to extract Aβ from brain homogenates. Briefly, the DEA solution was mixed with the tissue homogenate in a 1:1 ratio, using a glass pestle and dounce. The samples were spun at 100,000 x g for 1 hr at 4°C. The supernatant was collected and neutralized with 0.5 M Tris-HCl, pH 6.8 (Morales-Corraliza, et al., 2009; Schmidt, et al., 2012b).
2.2.3 Aβ sandwich ELISA
Quantification of human Aβ40 and Aβ42 was performed using ELISA, as described in greater detail elsewhere (Morales-Corraliza, et al., 2009; Schmidt, et al., 2012a). Briefly, 96-well plates were coated with capture monoclonal antibodies specific for Aβ40 and Aβ42 (for more details see Schmidt, et al., 2012a). The plates were incubated overnight at 4°C. Non-specific binding was blocked using 1% B lock Ace Solution (Dainippon Pharmaceutical Co., Osaka, Japan) in phosphate buffered saline (PBS) for 4 hrs at room temperature. Standards were prepared from synthetic human Aβ40 and Aβ42 (American Peptide Co., Sunnyvale, CA, U.S.A.) and DEA-extracted samples were added to the plates diluted in ELISA capture buffer (20 mM NaH2PO4, 2 mM EDTA, 400 mM NaCl, 0.2% bovine serum albumin, 0.05% 3 CHAPS, 0.4% Block Ace Solution, 0.05% NaN3, pH 7.0) and incubated overnight at 4°C. Detection was with a horseradish peroxidase-conjugated anti-human Aβ monoclonal antibody (prepared as described in Schmidt, et al., 2012a); plates were developed using the TMB Microwell Peroxidase Substrate System (Kirkegaard and Perry Laboratories, MD, U.S.A.). Standards were prepared from synthetic human Aβ40 and Aβ42 (American Peptide Co., Sunnyvale, CA, U.S.A.) and incorporated into every run.
2.3 Behavior
Procedures used to test OP were similar to those used previously (Scharfman, et al., 2007; Skucas, et al., 2011). Mice were acclimatized to a new cage (46 cm × 24 cm; plexiglass; no bedding) for 2 consecutive days before the day of the OP test. On the testing day, 2 identical objects were placed in the new cage, also without bedding. The objects consisted of identical small clear glass jars (55 mm in diameter × 48 mm high; VWR, Radnor, PA, U.S.A.) or blue plastic toy bricks (32 mm wide × 64 mm long × 32 mm high, Lego, Billund, Denmark). During training (Fig. 2A; “train”), each mouse was allowed to explore the objects for 5 min. Each session was video-recorded using a dome camera (Apex, Allen, TX, U.S.A.) and software (Pinnacle Technologies, Lawrence, KS, U.S.A.). Exploration was defined as a behavioral state where the mouse touched one of the objects (with forepaws, nose or teeth), or sniffed the object (indicated by movement of the nostrils or whisking). The same investigator evaluated all mice to reduce possible inter-investigator variability, and was blinded. After the training phase the mouse was removed and placed back in its original cage. The objects and the field were cleaned with 30% ethanol (Pharmco-Aaper, Brookfield, CT, U.S.A) followed by water to remove any scent of ethanol. After 60 min, the mouse was placed in the cage again. One of the objects was located at the same distance from cage walls, but at the opposite side of the cage. The other object was placed in the same location where it was before. Locations of the objects were counterbalanced. Exploration was examined for a 5 min testing session (Fig. 2A; “test”). Prior to the test we verified that the 2 objects were matched for saliency by showing, in both Tg2576 and WT mice, that an equivalent amount of time was spent exploring each object (see Results below). As noted previously (Ennaceur and Delacour, 1988), performance was considered normal when mice spent significantly more time exploring the moved (novel) object compared to the stationary (familiar) object in the test trial.
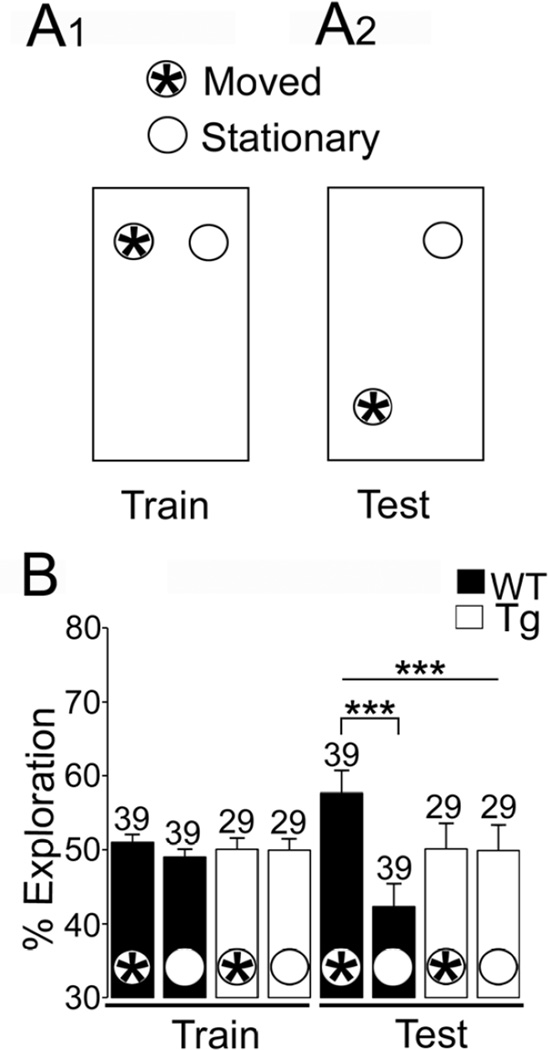
Fig. 2. Impaired object placement (OP) performance in young Tg2576 mice.
A. A schematic of the behavioral procedure is shown.
1. During an initial training session (Train) animals were placed in a cage containing 2 identical objects at one end of the cage. After 5 min they were returned to their home cage for 1 hr.
2. A schematic of a test session (Test) is shown. One object was moved (demarcated with a 5-pointed star) to the opposite end of the cage. After 5 min the mouse was removed.
B. Performance during Train and Test sessions is shown for wild type (WT; black) and Tg2576 (Tg; white) mice. During the Train session there were no differences in the percent of time spent exploring each object. However, during the Test session, WT animals explored the moved object more than the object in the familiar position (black bars with asterisks) but Tg2576 mice did not. In this and all subsequent figures, sample sizes are above the bars; single, double and triple asterisks reflect p<0.05, p<0.005 and p<0.001 respectively.
2.4 Anatomy
2.4.1 Perfusion-fixation
Mice were deeply anesthetized by isoflurane inhalation (Aerrane; Baxter Healthcare Corporation, Deerfield, IL, U.S.A.) followed by urethane (2.5 g/kg i.p.). A 26 gauge needle was inserted into the heart, followed by perfusion with a peristaltic pump (Minipuls 1, Gilson, Middleton, WI, U.S.A) of 10 ml of 0.9% NaCl in double-distilled water (ddH20), followed by 40 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed immediately and post-fixed for 24 hrs in 4% paraformaldehyde in 0.1 M PB at 4°C.
2.4.2 General preparation of tissue sections
After post-fixation, brains were hemisected and each hemisphere was cut (one in the coronal plane, the other in the horizontal plane) into 50 µm-thick sections using a vibratome (TPI 3000, Vibratome Co., St. Louis, MO, U.S.A.). Sections were collected sequentially so that, together with landmarks in the sections such as major nuclei and large myelinated pathways, it was possible to compare equivalent rostro-caudal, or dorso-ventral levels across animals.
2.4.3 Immunohistochemistry - NeuN
An antibody against a neuronal nuclear antigen, NeuN, was used to evaluate neurons because NeuN labels the nucleus of normal mature neurons (Mullen, et al., 1992). It should be noted that reduced expression of NeuN often occurs in neurons after insults or injury (e.g., aging, disruption of neurotrophin signaling, ischemia, toxicity; Buckingham, et al., 2008; Duffy, et al., 2011; Hayakawa, et al., 2008; Kadriu, et al., 2009; Matsuda, et al., 2009; Portiansky, et al., 2006; Won, et al., 2009). It has been reported that weak NeuN immunoreactivity (NeuN-ir) is caused by phosphorylation of the NeuN antigen in response to adverse conditions (Lind, et al., 2005) which leads to reduced ability of the NeuN antibody to recognize the antigen. Based on these findings and others, it has been suggested that weak NeuN-ir can be used as a marker of neurons which have been damaged but are not dead (Duffy, et al., 2011).
For each animal, one hemisphere was cut coronally and the other was cut in the horizontal plane. One section out of every 6 was selected. Unless otherwise stated, all washes and dilutions were performed in 0.1 M Tris buffer. In addition, all incubations were carried out at room temperature with continuous agitation on a rotator, and were followed by 3 washes in 0.1 M Tris buffer for 5 min. Similar to methods used previously (Duffy, et al., 2013), sections were incubated in 1% hydrogen peroxide for 2 min, to block endogenous peroxides. Sections were then incubated for 10 min, first in Tris A (0.1% Triton X-100 in 0.1 M Tris buffer), followed directly by an incubation in Tris B for 10 min (0.1% Triton X-100 and 0.005% bovine serum albumin in 0.1 M Tris buffer). Sections were blocked in 10% normal horse serum (Vector Laboratories, Burlingame, CA, U.S.A.) for 1 hr, to minimize non-specific binding of immunoreagents. The sections were incubated for 24 hrs in the primary antiserum (NeuN; 1:10,000; Clone A60, MAB 377, Chemicon, Temecula, CA, U.S.A.) followed by a 10 min wash in Tris A and a subsequent 10 min wash in Tris B. The sections were then incubated in the secondary antibody (horse anti-mouse; 1:400; Vector Laboratories) for 1 hr and subsequently washed for 10 min in Tris A followed by 10 min in Tris B. For signal amplification, the avidin-biotin-horseradish peroxidase complex (ABC; Vectastain Elite Kit, Vector Laboratories) method was used. Sections were incubated in ABC, diluted 1:100 in Tris B, for 2 hrs. Immunoreactivity was visualized using 3,3-diaminobenzidine (DAB). Sections were incubated in a solution containing; 0.022% DAB, 0.2% ammonium chloride, 0.1% glucose oxidase, and 0.8% D(+)-glucose in 0.1 M Tris buffer. To ensure that labeling was detected if it was present, sections were allowed to incubate with DAB for a long enough time that the background had a light level of staining. The reaction was stopped by washing sections in 0.1 M Tris buffer. The sections were mounted on 0.1% gelatin-coated slides, dehydrated in a graded series of alcohols (70%, 3 min; 95%, 3 min; 100%, 2×5 min) and xylene (2 × 5 min), and coverslipped with Permount (Fisher Chemical Co., Fair Lawn, NJ, U.S.A.).
