Abstract
HIV-associated neurocognitive disorders (HAND) continues to be prevalent (30–50 %) despite plasma HIVRNA suppression with combination antiretroviral therapy (cART). There is no proven therapy for individuals on suppressive cARTwith HAND. We have shown that the degree of HIV reservoir burden (HIV DNA) in monocytes appear to be linked to cognitive outcomes. HIV infection of monocytes may therefore be critical in the pathogenesis of HAND. A single arm, open-labeled trial was conducted to examine the effect of maraviroc (MVC) intensification on monocyte inflammation and neuropsychological (NP) performance in 15 HIV subjects on stable 6-month cART with undetectable plasma HIV RNA (<48 copies/ml) and detectable monocyte HIV DNA (>10 copies/106 cells). MVC was added to their existing cART regimen for 24 weeks. Post-intensification change in monocytes was assessed using multiparametric flow cytometry, monocyte HIV DNA content by PCR, soluble CD163 (sCD163) by an ELISA, and NP performance over 24 weeks. In 12 evaluable subjects, MVC intensification resulted in a decreased proportion of circulating intermediate (median; 3.06 % (1.93, 6.45) to 1.05 % (0.77, 2.26)) and nonclassical (5.2 % (3.8, 7.9) to 3.2 % (1.8, 4.8)) CD16-expressing monocytes, a reduction in monocyte HIV DNA content to zero log10 copies/106 cells and in levels of sCD163 of 43 % by 24 weeks. This was associated with significant improvement in NP performance among six subjects who entered the study with evidence of mild to moderate cognitive impairment. The results of this study suggest that antiretroviral therapy with potency against monocytes may have efficacy against HAND.
Keywords: HIV, DNA, HIV-associated neurocognitive disorders, Monocytes, Inflammation, CCR5, CD163
Introduction
Neurocognitive impairment continues to be prevalent in an estimated 30 % of HIV-infected individuals despite maximal plasma HIV RNA suppression on combination antiretroviral therapy (cART) (Cysique et al. 2004; Harezlak et al. 2011; Heaton et al. 2010). Various therapeutic modalities have been examined for HAND. These have included relatively large clinical trials of seleginine and minocycline (Nakasujja et al. 2013; Schifitto et al. 2007), but, to date, no effective preventive or therapeutic options have been identified for individuals already on cARTwho have neurocognitive impairment. While the majority of impairment is mild, this issue remains significant as even mild impairment negatively affects the ability of an individual to work or live fully independent and productive lives (Andrade et al. 2013; Benedict et al. 2000; Gorman et al. 2009; Heaton et al. 2010). Therefore, effective treatment strategies for HAND are needed.
It has been hypothesized that the pathogenesis of HAND involves trafficking of circulating bone marrow-derived monocytes, some of which are HIV infected, through the blood-brain barrier (BBB) into the brain parenchyma introducing HIV into the brain and triggering neuroimmune activation and inflammation, ultimately leading to neuronal degeneration and death (Gartner 2000; Gonzalez-Scarano and Martin-Garcia 2005). Histologic evaluation of brains obtained on autopsy of HIV-infected individuals who died while on fully suppressive cART continues to show substantial degree of macrophage infiltration and supports this hypothesis (Anthony et al. 2005). Furthermore, high levels of monocyte turnover in the bloodstream have been correlated with the presence of encephalitis in simian immunodeficiency virus (SIV) nonhuman primate model of HIV infection (Burdo et al. 2010).
HIV-infected monocytes are commonly found in blood and tissues of HIV patients with widespread distribution in all tissues including bone marrow (McElrath et al. 1989) and brain (Koenig et al. 1986). These cells of the myeloid lineage are potential reservoirs of HIV (Sonza et al. 1996; Ziegler-Heitbrock 2000). Circulating monocytes are a heterogeneous population, and current nomenclature has defined three distinct subsets based on CD14 and CD16 expression as classical (CD14++CD16−), nonclassical (CD14+/lowCD16++), and an intermediate (CD14++CD16+) monocyte subset (Zawada et al. 2011; Ziegler-Heitbrock 2000; Ziegler-Heitbrock et al. 2010). It is suggested that CD16-expressing monocyte (MO) subsets in the periphery preferentially migrate into tissues and display proinflammatory features after stimulation with toll-like receptor (TLR) ligands (Farina et al. 2004; Leavy 2011). This is of relevance as neurocognitive impairment has specifically been linked to increased CD16-bearing monocytes in circulation (Pulliam et al. 1997, 2004).
Several circulating cerebrospinal fluid (CSF) and plasma biomarkers have been associated with central nervous system (CNS) inflammation and neurocognitive impairment in the setting of suppressive cART. CD163 is a scavenger receptor found on monocytes, shed by proteolytic cleavage after pro-inflammatory stimulation with TLRs and lipopolysaccharide (LPS) (Droste et al. 1999). Soluble CD163 (sCD163) and membrane CD163 (mCD163) appear to be correlated with the expansion of monocytes and rapid onset of SIV and HIV infections (Burdo et al. 2011) and have recently been reported to be associated with neurocognitive impairment in HIV infection (Burdo et al. 2013). Neopterin, a soluble pteridine, is produced principally by myeloid-derived cells. While levels of CSF neopterin are elevated in HIV disease, this level is decreased markedly following cART, but, despite long-term cART, the levels in some patients remain elevated (Abdulle et al. 2002; Eden et al. 2007; Yilmaz et al. 2008).
