Abstract
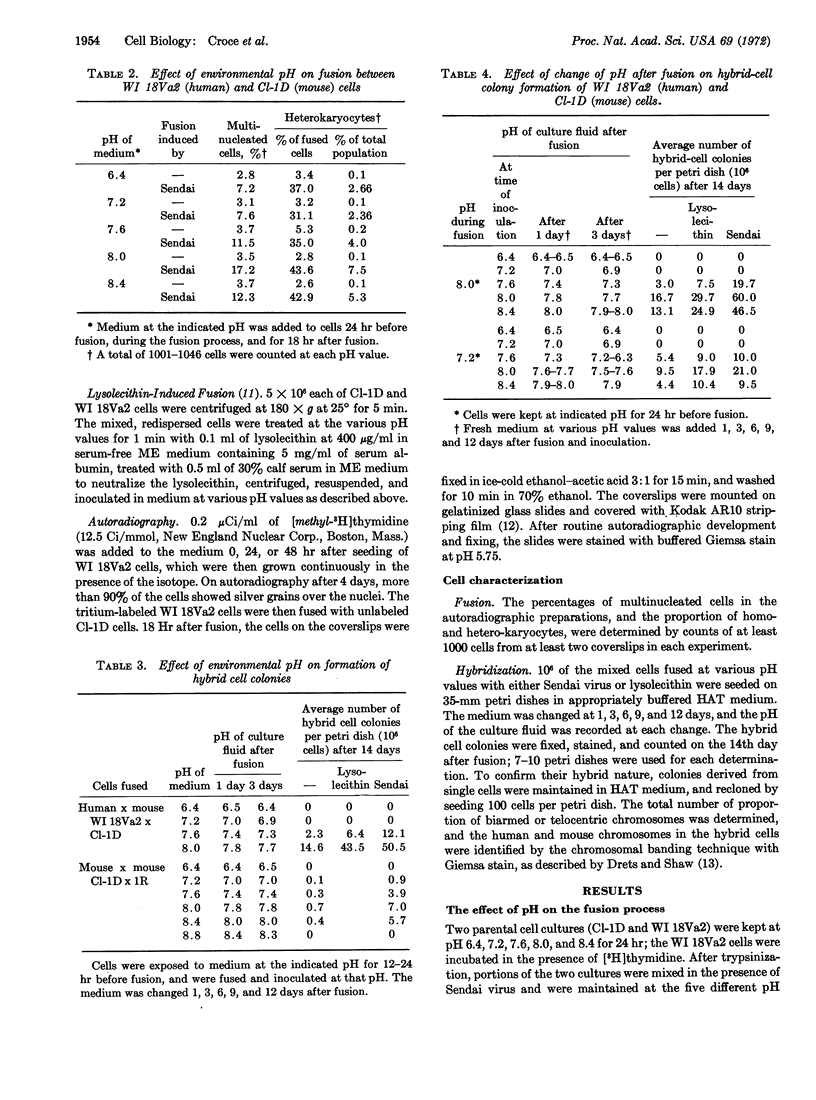
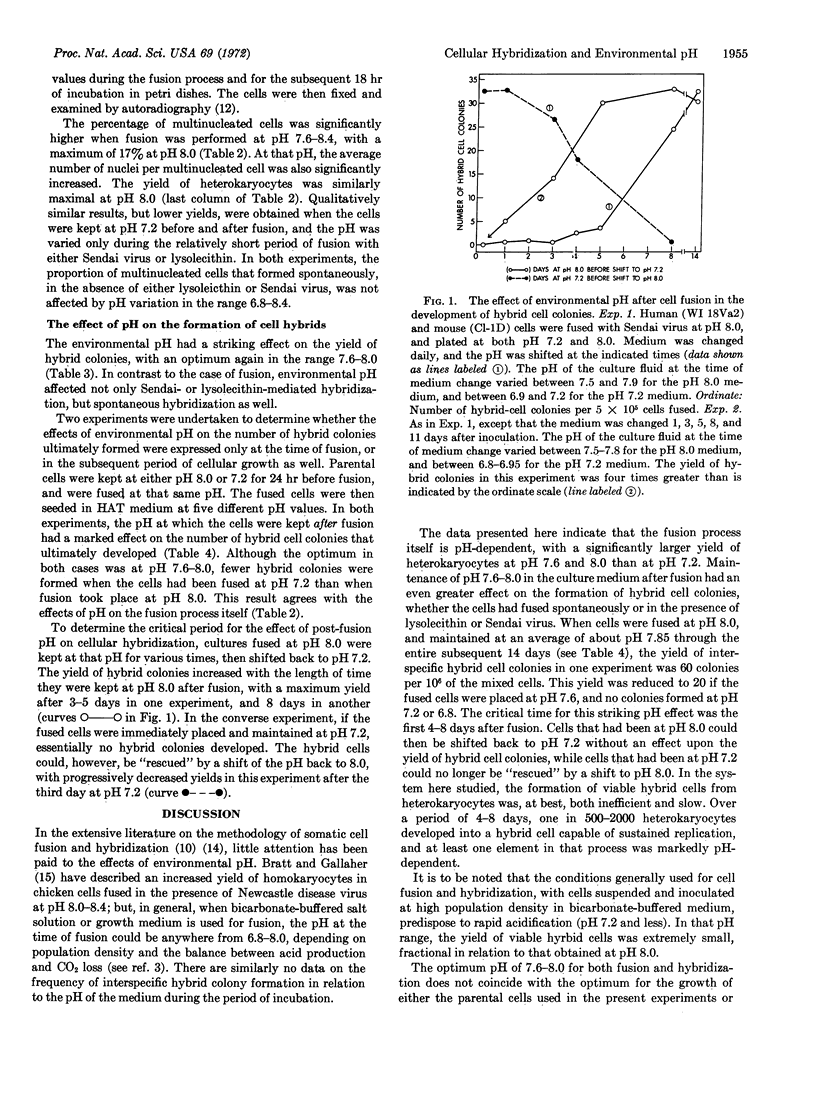
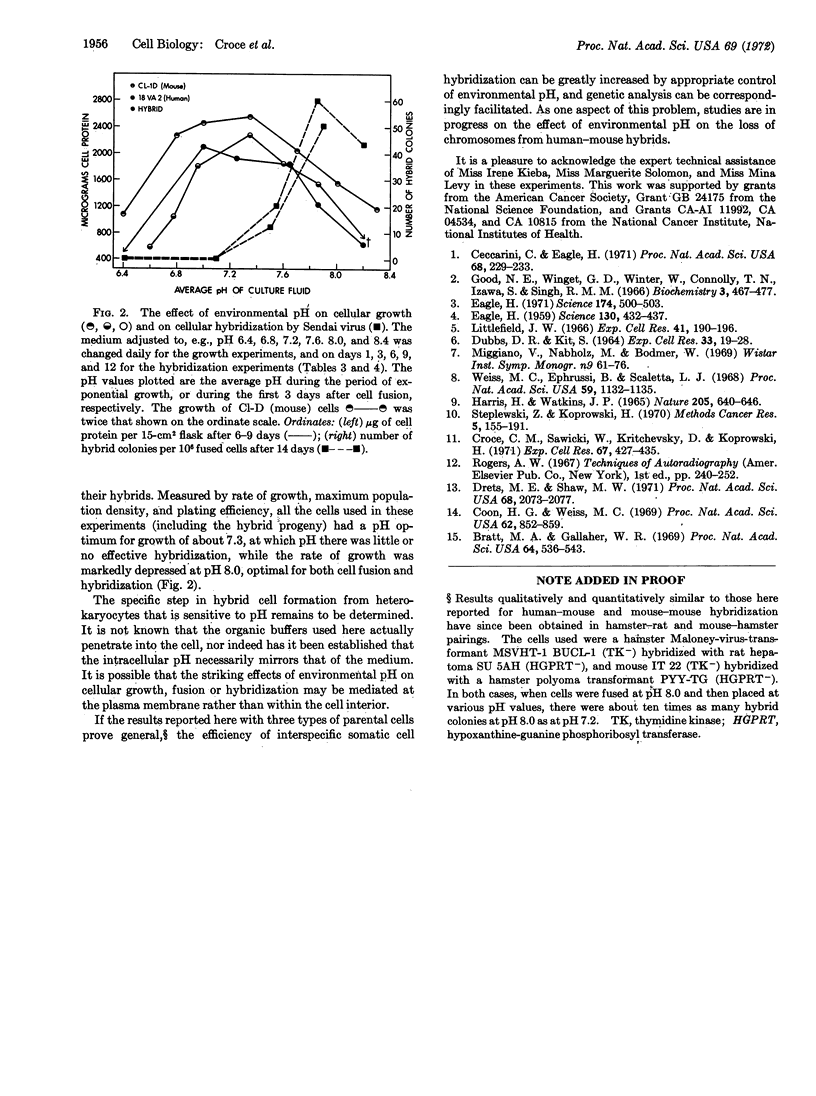
The hybridization of a human and mouse cell was strikingly pH-dependent, with a well defined optimum at (about) pH 7.6-8.0. The yield of hybrid cell colonies (1 per 500-2000 heterokaryocytes) was several hundred times greater than that obtained at pH 6.8-7.2. Although there was a significant effect on the efficiency of cell fusion, the critical time for the pH effect was in the first 4-8 days after fusion, presumably while viable hybrids were being formed from the multinucleated heterokaryocytes.
Keywords: human-mouse, heterokaryocytes, multinucleate, Sendai virus fusion, lysolecithin
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini C., Eagle H. pH as a determinant of cellular growth and contact inhibition. Proc Natl Acad Sci U S A. 1971 Jan;68(1):229–233. doi: 10.1073/pnas.68.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon H. G., Weiss M. C. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc Natl Acad Sci U S A. 1969 Mar;62(3):852–859. doi: 10.1073/pnas.62.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Sawicki W., Kritchevsky D., Koprowski H. Induction of homokaryocyte, heterokaryocyte and hybrid formation by lysolecithin. Exp Cell Res. 1971 Aug;67(2):427–435. doi: 10.1016/0014-4827(71)90428-9. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. EFFECT OF HALOGENATED PYRIMIDINES AND THYMIDINE ON GROWTH OF L-CELLS AND A SUBLINE LACKING THYMIDINE KINASE. Exp Cell Res. 1964 Jan;33:19–28. doi: 10.1016/s0014-4827(64)81006-5. [DOI] [PubMed] [Google Scholar]
- Drets M. E., Shaw M. W. Specific banding patterns of human chromosomes. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2073–2077. doi: 10.1073/pnas.68.9.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HARRIS H., WATKINS J. F. HYBRID CELLS DERIVED FROM MOUSE AND MAN: ARTIFICIAL HETEROKARYONS OF MAMMALIAN CELLS FROM DIFFERENT SPECIES. Nature. 1965 Feb 13;205:640–646. doi: 10.1038/205640a0. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Ephrussi B., Scaletta L. J. Loss of T-antigen from somatic hybrids between mouse cells and SV40-transformed human cells. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1132–1135. doi: 10.1073/pnas.59.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]