Sections were examined using a brightfield microscope (BX61, Olympus, Center Valley, PA, U.S.A.), and photographed using a digital camera (RET 2000R–F-CLR-12, Q Imaging, Surrey, BC, Canada). Quantification of NeuN-ir was performed using Bioquant software (Bioquant Image Analysis Corporation, Nashville, TN, U.S.A.), as previously described (Duffy, et al., 2011). Briefly, the edges of each section were demarcated using a digital tool and areas displaying weak cortical NeuN-ir were evaluated by computerized thresholding. For each animal, both hemispheres were rendered in 3-D based on the z-coordinates of all sections from a given hemisphere. A surface-rendering tool (Topographer Plug-in, Bioquant Image Analysis Corporation) was used to construct a virtual skin over the 3-D image using a mesh modeling algorithm. This procedure resulted in images where areas containing weak NeuN-ir could be quantified as a fraction of the total volume of the hemisphere.
2.4.4 Myelin histochemistry
Sections were stained for myelin with iron-hematoxylin, using a modification of Mahon’s method (Jebb and Woolsey, 1977). Following hydration for 1 hr in ddH2O, free-floating sections were mordanted for 30 min in a 2.5% solution of iron ammonium sulfate (dissolved in ddH2O). After washes in ddH2O (3 × 5 min), sections were stained for myelin by incubating the sections in a 1% alcoholic hematoxylin/0.075% lithium carbonate solution for 1 hr. Myelinated fiber tracts such as the corpus callosum were stained dark blue. The sections were washed in ddH2O (3 × 5 min), mounted and dehydrated as described above.
2.4.5 Toluidine blue staining of semi-thin sections
Sections were prepared as described above, except 2% glutaraldehyde was added to the perfusion medium. Sections were post-fixed for 1 hr in 1% osmium tetroxide (Electron Microscopy Sciences; Fort Washington, PA, U.S.A.) in 0.1M PB, dehydrated in ethanol and cleared using propylene oxide (Electron Microscopy Sciences). Sections were embedded in Epon (Electron Microscopy Sciences) and polymerized in a 60°C oven for 48 hrs, as previously described (Duffy, et al., 2011; Duffy, et al., 2009). Semi-thin sections (1 or 2 µm) were cut using a TPI 3000 vibratome (Vibratome Co., St. Louis, MO, U.S.A.) and stained with 0.2% toluidine blue (dissolved in a 2.5% solution of sodium carbonate in ddH2O) as previously described (Sloviter, et al., 1996).
2.5 Electrophysiology
2.5.1 Slice preparation
Following deep anesthesia with isoflurane, mouse brains were excised and placed in ice-cold oxygenated (95% O2/5% CO2) sucrose-based artificial cerebrospinal fluid (sucrose-ACSF; containing, in mM: 252.0 sucrose, 5.0 KCl, 2.4 CaCl2, 2.0 MgSO4, 26.0 NaHCO3, 1.25 NaH2PO4, 10.0 D-glucose) for approximately 60 sec. One hemisphere was glued using cyanoacrylate (Krazy glue, Elmer’s Products Inc., Westerville, OH, U.S.A.) to the stage of a tissue slicer (HM 650 V, Microm International GmbH, Walldrof, Germany) and 400 µm-thick slices were cut in the horizontal plane while immersed in ice-cold ACSF. Slices were immediately placed in sucrose-ACSF at room temperature, and transferred by a wide-bore pipette to a nylon mesh in an interface-style recording chamber where slices were submerged in pre-heated (30°C) oxygenated (95% O2, 5% CO2) sucrose-ACSF except for their upper surfaces (Scharfman, et al., 2001; www.healthresearch.org/technology-transfer/brain-and-tissue-slice-recording). Slices were maintained at 30–32°C using a temperature controller with feedback (PTCO3, Scientific Systems Design, Ontario, Canada). Perfusion (∼1 ml/min with a peristaltic pump, Minipuls 2, Gilson) was switched 30 min after slices were placed in the recording chamber from sucrose-ACSF to NaCl-based ACSF (NaCl-ACSF; 126 mM NaCl instead of sucrose but otherwise the same as sucrose-ACSF), which was used for the rest of the experiment. After 30 min perfusion with NaCl-ACSF, recordings were initiated.
2.5.2 Recording and stimulation
Procedures were similar to those previously published (Skucas, et al., 2013; Skucas, et al., 2011). For recordings, 3–5 MΩ electrodes were made from borosilicate glass with a capillary in the lumen (1.0 mm outer diameter, 0.75 mm inner diameter, World Precision Instruments, Sarasota, FL, U.S.A.), pulled horizontally (Model P87, Sutter Instruments, Novato, CA, U.S.A.) and filled with NaCl-ACSF. Recordings were amplified (Axoclamp 2B, Molecular Devices, Sunnyvale, CA, U.S.A.), digitized (Digidata 1440A, Molecular Devices), and acquired using pClamp (Molecular Devices).
For stimulation, a monopolar stimulating electrode made from Teflon-coated stainless steel wire (diameter, including Teflon, 75 µm; A–M Systems, Carlsborg, WA, U.S.A) was controlled using a stimulus isolation unit (IsoFlex, AMPI Instruments, Jerusalem, Israel) that generated current pulses (100 µA), and pClamp, which allows digital control of stimulus duration (10–100 µsec) and frequency (0.05 Hz). The electrode was placed under visual control (Stemi SV6, Carl Zeiss, Thornwood, NY, U.S.A) on the angular bundle, at the border of the white matter and layer VI of the most medial part of the medial EC (MEC).
Slices were not accepted unless recordings in layer III exhibited a field potential in response to stimulation, because intracellular recordings have shown that few neurons are detected in superficial layers when no field potential is detected there (HES, unpublished). Stimulus strength was chosen to evoke a 7 mV antidromic population spike in layer VI because this stimulus was sufficient to activate most deep layer neurons, based on intracellular recordings (HES, unpublished). Paired pulse stimulation was triggered with an interstimulus interval of 40 msec because this interstimulus interval elicits robust paired pulse facilitation in hippocampus (Skucas, et al., 2013).
2.5.3 Current source density (CSD) analysis
Methods were similar to those described previously (Skucas et al., 2013). The depth of the recording electrode tip in a given slice was determined by a micromanipulator with precision to the nearest micron (Leica Microsystems, Buffalo Grove, IL, U.S.A.) and was the same for all recording sites, typically 50 µm. Recordings were made at ∼30 µm intervals along a tangent to the pia that intersected the stimulation site (Croll, et al., 1999; Scharfman, 1996).
2.5.4 Exposure to NaCl-ACSF containing 0 mM [Mg2+]o
Slices were exposed to a reduced concentration of [Mg2+]o by switching NaCl-ACSF containing 2 mM MgSO4 to NaCl-ACSF containing nominal 0 mM MgSO4 (0 Mg2+-ACSF). Responses were recorded sequentially in each layer with a fixed stimulus (stimulus frequency ∼0.017 Hz), starting in layer VI and continuing along a tangent to the white matter until reaching the pia. This laminar profile was conducted before and 20–40 min after the onset of exposure to the 0 Mg2+-ACSF.
Previous studies have shown, in the rat, that spontaneous epileptiform activity occurs in the EC after exposure to 0 Mg2+-ACSF for 15 min (or less), using a similar perfusion system and tissue chamber (Scharfman and Ofer, 1997). However, in slices from WT mice we found that spontaneous field potentials did not occur until after 40 min exposure to 0 Mg2+-ACSF. Presumably the species/strain, which is partly C57BL6/J (a strain known to be resistant to epilepsy; Schauwecker, 2011) was the reason that a long exposure to 0 Mg2+-ACSF was required to induce spontaneous epileptiform activity. Therefore, evoked responses could be monitored before, and 20–40 min after exposure to 0 Mg2+-ACSF without confounding spontaneous epileptiform discharges.
For the experiments described above, recordings were made sequentially throughout the EC before and after 0 Mg2+-ACSF. To be sure that the slight differences in recording positions before and after 0 Mg2+-ACSF did not influence the results, we also used another procedure with a fixed recording position.
2.6 Data analysis
Analysis was conducted by an investigator who was blinded to genotype. For sandwich ELISA, data are reported as fmol of Aβ/g wet brain, based on standard curves for synthetic human Aβ40 and Aβ42. For OP, the time an animal spent exploring each object for the first 5 min of the training and testing sessions was measured from video. Exploration of each object is expressed as a percentage of the total time spent exploring both objects. For NeuN-ir, the threshold for NeuN-ir was defined as the mean NeuN-ir of nuclei in the GC layer of the DG, which always displayed robust NeuN-ir. The threshold was set so that the background around GCs did not meet threshold; only the level of NeuN-ir in GCs met the threshold. Field potential analysis was performed using Clampfit (Version 10.3, Molecular Devices). The area under the curve (AUC) was defined as the integrated area of the negative portion of the field potential (diagrammed in the Results). The amplitude of the antidromic population spike in layer VI was defined as the difference between the prestimulus baseline and the peak of the population spike. The latency of the field excitatory postsynaptic potential (fEPSP) was defined as the time from the middle of the stimulus artifact to the negative peak of the fEPSP. For CSD, the CSD was calculated offline using MATLAB (Mathworks, Natick, MA, U.S.A.) by the second spatial derivative of the local field potential, using a 3-point formula, as previously published (Skucas, et al., 2013).
All data are presented as mean ± standard error of the mean (SEM) and p<0.05. Statistical comparisons were made using two-tailed Student’s t-test (Microsoft Excel 2007, Microsoft Corporation), Fisher’s exact test or analysis of variance (ANOVA; Statview v5.0.1, SAS Institute, Cary, NC, U.S.A.), with Bonferroni/Dunn post-hoc test.
3. RESULTS
The average age of all mice used in this study was 2.9 ± 0.04 months (range 1.4 – 3.8 months; n=151). There was no significant age difference between WT (3.0 ± 0.05 months, n=69) and Tg2576 mice (2.8 ± 0.06 months, n=82; Student’s t-test, p=0.089).
3.1 Aβ in young Tg2576 mice
We first determined if there were detectable levels of Aβ in Tg2576 mice at 2 months of age. Notably, previous studies showed that β-amyloid plaque is not detected until at least 6 months (Hsiao, et al., 1996; Irizarry, et al., 1997; Kawarabayashi, et al., 2001; Westerman, et al., 2002). One study of Tg2576 mice did show that Aβ was detected with immunohistochemistry in the olfactory bulb at 3–4 months of age, but the levels of Aβ that were present in the EC, prefrontal cortex (PFC) or adjacent regions were almost undetectable in that study (Wesson, et al., 2010).
We conducted immunohistochemistry using a different antibody to Aβ (6E10, Covance, Princeton, NJ, U.S.A.; see Supplemental Methods for details), as well as thioflavin-s staining (to examine β-amyloid plaques). The average age for the Tg2576 mice used with the antibody to Aβ was 3.9 ± 0.1 months (range 3.4 – 4.4 months, n=10). For thioflavin-s, these animals as well as additional animals were used (mean age, 3.4 ± 0.1 months; range 2.9–4.4, n=32). For both stains, 8 sections were used throughout the dorso-ventral axis of each animal (300 µm apart). As expected, no labeling could be detected with these methods (Suppl. Fig. 1). However, labeling by the Aβ antibody and thioflavin-s was extensive at older ages (average age: 12.9 ± 1.4 months; range 10.6 – 16.4 months, n=5; Suppl. Fig. 1), suggesting that the methods used were adequate for detection.
We also used sandwich ELISA to assess the levels of human Aβ40 and Aβ42 in the EC and other regions of young Tg2576 mice. The average age of mice used for ELISA was 2.1 ± 0.09 months (n=10). We detected Aβ40 and Aβ42 in all regions examined (Fig. 1). Differences in concentration (fmol of Aβ/g wet brain) were not significant (one-way ANOVAs, Aβ40: F4,20=0.49, p=0.745; Aβ42: F4,20=1.13, p=0.370). There were no regional differences in the ratios of Aβ40/Aβ42 (one-way ANOVA: F4,20=0.27, p=0.893; Fig. 1). Therefore, there were detectable levels of Aβ40 and Aβ42 in all the regions tested, even at 2 months of age, whereas β-amyloid plaque was not detected.
Fig. 1. Regional analysis of human Aβ40 and Aβ42 levels in 2-month-old Tg2576 mice.
A. The levels of Aβ40 and Aβ42 are shown in fmol/g of wet brain region (n=10 mice; 2 mice/group). EC = entorhinal cortex; HC = hippocampus; FA = frontal areas; BS = brainstem; CB = cerebellum. Regional differences were not significant. For this and all other figures, statistical comparisons are provided in detail in the text.
B. The ratios of Aβ40/Aβ42 are shown for each region. Regional differences were not significant.
3.2 Young Tg2576 mice display early deficits in memory for object location
For OP, the average age of the mice was 3.0 ± 0.03 months (range 2.6 – 3.7 months, n=68). There was no significant age difference between WT (3.0 ± 0.04 months, n=39) and Tg2576 mice (3.0 ± 0.05 months, n=29; Student’s t-test, p=0.986). The mice used for behavior were distinct from those used for anatomy and electrophysiology (described below) but ages were comparable (Suppl. Fig. 2).
During the training phase of the OP task, a two-way ANOVA showed that there was no effect of genotype (F1,128=1.12, p=0.292; Fig. 2B). There was no interaction of factors (genotype x object location: F1,128=0.63, p=0.427). Therefore, both WT and Tg2576 mice performed well in the training phase, i.e., they spent a similar amount of time exploring each object.
In the test phase, a two-way ANOVA showed that there was an effect of genotype (F1,128=0.00007, p<0.001) and a significant interaction between genotype and object location (F1,128=5.41, p=0.0216). Post-hoc analysis showed that there was an adverse effect of genotype (p<0.01; Fig. 2B), i.e., Tg2576 mice did not distinguish novel and familiar object locations well.
3.3 Weak NeuN-ir in young Tg2576 mice
The mean age of animals used for anatomical studies was 3.1 ± 0.04 months (range 2.9 – 3.6 months, n=22). There was no significant age difference between WT (3.1 ± 0.1 months, n=10) and Tg2576 mice (3.2 ± 0.05, n=12 months; Student’s t-test, p=0.575).
Areas of weak NeuN-ir were observed in Tg2576 mice in the EC, especially in superficial layers (Fig. 3). When NeuN labeling was weak, cells were either very lightly labeled with the NeuN antibody, or there was no detectable immunoreactivity (Fig. 3). In previous studies, this pattern of weak NeuN-ir corresponded to pyknotic cells using cresyl violet staining and the pyknotic cells that lacked NeuN-ir were neurons based on electron microscopic examination (Duffy, et al., 2011). In the present study, findings were similar. For example, when there was an area of weak NeuN-ir (e.g., Fig. 5), the adjacent section (Suppl. Fig. 3) showed numerous pyknotic cells in that same area using cresyl violet. We also labeled sections adjacent to those used for NeuN-ir with an antibody to glial fibrillary acidic protein (GFAP) and counterstained with cresyl violet to determine if GFAP labeled profiles were pyknotic. GFAP labeled cells appeared to be normal, not pyknotic (Suppl. Fig. 3). Taken together the data suggest that the pyknotic cells were likely to be neurons, not astrocytes.
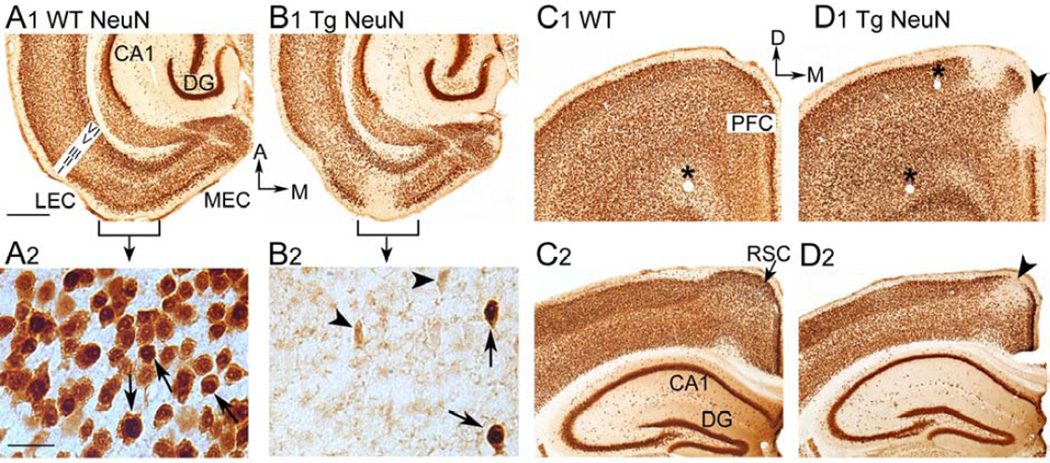
Fig. 3. Weak immunoreactivity (ir) for the neuronal nuclear antigen NeuN in the EC, retrosplenial cortex (RSC) and prefrontal cortex (PFC) of Tg2576 mice.
A. 1. A horizontal section containing the EC from a WT mouse, labeled using an antibody to NeuN illustrates normal NeuN-ir. The area of the lateral EC (LEC) indicated by the bracket is expanded in A2. MEC = medial EC; CA1 = area CA1; DG = dentate gyrus; A = anterior; M = medial.
2. Neurons with normal NeuN-ir (arrows) have neuronal nuclei that are densely stained, and lighter cytoplasmic labeling is also present.
B. 1. A NeuN-labeled horizontal section at a similar dorso-ventral level to the one shown in A1 demonstrates weak NeuN-ir in the superficial layers of part of the LEC of a Tg2576 mouse. The area of weak NeuN-ir that is expanded in B2 is indicated by a bracket.
2. Part of layer II/III of the LEC (indicated by the bracket in B1) is shown at higher magnification. Many cells show no NeuN-ir or weak NeuN-ir (arrowheads) whereas other cells have normal NeuN-ir (arrows).
C. 1. A coronal section from the PFC of a WT mouse shows normal NeuN-ir. D = dorsal. An asterisk demarks a blood vessel.
2. A coronal section from a WT mouse shows normal NeuN labeling in the hippocampal formation and RSC.
D. 1. A NeuN-labeled coronal section at a similar rostro-caudal level to the one in C1 shows weak NeuN-ir, primarily in the superficial layers of the PFC (arrowhead) of a Tg2576 mouse.
2. A NeuN-labeled coronal section at a similar rostro-caudal level to the one in C2 shows weak NeuN-ir in the superficial layers of the RSC (arrowhead).
Calibration in A1 (500 µm) is for all images except A2 and B2. Calibration in A2 (50 µm) is for A2 and B2.
Fig. 5. Weak NeuN-ir corresponds to areas of abnormal myelin staining in the EC and PFC of Tg2576 mice.
A. 1. A horizontal section from a WT mouse showing NeuN-ir in the EC. 2–3. An adjacent section stained for myelin. The area marked by a bracket in A2 is shown at higher power in A3. Arrows point to normal staining of myelinated fibers.
B. 1. A section from a Tg2576 mouse at a similar dorso-ventral level to A demonstrates weak NeuN-ir (arrowhead) in the superficial layers of a part of the EC.
2. An adjacent section stained for myelin shows a patch of dark myelin stain in the area where there was weak NeuN-ir. The area, marked with a bracket, is shown at higher power in B3.
3. Myelin-stained cells are marked by arrowheads.
C. 1. The PFC is shown in a coronal section from a WT mouse, labeled using an antibody to NeuN.
2. An adjacent section stained for myelin is shown. Myelinated fibers are stained (arrow). Blood vessels are marked by asterisks. The area marked with a bracket is shown at higher power in C3.
D. 1. A coronal section from a Tg2576 mouse at a similar dorso-ventral level to the one shown in C demonstrates weak NeuN-ir (arrow), primarily in the superficial layers.
2. An adjacent section stained for myelin shows that there is myelin stain in the location corresponding to weak NeuN-ir. The area marked with a bracket is shown at higher power in 3.
3. Cells stained with myelin are labeled by arrowheads.
E. 1. A toluidine blue-stained semi-thin section from a WT mouse is shown, illustrating normal staining. Arrows point to somata.
2. A toluidine blue-stained semi-thin section from a Tg2576 mouse, adjacent to a section with weak NeuN-ir. Arrowheads point to cells with abnormal morphology. Calibration in A2 (500 µm) is for all images except A3-D3 and E1–2. Calibration in A3 (100 µm) is for A3-D3. Calibration in E1 (50 µm) is for E1–2.
In addition to the EC, the retrosplenial cortex (RSC), and PFC also showed areas of weak NeuN-ir in Tg2576 mice (Figs. 3–4). A two-way ANOVA with brain region (EC or RSC+PFC) and genotype as factors showed that there was a significant effect of genotype (F1,37=11.77, p=0.002) with Tg2576 mice exhibiting the greater defect in NeuN expression (post-hoc test, p<0.01; Fig. 4C). There was no effect of region (F1,37=2.11, p=0.155; Fig. 4C), and no interaction of factors (F1,37=1.34, p=0.255), indicating that both regions showed loss of NeuN-ir in Tg2576 mice and one was not statistically greater than the other.
Fig. 4. Quantification of NeuN-ir in Tg2576 mice.
A. 1. Schematic illustrations of coronal sections adapted from Paxinos and Watson (2007).
2. NeuN-ir in the forebrain of a WT mouse. All sections from the WT animal used for Fig. 3 were merged to create a 3-D image. Orientation is indicated by the axes shown above A2 (D = dorsal; P = posterior; M = medial). Areas of robust NeuN-ir are shown in gray. Areas of NeuN-ir that did not meet a threshold intensity (see Methods) are considered areas of weak expression and are shown in color (pink = EC and adjacent areas, blue = RSC+PFC). Calibration in A2 (1 mm) is for A2–3 and B2–3.
3. NeuN-ir in the forebrain of the Tg2576 mouse in Fig. 3.
C. The percentage of NeuN-ir that did not meet threshold intensity is shown for WT (black bars) and Tg2576 mice (white bars). Differences between genotypes were significant for the total and also the regions (EC, RSC+PFC).
In contrast, there was no evidence of weak NeuN-ir in the hippocampal pyramidal cell layers or the granule cell layer of the DG of young Tg2576 mice (Fig. 3). Notably, NeuN-ir was reduced in the hippocampal cell layers in older Tg2576 mice (12 months; Suppl. Fig. 4). These data suggest that early defects in NeuN-ir occur in the EC before the hippocampus, and ultimately both areas develop reduced NeuN-ir.
3.4 Abnormal myelin staining of neurons in the EC of young Tg2576 mice
The same animals that were used for anatomical studies were used to examine myelin stain. As shown in Fig. 5, there were cells in the EC and frontal cortex of young Tg2576 mice that were densely labeled by the myelin stain (Fig. 5B, D) despite the lack of myelin stain in other areas of the section. Within the EC, the myelin-stained cells were primarily in the superficial layers (Fig. 5B). The locations within the superficial layers in which the myelin-stained cells were present corresponded to areas where there was weak NeuN-ir in adjacent sections (Fig. 5). Of the 12 Tg2576 mice, 9 mice exhibited myelin uptake (92%). Of the ten WT mice, 2 animals exhibited myelin uptake in EC cells (20%), which was significantly different from Tg2576 mice (9/12 vs. 2/10; Fisher’s exact test, p=0.030).
To determine the type of cell labeled by myelin stain, we examined 1 µm-thick toluidine blue-stained ultrathin sections in a subset of Tg2576 mice (n=4; Fig. 5E). The only cells that appeared to be abnormal were neurons: they were densely stained with toluidine blue, and typically had an invaginated nucleus and cytoplasmic vacuoles (Fig. 5E).
3.5 Repetitive field potentials in the EC of Tg2576 mice
The data described above suggested that there were anatomical abnormalities in the EC of Tg2576 mice. To examine whether physiological abnormalities also were present, we used slices containing the EC. The average age of all mice used for these experiments was 2.7 ± 0.1 months (range 1.4 – 3.8 months, n=35). There was no significant age difference between WT (2.7 ± 0.2 months, n=16) and Tg2576 mice (2.7 ± 0.2 months, n=19; Student’s t-test, p=0.995).
As shown in Fig. 6C, the initial portion (0–10 msec) of the evoked responses to white matter stimulation in WT mice had similar components as the normal adult rat or mouse, as previously described (Croll, et al., 1999; Scharfman, 1996), consistent with similar circuitry in rats and mice (Moser, et al., 2010; van Groen, et al., 2003). Thus, stimulation of the underlying white matter led to a large antidromic population spike using a recording site in deep layers (Fig. 6A). Afterwards, i.e., with a greater delay following the stimulus, there was a smaller fEPSP in the superficial layers (Fig. 6B–C).
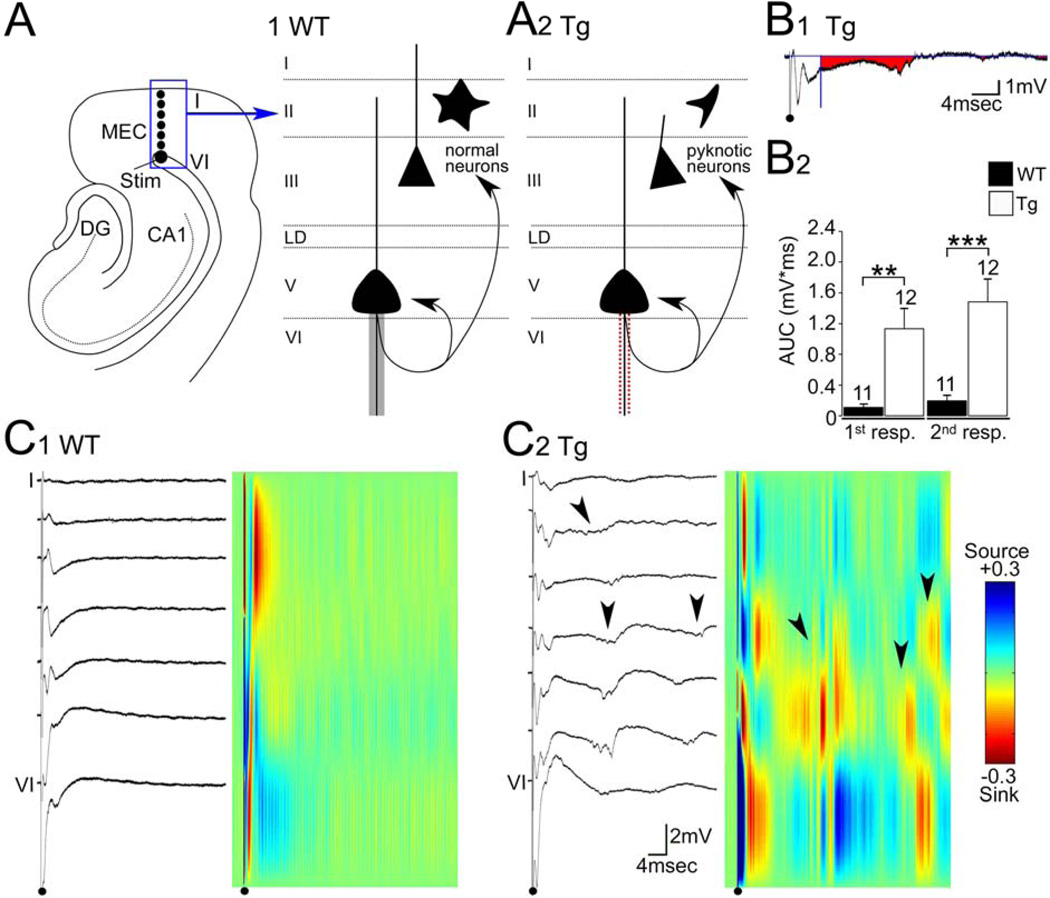
Fig. 6. Laminar analysis of field potential recordings in the EC.
A. Left: A diagram of the EC and hippocampus in a horizontal section illustrates the stimulation site (stim; large black circle), and typical sites used to record (smaller black circles). The stimulation site was in the angular bundle (AB) on the border of layer VI of the most medial part of the MEC. Recordings for current source density (CSD) analysis were made along an axis perpendicular to the white matter, from layer VI to the pia. Not all recording sites are shown.
1. A schematic diagram of the layers of the EC in a WT mouse, showing the location of cells and the inputs to deep and superficial layers.
2. A schematic diagram illustrates abnormalities in a young Tg2576 mouse. Pyknotic neurons are present in superficial layers, and a defect related to axons is schematically depicted in deep layer neurons, reflecting slower latencies of antidromic population spikes (see Fig. 7).
B. 1. A representative field potential recorded from layer III of a Tg2576 mouse. In this and subsequent figures, the black dot marks the stimulus and the stimulus artifact is truncated. A horizontal blue line is used to mark the baseline potential (0 mV). A vertical blue line at 6 msec represents the point where area under the curve (AUC) measurement began. This time point was selected to avoid the early components of the evoked response, which did not reflect hyperexcitability, and were typically over by 6 msec. The area used for measurements is shown in red.
2. AUC was measured for responses to paired stimuli (100 msec interval). The AUC of both the first and second response were significantly different in Tg2576 vs. WT mice.
C. 1. Left: For a slice of a WT mouse, representative field potentials are shown. Responses were elicited by a fixed stimulus eliciting a 7 mV antidromic population spike in layer VI, and were recorded sequentially in 7 locations spanning the layers of the EC. Right: Corresponding CSD.
2. Left: For a slice of a Tg2576 mouse, repetitive potentials (arrowheads) are shown. The stimulus was similar to the one used for the WT recordings.
Right: Corresponding CSD. Sinks (red) were located in layers III-VI (arrowheads). Calibration for CSD: +0.3 (sink) to −0.3 (source) mV/mm2.
In contrast to WT mice, there were repetitive field potentials evoked by a pair of stimuli in the Tg2576 mice (Fig. 6C), suggesting that there was hyperexcitability. Measurements of the area corresponding to the repetitive potentials as shown in Fig. 6B confirmed that there was a greater area in Tg2576 mice. In fact, WT mice had almost no detectable area, suggesting normal excitability (WT area: 0.11 ± 0.04 mV*ms, n=11; Tg2576 area: 1.13 ± 0.26, n=12; Student’s t-test, p=0.002). Tg2576 mice also exhibited a greater area when the responses to the second stimulus of a pair were compared (WT area: 0.20 ± 0.07 mV*ms; Tg2576 area: 1.48 ± 0.3 mV*ms; Student’s t-test, p<0.001).
When measurements were made of the fEPSP recorded in layer III in response to single stimuli (Fig. 6–7), there was a longer latency in Tg2576 mice compared to WT (WT: 1.66 ± 0.10 msec, n=17; Tg2576: 2.56 ± 0.21 msec, n=27; Student’s t-test, p=0.002). The latency to the peak of the antidromic population spike, recorded in layer VI, was also longer in Tg2576 mice compared to WT (WT: 0.67 ± 0.04 msec, n=17; Tg2576: 0.83 ± 0.36; Student’s t-test, p=0.002; Fig. 7). There did not appear to be any difference in the stimulus strength needed to evoke a large antidromic population spike (7 mV; WT: 40.94 ± 3.23 µsec; Tg2576: 39.17 ± 3.36 µsec; Student’s t-test, p=0.706), suggesting specificity of the latency effect.
Fig. 7. Analysis of orthodromic and antidromic responses in layers III and VI of the EC.
A. Left: A diagram of the stimulation (STIM) and recording sites (layer III and layer VI) similar to Fig. 6A.
Right: Representative field potentials demonstrating the measurements used for latency in part C. Measurements were made from the middle of the stimulus artifact to the point of greatest amplitude in the orthodromic response recorded in layer III (C1) and antidromic population spike recorded in layer VI (C2).
B. 1–2. Representative field potentials from a WT (black line) and Tg2576 mouse (red line), in layer III (1) and layer VI (2).
C. 1–2. Bar graphs show the latency to the layer III orthodromic (1) and layer VI antidromic (2) response. WT: black bars; Tg2576: white bars. The field potentials were significantly longer in latency in Tg2576 mice.
3.6 Greater effect of ACSF containing 0 mM Mg2+ in Tg2576 compared to WT mice
The repetitive field potentials evoked by a single stimulus in Tg2576 mice were similar to previous recordings in the EC in response to manipulations that produce epileptiform discharges in the EC (Heinemann, et al., 2000; Lucke, et al., 1995; Nagao, et al., 1996; Scharfman and Ofer, 1997) such as a low concentrations of extracellular magnesium ([Mg2+]o), which is typically attributed to the removal of the Mg2+-dependent block of the NMDA receptor (Heinemann, et al., 2000; Jones and Lambert, 1990). Therefore, we examined the EC after adding ACSF containing 0 mM Mg2+.
We predicted that the EC of Tg2576 mice would be more susceptible to seizure activity because single stimuli evoked repetitive responses in Tg2576 mice even under normal conditions. In addition, hAPP mice are susceptible to seizures (Minkeviciene, et al., 2009; Noebels, 2011; Palop, et al., 2007; Scharfman, 2012). The results supported this hypothesis.
In these experiments, evoked responses to white matter stimuli were recorded 20–40 min after the normal ACSF containing 2 mM Mg2+ was replaced with ACSF containing 0 mM Mg2+. The stimulus strength that was used was chosen so that it elicited a large amplitude (7 mV) antidromic population spike in layer VI. A large spike was chosen based on the assumption that the EC circuitry would be activated in a robust manner if deep layer neurons were activated strongly.
After 20 min exposure to 0 mM Mg2+ ACSF, there was a very large effect in the Tg2576 mice compared to WT mice (Fig. 8B). The area of the field potential corresponding to repetitive potentials was quantified as shown in Fig. 6B. A two-way ANOVA with genotype and condition (pre or post 0 mM Mg2+) as factors showed that there was a significant effect of genotype (F1,42=22.78, p<0.0001) and an interaction of factors (F1,42=6.71, p=0.013) with the Tg2576 mice showing the greater area (post-hoc analysis for genotype, p<0.0001; Fig. 8B). These results indicate that decreasing [Mg2+]o had a greater effect in Tg2576 mice. The same result was obtained when the response to the second stimulus of a pair was analyzed (genotype: F1,42=21.23, p<0.001; genotype x condition: F1,42=5.78, p=0.008; post-hoc analysis for genotype, p<0.0001).
Fig. 8. Laminar analysis of field potential recordings in the EC with nominal 0 mM [Mg2+]o in the artificial cerebrospinal fluid (ACSF; 0 Mg2+-ACSF).
A. 1–2. Representative field potentials (left) and CSD analysis (right) from a WT (1) and Tg2576 mouse (2), 20–40 min after exposure to 0 Mg2+-ACSF. Calibration: +0.3 to −0.3 mV/mm2. Recordings were made using a stimulus that evoked a 7 mV antidromic population spike in layer VI. Repetitive activity in layer III corresponds to long latency current sinks in the CSD (right; black arrowhead).
B. 1. AUC of responses recorded in layers III of WT (black bars) and Tg2576 mice (white bars) 20 min after exposure to 0 Mg2+-ACSF. Tg2576 mice had greater area.
2. A bar graph of the latency to evoked and spontaneous epileptiform activity in slices from WT (black bars) and Tg2576 mice (white bars) after changing normal ACSF to 0 Mg2+-ACSF. Tg2576 mice had a shorter latency to evoked epileptiform activity but there was no significant difference in the latency to spontaneous epileptiform activity.
To determine the part of the EC circuitry that might be responsible for the repetitive field potentials in Tg2576 mice, sequential recordings were made in each layer of the EC (see Methods). CSD analysis showed that sinks corresponding to the repetitive potentials were primarily located in deep layer III (Fig. 8B), implicating synapses in that layer. Therefore, we recorded in layer III throughout the experiment, so that the recording position would be in the location of the sink for the entire experiment. Different mice were used for these experiments (n=15). The results were the same when the first stimulus was examined: there was a significant effect of genotype (F1,26=36.46, p<0.0001), genotype x condition interaction (F1,26=5.01, p=0.034) and post-hoc analysis for genotype showed the greatest effect in Tg2576 mice (p<0.0001; Fig. 8B). The results were also the same for the response to the second stimulus of each pair (genotype, F1,26=34.10, p<0.0001; genotype x condition, F1,26=6.45, p=0.016; post-hoc analysis for genotype, p<0.0001). Therefore, in EC slices, layer III of Tg2576 mice showed greater hyperexcitability after exposure to 0 Mg2+-ACSF than WT mice.
We also measured the latency between the onset of exposure to 0 Mg2+-ACSF and the time when fEPSPs in layer III increased. For this measurement, the number of consecutive fEPSPs evoked by a single stimulus was monitored. In slices of WT mice, which normally exhibited only 1 fEPSP/stimulus, the latency was measured to the time when 2 fEPSPs occurred in succession. In Tg2576 mice, which could exhibit >1 consecutive fEPSP/stimulus before 0 mM Mg2+-ACSF, the latency was measured to the time when an additional fEPSP was elicited. The latency was shorter in Tg2576 mice (WT: 44 ± 3 min, n=11; Tg2576: 16 ± 3 min, n=12; Student’s t-test, p<0.001; Fig. 8B). However, there was no difference in the latency to onset of spontaneous epileptiform activity in layer III (WT: 54 ± 4 min; Tg2576: 54 ± 7 min; Student’s t-test, p=0.254; Fig. 8B).
4. DISCUSSION
The results demonstrate that abnormalities develop in the EC of Tg2576 mice at extremely early ages using assays that are sensitive to EC impairments such as OP, NeuN expression, myelin uptake, and recordings from the EC in slices. Thus, young Tg2576 mice had impaired OP, weak EC NeuN-ir, EC cells showed myelin uptake, and evoked responses in the EC exhibited abnormal excitability. The alterations in the EC developed when human Aβ40 and Aβ42 were detected but before β-amyloid plaque pathology.
These observations suggest that one of the earliest areas of the brain to become impaired in the Tg2576 mouse is the EC – reminiscent of human AD. These studies are important because they provide validation for the mouse model; i.e., the Tg2576 mouse EC resembles the EC in human AD in having early impairments, which was not previously clear. These similarities make the Tg2576 mouse possible to use to clarify mechanisms of EC vulnerability that could be relevant to clinical AD. Another implication is that AD may begin earlier than previously considered, an idea that has been proposed before (Balietti, et al., 2013; Francis, et al., 2012; Jacobsen, et al., 2006; Reiman, et al., 2012).
The results also showed that additional brain regions were affected. These areas, the RSC and PFC, are interesting in light of the evidence that they are affected clinically in AD also (DeKosky and Scheff, 1990; Hof, et al., 1990; Minoshima, et al., 1997; Nestor, et al., 2003).
4.1 Human Aβ40 and Aβ42 in Tg2576 mice at 2 months of age
Previous studies have shown that levels of human Aβ40 and Aβ42 in whole brain homogenates are detectable at 2 months of age and increase significantly between 7–12 months of age in Tg2576 mice (Holcomb, et al., 1998; Jacobsen, et al., 2006). Here we show that human Aβ40 and Aβ42 levels are detected at 2 months of age in subregions rather than the whole brain. We did not detect significant differences between the regions that we examined. The data are consistent with the idea that Aβ40 and Aβ42 contribute to impairments in mouse models of AD before β-amyloid plaque is detected (Balietti, et al., 2013; Jacobsen, et al., 2006; Palop, et al., 2007; Wesson, et al., 2010; Wykes, et al., 2012). Our results show the average regional percentage of Aβ42 (Aβ42/totalAβ=12.35 ± 0.66%) was similar to data from whole brain homogenates in 2-month-old Tg2576 mice (Jacobsen, et al., 2006). The results suggest that by 2 months of age most areas of the Tg2576 mouse brain have detectable Aβ, and the abnormalities in the EC are not necessarily due to a rise in Aβ in the EC specifically.
4.2 Early behavioral deficits exist in Tg2576 mice
In previous studies, it has been suggested that Tg2576 mice exhibited deficits in tests of spatial memory after 6 months of age (Hsiao, et al., 1996; King, et al., 1999; Westerman, et al., 2002), when β-amyloid plaques becomes prominent (Hsiao, et al., 1996; Lee and Han, 2013). Our results suggest that when a task of spatial memory is used that is dependent on the EC, such as OP, impairments can be detected earlier. These data are consistent with studies showing that object recognition (OR) – also dependent on the EC in part - is impaired relatively early (2 months) in the TgCRND8 mouse, where β-amyloid deposition occurs earlier (3 months) than the Tg2576 mouse (Francis, et al., 2012).
We cannot be sure that the behavioral impairments were dependent entirely on defects in the EC. One reason is that the task does not only depend on the EC. There is likely to be a requirement for the olfactory bulb, which is relevant because there are impairments very early in life in olfactory bulb circuitry in Tg2576 mice (Cao, et al., 2012). These early defects in the olfactory bulb could directly influence behavior and also exert an indirect influence because the olfactory bulb is a major input to the EC (Burwell and Amaral, 1998; Canto, et al., 2008; Luskin and Price, 1983; Room, et al., 1984; Wouterlood and Nederlof, 1983). Furthermore, the inputs from the olfactory bulb terminate in the superficial layers of the EC (Burwell and Amaral, 1998; Luskin and Price, 1983) - the layers where we saw the greatest NeuN and myelin abnormalities. Regardless, the results have the intriguing clinical implication that tasks like OP or OR (Francis, et al., 2012) could be early cognitive tasks that identify patients who will develop AD (Hort, et al., 2007; Iachini, et al., 2009; Kessels, et al., 2010).
4.3 Weak NeuN-ir and myelin uptake at early ages in Tg2576 mice
Weak NeuN-ir has been observed in rodents after diverse types of insults or injuries (Buckingham, et al., 2008; Duffy, et al., 2011; Kadriu, et al., 2009; Matsuda, et al., 2009; McPhail, et al., 2004; Portiansky, et al., 2006; Unal-Cevik, et al., 2004; Won, et al., 2009; Wu, et al., 2010). The mechanism that causes reduced NeuN-ir appears to be phosphorylation of NeuN (Lind, et al., 2005). In neurons with weak NeuN-ir, it has been suggested that oxidative stress plays a role, because of an increase in superoxide dismutase (Matsuda, et al., 2009), and another study suggests there is an increase in an enzyme that catalyzes the production of reactive oxygen species (Won, et al., 2009).
The idea that some kind of injury develops in neurons of the Tg2576 mouse with weak NeuN-ir is consistent with the demonstration that there are signs of oxidative and mitochondrial damage early in AD, before widespread accumulation of β-amyloid plaques (Gibson, et al., 2010; Keller, et al., 2005; Leuner, et al., 2007; Mancuso, et al., 2010; Swerdlow and Khan, 2004; Young and Bennett, 2010). In the EC specifically, it has been shown that the α-subunit of mitochondrial adenosine triphosphate-synthase is damaged before clinical signs of AD (Terni, et al., 2010). In studies of the PFC, increases in markers of oxidative stress have been correlated with an increase in severity of AD symptoms (Ansari and Scheff, 2010; Korolainen, et al., 2006). In the RSC, a decrease in mitochondrial activity was observed in patients with a genetic risk of AD, before there were any symptoms of AD (Valla, et al., 2001; Valla, et al., 2010). Increased Aβ levels could play a role, because Aβ42 induces oxidative stress in neuronal cell cultures (De Felice, et al., 2007; Figueiredo, et al., 2013). However, it has also been suggested that Aβ42 has antioxidant effects at low concentrations (Butterfield, 2002; Butterfield and Bush, 2004; Greenough, et al., 2013; Hensley, et al., 1994; Sutherland, et al., 2013).
It is not known why the EC, RSC and PFC are more vulnerable than other regions in AD, but one suggestion is that these areas have a high metabolic rate (Hevner, et al., 1995; Hevner and Wong-Riley, 1992; Mutisya, et al., 1994). Another suggestion is that the neurons in the EC in AD are vulnerable to loss of neurotrophic support which occurs in AD (Connor, et al., 1997; Duffy, et al., 2011; Nagahara, et al., 2009; Peng, et al., 2005; Phillips, et al., 1991; Scharfman and Chao, 2013). A loss of neurotrophic support could play a role in the reduction in NeuN-ir because a transgenic mouse with a reduction in a critical protein in neurotrophin signaling, ankyrin rich membrane spanning protein (ARMS/Kiddins2) exhibited weak NeuN-ir in the EC (Duffy, et al., 2011; Scharfman and Chao, 2013). Interestingly, the ARMS+/− mouse had a reduction in NeuN preferentially in the EC, RSC and PFC (Duffy, et al., 2011) like the EC, RSC and PFC of the Tg2576 mouse (Fig. 3).
Myelin staining of cells in the EC is interesting in light of observations made in the cortex of mice where myelin stained cells following severe continuous seizures (status epilepticus; Carpentier, et al., 2012). This finding raises the possibility that increased neuronal activity causes myelin uptake. If so, the hyperexcitability we detected in EC slices could be related to the myelin uptake we found. However, the hyperexcitability in EC slices that we recorded was much weaker neuronal activity than status epilepticus.
4.4 Increased excitability in the EC of young Tg2576 mice
There are many potential factors that could contribute to increased excitability in the EC of young Tg2576 mice, before widespread β-amyloid deposition. Aβ may play a role, because adding synthetic Aβ to hippocampal slices increases fEPSP slope in responses to electrical stimulation in area CA1 (Puzzo, et al., 2008). APP may also play a role because APP has been shown to increase NMDA surface expression (Cousins, et al., 2009; Hoe, et al., 2009). This effect is particularly interesting because it could explain the increased sensitivity of the EC of young Tg2576 mice to 0 mM Mg2+-ACSF.
Also, there is evidence of increased caspase-3 activity in cholinergic neurons in the pedunculopontine tegmentum (PPT) at only 2 months of age in Tg2576 mice (Zhang, et al., 2005). The PPT projects to the EC, via the basal forebrain, and the EC receives the densest cholinergic input of the cortex (Mesulam, et al., 1983; Newman, et al., 2012; Rye, et al., 1987). Dysfunction of cholinergic input to the EC could lead to alteration in firing properties of EC neurons because in 4-month-old Tg2576 mice, there was impaired spike frequency adaptation (Marcantoni, et al., 2013), which normally is supported by Kv7 potassium channels/M current; M current is mediated by muscarinic receptor activation. Weak spike frequency adaptation in EC principal neurons due to cholinergic dysfunction and impaired M current would be likely to increase firing, contributing to increased excitability.
4.5 Increased latency to evoked responses in the EC of Tg2576 mice
In slices from the EC of Tg2576 mice we observed a longer latency in evoked responses. This observation could be due to abnormalities in sodium channels and axonal conduction because the longer latency was observed in an antidromic potential – not requiring synaptic transmission. Because the stimulus intensity that was required to elicit a fixed amplitude, large (7 mV) antidromic spike was not influenced by genotype, sodium channel defects seem less likely to explain the result than a defect of another kind related to axonal structure and function. This idea is consistent with previous findings in mouse models of AD neuropathology showing defects in myelin and axonal function (e.g., transport, Bartzokis, 2004; Stokin and Goldstein, 2006; Sun, et al., 2005). However, internalization or other defects in sodium channels have been shown (Corbett, et al., 2013; Scharfman, 2012; Verret, et al., 2012). Notably, we cannot exclude the possibility that longer latencies – and other impairments in Tg2576 mice described above – were due to neurodevelopmental abnormalities caused by effects of overexpressed mutant hAPP on development. Indeed, abnormal wiring in the olfactory bulb has been observed in Tg2576 mice as early as postnatal day 10 (Cao, et al., 2012).
4.6 Implications
Characterizing the early changes in AD can potentially lead to better opportunities for intervention by targeting the most sensitive and earliest aspects of the disease. In addition, treatment early in AD, when extensive pathology has not yet developed, may be most effective. Our results support the hypothesis that one of the locations that is affected first is the EC, making it an important area to target.
Supplementary Material
HIGHLIGHTS.
Entorhinal cortical (EC) impairments occur at 2–4 months of age in Tg2576 mice.
Object placement, a task that involves the EC, was impaired in young Tg2576 mice.
NeuN immunoreactivity and myelin uptake were abnormal in the EC of young Tg2576 mice.
The EC showed abnormal excitability in slices of young Tg2576 mice.
ACKNOWLEDGEMENTS
We thank Thomas Radman and Charlie Schroeder for implementation of current source density analysis. This study was supported by the Alzheimer’s Association, New York Office of Mental Health, the NYU Langone Medical Center of Excellence Seed Grant program, NIH MH-084215 and P01 AG017617.
ABBREVIATIONS
- A
anterior
- Aβ
amyloid-β
- AB
angular bundle
- ACSF
artificial cerebrospinal fluid
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- APP
amyloid precursor protein
- ARMS
ankyrin repeat membrane spanning protein
- ABC
avidin-biotin-horseradish peroxidase complex
- AUC
area under the curve
- BS
brainstem
- CB
cerebellum
- CHAPS
3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate hydrate
- CSD
current source density
- D
dorsal
- DAB
3,3-diaminobenzidine
- ddH2O
double-distilled H2O
- DEA
diethylamine
- DG
dentate gyrus
- EC
entorhinal cortex
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- ELISA
enzyme linked immunosorbent assay
- FA
frontal area
- fEPSP
field excitatory postsynaptic potential
- GC
granule cell
- GFAP
glial fibrillary acidic protein
- hAPP
human APP
- HC
hippocampus
- Hz
hertz
- ir
immunoreactivity
- LEC
lateral EC
- M
medial
- MEC
medial EC
- [Mg2+]o
concentration of extracellular magnesium
- M
medial
- mon
month
- mV
millivolt
- MΩ
megaohm
- NeuN
neuronal nuclear antigen
- OP
object placement
- OR
object recognition
- P
posterior
- PFC
prefrontal cortex
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PPT
pedunculopontine tegmentum
- RSC
retrosplenial cortex
- SEM
standard error of the mean
- stim
stimulus
- Tg
transgenic
- Tris
tris(hydroxymethyl)aminomethane
- V
ventral
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no actual or potential conflicts of interest to disclose.
REFERENCES
- Ansari M, Scheff S. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balietti M, Giorgetti B, Casoli T, Solazzi M, Tamagnini F, Burattini C, Aicardi G, Fattoretti P. Early selective vulnerability of synapses and synaptic mitochondria in the hippocampal CA1 region of the Tg2576 mouse model of Alzheimer's disease. J Alzheimers Dis. 2013;34:887–896. doi: 10.3233/JAD-121711. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MAC, De Santi S, Wegiel J, Tarshish C, Saint Louis L, Wisniewski H. MRI of entorhinal cortex in mild Alzheimer's disease. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. On areas of transition between entorhinal allocortex and temporal isocortex in the human brain. Normal morphology and lamina-specific pathology in Alzheimer's disease. Acta Neuropathol. 1985;68:325–332. doi: 10.1007/BF00690836. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Bush AI. Alzheimer's amyloid β-peptide (1–42): involvement of methionine residue 35 in the oxidative stress and neurotoxicity properties of this peptide. Neurobiol Aging. 2004;25:563–568. doi: 10.1016/j.neurobiolaging.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Schrank BR, Rodriguez S, Benz EG, Moulia TW, Rickenbacher GT, Gomez AC, Levites Y, Edwards SR, Golde TE, Hyman BT, Barnea G, Albers MW. Aβ alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo . Nat Commun. 2012;3:1009. doi: 10.1038/ncomms2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier P, Foquin A, Dorandeu F. A new use for an old method: The Woelcke myelin stain for counting degenerating neurons in the brain of mice following status epilepticus. Neurotoxicology. 2012;33:789–795. doi: 10.1016/j.neuro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Magagna-Poveda A, Parratt C, Umans JG, MacLusky NJ, Scharfman HE. Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil (PTU) Endocrinology. 2012;153:1311–1316. doi: 10.1210/en.2011-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, Bender A, Mucke L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer's disease. J Neurosci. 2007;27:2727–2733. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Mol Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Corbett BF, Leiser SC, Ling HP, Nagy R, Breysse N, Zhang X, Hazra A, Brown JT, Randall AD, Wood A, Pangalos MN, Reinhart PH, Chin J. Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2013;33:7020–7026. doi: 10.1523/JNEUROSCI.2325-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SL, Hoey SE, Anne Stephenson F, Perkinton MS. Amyloid precursor protein 695 associates with assembled NR2A- and NR2B–containing NMDA receptors to result in the enhancement of their cell surface delivery. J Neurochem. 2009;111:1501–1513. doi: 10.1111/j.1471-4159.2009.06424.x. [DOI] [PubMed] [Google Scholar]
- Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, Wiegand SJ, Rudge JS, Scharfman HE. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz David H, Kopeikina Kathy J, Pitstick R, Sahara N, Ashe Karen H, Carlson George A, Spires-Jones Tara L, Hyman Bradley T. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer's disease. Prog Brain Res. 2007;163:741–753. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Schaner MJ, Chin J, Scharfman HE. Expression of c-fos in hilar mossy cells of the dentate gyrus in vivo. Hippocampus. 2013;23:649–655. doi: 10.1002/hipo.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy AM, Schaner MJ, Wu SH, Staniszewski A, Kumar A, Arevalo JC, Arancio O, Chao MV, Scharfman HE. A selective role for ARMS/Kidins220 scaffold protein in spatial memory and trophic support of entorhinal and frontal cortical neurons. Exper Neurol. 2011;229:409–420. doi: 10.1016/j.expneurol.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy AM, Zhou P, Milner TA, Pickel VM. Spatial and intracellular relationships between the α7 nicotinic acetylcholine receptor and the vesicular acetylcholine transporter in the prefrontal cortex of rat and mouse. Neuroscience. 2009;161:1091–1103. doi: 10.1016/j.neuroscience.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Figueiredo CP, Clarke JR, Ledo JH, Ribeiro FC, Costa CV, Melo HM, Mota-Sales AP, Saraiva LM, Klein WL, Sebollela A, De Felice FG, Ferreira ST. Memantine rescues transient cognitive impairment caused by high-molecular-weight Aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci. 2013;33:9626–9634. doi: 10.1523/JNEUROSCI.0482-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis BM, Kim J, Barakat ME, Fraenkl S, Yucel YH, Peng S, Michalski B, Fahnestock M, McLaurin J, Mount HTJ. Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol Aging. 2012;33:555–563. doi: 10.1016/j.neurobiolaging.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Starkov A, Blass J, Ratan R, Beal MF. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough MA, Camakaris J, Bush AI. Metal dyshomeostasis and oxidative stress in Alzheimer's disease. Neurochem Int. 2013;62:540–555. doi: 10.1016/j.neuint.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, Kim D, Hamto P, Lo I, Yu GQ, Palop JJ, Masliah E, Mucke L. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68:428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa N, Yokoyama H, Kato H, Araki T. Age-related alterations of oxidative stress markers in the mouse hippocampal CA1 sector. Exp Mol Pathol. 2008;85:135–140. doi: 10.1016/j.yexmp.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Schmitz D, Eder C, Gloveli T. Properties of entorhinal cortex projection cells to the hippocampal formation. Ann N Y Acad Sci. 2000;911:112–126. doi: 10.1111/j.1749-6632.2000.tb06722.x. [DOI] [PubMed] [Google Scholar]
- Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for β-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Liu S, Wong-Riley MTT. A metabolic map of cytochrome oxidase in the rat brain: Histochemical, densitometric and biochemical studies. Neuroscience. 1995;65:313–342. doi: 10.1016/0306-4522(94)00514-6. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Wong-Riley MT. Entorhinal cortex of the human, monkey, and rat: metabolic map as revealed by cytochrome oxidase. J Comp Neurol. 1992;326:451–7469. doi: 10.1002/cne.903260310. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Fu Z, Makarova A, Lee JY, Lu C, Feng L, Pajoohesh-Ganji A, Matsuoka Y, Hyman BT, Ehlers MD, Vicini S, Pak DT, Rebeck GW. The effects of amyloid precursor protein on postsynaptic composition and activity. J Biol Chem. 2009;284:8495–8506. doi: 10.1074/jbc.M900141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR. Morphology and neurochemical characteristics of the vulnerable neurons in brain aging and Alzheimer's disease. Eur Neurol. 1997;37:71–81. doi: 10.1159/000117414. [DOI] [PubMed] [Google Scholar]
- Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Hort J, Laczo J, Vyhnalek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci USA. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Iachini I, Iavarone A, Senese V, Ruotolo F, Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: A review. Curr Aging Sci. 2009;2:43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Jacobsen JS, Wu C-C, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebb AH, Woolsey TA. A simple stain for myelin in frozen sections: a modification of Mahon's method. Stain Technol. 1977;52:315–318. doi: 10.3109/10520297709116805. [DOI] [PubMed] [Google Scholar]
- Jones RS, Lambert JD. The role of excitatory amino acid receptors in the propagation of epileptiform discharges from the entorhinal cortex to the dentate gyrus in vitro. Exp Brain Res. 1990;80:310–322. doi: 10.1007/BF00228158. [DOI] [PubMed] [Google Scholar]
- Kadriu B, Guidotti A, Costa E, Auta J. Imidazenil, a non-sedating anticonvulsant benzodiazepine, is more potent than diazepam in protecting against DFP-induced seizures and neuronal damage. Toxicol. 2009;256:164–174. doi: 10.1016/j.tox.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Rijken S, Joosten-Weyn Banningh LWA, Van Schuylenborgh-Van Es N, Olde Rikkert MGM. Categorical spatial memory in patients with mild cognitive impairment and Alzheimer dementia: positional versus object-location recall. J Int Neuropsychol Soc. 2010;16:200–204. doi: 10.1017/S1355617709990944. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. Behav Brain Res. 2001;103:145–162. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Kordower J, Chu Y, Stebbins G, DeKosky S, Cochran E, Bennett D, Mufson E. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Korolainen M, Goldsteins G, Nyman T, Alafuzoff I, Koistinaho J, Pirttil T. Oxidative modification of proteins in the frontal cortex of Alzheimer's disease brain. Neurobiol Aging. 2006;27:42–53. doi: 10.1016/j.neurobiolaging.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released β-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Han PL. An update of animal models of Alzheimer disease with a reevaluation of plaque depositions. Exp Neurobiol. 2013;22:84–95. doi: 10.5607/en.2013.22.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Hauptmann S, Abdel Kader R, Scherping I, Keil U, Strosznajder J, Eckert A, Mller W. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke A, Nagao T, Kohling R, Avoli M. Synchronous potentials and elevations in [K+]o in the adult rat entorhinal cortex maintained in vitro. Neurosci Lett. 1995;185:155–158. doi: 10.1016/0304-3940(95)11248-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The laminar distribution of intracortical fibers originating in the olfactory cortex of the rat. J Comp Neurol. 1983;216:292–302. doi: 10.1002/cne.902160306. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Orsucci D, LoGerfo A, Calsolaro V, Siciliano G. Clinical features and pathogenesis of Alzheimer's disease: involvement of mitochondria and mitochondrial DNA. Adv Exp Med Biol. 2010;685:34–44. doi: 10.1007/978-1-4419-6448-9_4. [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Raymond EF, Carbone E, Marie H. Firing properties of entorhinal cortex neurons and early alterations in an Alzheimer's disease transgenic model. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1368-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Umeda M, Uchida H, Kato H, Araki T. Alterations of oxidative stress markers and apoptosis markers in the striatum after transient focal cerebral ischemia in rats. J Neural Transm. 2009;116:395–404. doi: 10.1007/s00702-009-0194-0. [DOI] [PubMed] [Google Scholar]
- McPhail LT, McBride CB, McGraw J, Steeves JD, Tetzlaff W. Axotomy abolishes NeuN expression in facial but not rubrospinal neurons. Exp Neurol. 2004;185:182–190. doi: 10.1016/j.expneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Morales-Corraliza J, Mazzella MJ, Berger JD, Diaz NS, Choi JHK, Levy E, Matsuoka Y, Planel E, Mathews PM. In vivo turnover of tau and APP metabolites in the brains of wild type and Tg2576 mice: Greater stability of sAPP in the beta-amyloid depositing mice. PLoS ONE. 2009;4:e7134. doi: 10.1371/journal.pone.0007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer's disease. Prog Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Moser EI, Witter MP, Moser M-B. Entorhinal cortex. In: Shepherd G, Grillner S, editors. Handbook of Brain Microcircuits. New York: Oxford University Press; 2010. pp. 175–193. [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem. 1994;63:2179–2784. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease) Eur J Neurosci. 2003;18:2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME. Cholinergic modulation of cognitive processing: insights drawn from computational models. Front Behav Neurosci. 2012;6:24. doi: 10.3389/fnbeh.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia. 2011;(52 Suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, Save E. Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiol Learn Mem. 2004;82:1–11. doi: 10.1016/j.nlm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Portiansky EL, Barbeito CG, Gimeno EJ, Zuccolilli GO, Goya RG. Loss of NeuN immunoreactivity in rat spinal cord neurons during aging. Exper Neurol. 2006;202:519–521. doi: 10.1016/j.expneurol.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum JB, Kosik KS, Tariot PN, Lopera F. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room P, Groenewegen HJ, Lohman AH. Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. I. Anatomical observations. Exp Brain Res. 1984;56:488–496. doi: 10.1007/BF00237989. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Hintz T, Gomez J, Stormes K, Barouk S, Malthankar Phatak G, McCloskey D, Luine V, Maclusky N. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability of entorhinal cortex and hippocampus after application of aminooxyacetic acid (AOAA) to layer III of the rat medial entorhinal cortex in vitro. J Neurophysiology. 1996;76:2986–3001. doi: 10.1152/jn.1996.76.5.2986. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. "Untangling" Alzheimer's disease and epilepsy. Epilepsy Curr. 2012;12:178–183. doi: 10.5698/1535-7511-12.5.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Chao MV. The entorhinal cortex and neurotrophin signaling in Alzheimer’s disease and other disorders. Cog Neurosci. 2013;4:125–135. doi: 10.1080/17588928.2013.826184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Ofer A. Pretreatment with l-kynurenine, the precursor to the excitatory amino acid antagonist kynurenic acid, suppresses epileptiform activity in combined entorhinal/hippocampal slices. Neurosci Lett. 1997;224:115–118. doi: 10.1016/s0304-3940(97)13472-3. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Smith KL, Goodman JH, Sollas AL. Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyramidal cells. Neuroscience. 2001;104:741–759. doi: 10.1016/s0306-4522(01)00132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker P. The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res. 2011;97:1–11. doi: 10.1016/j.eplepsyres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SD, Mazzella MJ, Nixon RA, Mathews PM. Aβ measurement by enzyme-linked immunosorbent assay. Methods Mol Biol. 2012a;849:507–527. doi: 10.1007/978-1-61779-551-0_34. [DOI] [PubMed] [Google Scholar]
- Schmidt SD, Nixon RA, Mathews PM. Tissue processing prior to analysis of Alzheimer's disease associated proteins and metabolites, including Aβ. Methods Mol Biol. 2012b;849:493–506. doi: 10.1007/978-1-61779-551-0_33. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G, Schroeder CE, MacLusky NJ, Scharfman HE. Testosterone depletion in adult male rats increases mossy fiber transmission, LTP, and sprouting in area CA3 of hippocampus. J Neurosci. 2013;33:2338–2355. doi: 10.1523/JNEUROSCI.3857-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL, Wood MA, Binder DK, Scharfman HE. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci. 2011;31:6392–6397. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter R, Dean E, Sollas A, Goodman J. Apoptosis and necrosis induced in different hippocampal neuron populations by repetitive perforant path stimulation in the rat. J Comp Neurol. 1996;366:516–533. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser E. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Stokin GB, Goldstein LS. Axonal transport and Alzheimer's disease. Annu Rev Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer's disease. Neural Plast. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Song SK, Harms MP, Lin SJ, Holtzman DM, Merchant KM, Kotyk JJ. Detection of age-dependent brain injury in a mouse model of brain amyloidosis associated with Alzheimer's disease using magnetic resonance diffusion tensor imaging. Exp Neurol. 2005;191:77–85. doi: 10.1016/j.expneurol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Chami B, Youssef P, Witting PK. Oxidative stress in Alzheimer's disease: Primary villain or physiological by-product? Redox Rep. 2013;18:134–141. doi: 10.1179/1351000213Y.0000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R, Khan S. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Terni B, Boada J, Portero Otin M, Pamplona R, Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer's disease pathology. Brain Pathol. 2010;20:222–233. doi: 10.1111/j.1750-3639.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Valla J, Berndt JD, Gonzalez Lima Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Yaari R, Wolf A, Kusne Y, Beach T, Roher A, Corneveaux J, Huentelman M, Caselli R, Reiman E. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE ε4 allele, the major late-onset Alzheimer's susceptibility gene. J Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–149. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadghiri YZ, Li J, Wang J, Hoang DM, Sun Y, Xu H, Tsui W, Li Y, Boutajangout A, Wang A, de Leon M, Wisniewski T. Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer's disease transgenic mice using magnetic resonance microimaging. PLoS One. 2013;8:e57097. doi: 10.1371/journal.pone.0057097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson D, Borkowski A, Landreth G, Nixon R, Levy E, Wilson D. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's β-amyloidosis mouse model. J Neurosci. 2011;31:15962–15971. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-β burden in an Alzheimer's disease mouse model. J Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Age and gene overexpression interact to abolish nesting behavior in Tg2576 amyloid precursor protein (APP) mice. Behav Brain Res. 2011;216:408–413. doi: 10.1016/j.bbr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M, Moser E. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Won SY, Choi SH, Jin BK. Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro: role of microglial NADPH oxidase. J Neuroimmunol. 2009;214:83–92. doi: 10.1016/j.jneuroim.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Nederlof J. Terminations of olfactory afferents on layer II and III neurons in the entorhinal area: degeneration-Golgi-electron microscopic study in the rat. Neurosci Lett. 1983;36:105–110. doi: 10.1016/0304-3940(83)90250-1. [DOI] [PubMed] [Google Scholar]
- Wu KL, Li YQ, Tabassum A, Lu WY, Aubert I, Wong CS. Loss of neuronal protein expression in mouse hippocampus after irradiation. J Neuropathol Exp Neurol. 2010;69:272–280. doi: 10.1097/NEN.0b013e3181d1afe4. [DOI] [PubMed] [Google Scholar]
- Wykes R, Kalmbach A, Eliava M, Waters J. Changes in the physiology of CA1 hippocampal pyramidal neurons in preplaque CRND8 mice. Neurobiol Aging. 2012;33:1609–1623. doi: 10.1016/j.neurobiolaging.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K, Bennett J. The mitochondrial secret(ase) of Alzheimer's disease. J Alzheimers Dis. 2010;20:S381–S400. doi: 10.3233/JAD-2010-100360. [DOI] [PubMed] [Google Scholar]
- Zhang B, Veasey SC, Wood MA, Leng LZ, Kaminski C, Leight S, Abel T, Lee VM, Trojanowski JQ. Impaired rapid eye movement sleep in the Tg2576 APP murine model of Alzheimer's disease with injury to pedunculopontine cholinergic neurons. Am J Pathol. 2005;167:1361–1369. doi: 10.1016/S0002-9440(10)61223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.