The degree of HIV reservoir burden (HIV DNA) within monocytes (CD14+ cells) in the bloodstream have also been linked to cognitive outcomes (Kallianpur et al. 2013; Kusao et al. 2012; Shiramizu et al. 2012), to structural changes in brain MRI (Kallianpur et al. 2012, 2013), and to altered evidence of brain injury and glial dysfunction by magnetic resonance spectroscopy (Valcour et al. 2013). Levels of monocyte HIV DNA correlates with global neuropsychological test performance (Shiramizu et al. 2012), and a stepwise increase in levels of HIV DNA within circulating monocytes are seen with worsening clinical cognitive status (Kusao et al. 2012; Valcour et al. 2013). Monocyte HIV DNA levels are higher in ART-naïve individuals with HIV-associated dementia (HAD) than in individuals without HAD prior to initiation of first-time cART and, more importantly, remain higher over 4 years of cART even among subjects whose HIV is fully suppressed (Valcour et al. 2009). The monocyte HIV DNA levels correlating to HAND appear to be primarily within CD14+CD16+ subsets of monocytes (Kusao et al. 2012). Levels of HIV-infected inflammatory monocytes/macrophages may therefore be critical in the pathogenesis of neurocognitive impairment.
The reasons for the failure of current-day cART to control HAND have been a topic of much interest. It has been hypothesized that the failure of cART to control HAND may be secondary to the failure of many current antiretroviral medications to penetrate into the CNS in adequate concentration; however, data on the association between CNS penetrance as assessed by the CNS penetration efficacy (CPE) score and neurocognitive function has been mixed (Cysique et al. 2009; Giancola et al. 2006; Letendre et al. 2008; Smurzynski et al. 2011; Tozzi et al. 2007; Tozzi et al. 2009). More recently, we have proposed an alternative complementary hypothesis that the lack of potency of antiviral agents into monocytes/ macrophages may be associated with poorer neurocognitive performance (Shikuma et al. 2012).
Maraviroc (MVC) is an orally administered noncompetitive human CC chemokine receptor 5 (CCR5) inhibitor, currently licensed as an HIVantiretroviral medication (Meanwell and Kadow 2007). It is recommended by the most recent (February 2013) US Department of Health and Human Services Antiretroviral Therapy Guidelines as an acceptable alternative agent in combination with a dual nucleoside (tide) backbone. The chemokine receptor CCR5 serves as the principal co-receptor for entry of M-tropic HIV into monocytes/ macrophages (Alkhatib et al. 1996; Deng et al. 1996). MVC has been shown in vitro to suppress monocyte migration and may be able to block ligation by CCR5 ligands, and alter CCR5 recycling and signaling (Rossi et al. 2010, 2011). Based on these findings, we reasoned that intensification of cART regimens with MVC may decrease cellular monocyte immune activation and inflammation, and HIV DNA burdens within this myeloid lineage with implication for HAND.
Methods
Patients and study design
This was a single arm, open-label 24-week study of MVC intensification in HIV-infected subjects on cART. Entry criteria mandated that the subjects be on stable cART for >6 months prior to entry, have a plasma HIV RNA <48 copies/ml, and have PBMC HIV DNA above the limit of detection of our research assay defined as HIV DNA >10 copies/106 cells. The study excluded individuals with previous use of MVC, abnormal chemistries, or blood counts likely to be clinically concerning for significant underlying pathology such as hepatic or renal disease, current active substance abuse, uncontrolled chronic illnesses, major affective disorders, cardiovascular disease, or non-HIV risk factors that may impact cognitive performance. There were no CD4 count entry restrictions. The study was approved by the University of Hawaii, Human Studies Program, and written informed consent was obtained from all enrolled subjects. MVC was given for 24 weeks in addition to the subject's current antiretroviral therapy with dose adjustment as recommended by the package insert based on each subject's antiretroviral and other concomitant medications. Following entry into the study, each subject returned for follow-up visits at weeks 2, 4, 8, 12, and 24 for safety monitoring. Research bloods were drawn at weeks 4, 12, and 24 study visits.
Neuropsychological test assessment
Subjects were tested at entry and at 24 weeks by a trained psychometrist. The neuro-psychological battery assessed multiple cognitive domains typically affected by HIV including attention/concentration (choice and sequential reaction time—California Computerized Assessment Package [CalCAP], WAIS-R Digit Span), learning/memory (Rey Auditory Verbal Learning Test [RAVLT], Rey Complex Figure Test); psychomotor speed (Trail Making Test–Part A, WAIS-R Digit Symbol, Grooved Pegboard), executive functioning (Verbal Fluency Test [FAS], Trail Making Test–Part B, DKEFS Color-Word interference), language (Animal Naming, Boston Naming Test), and gross motor (Timed Gait). The National Adult Reading Test was used as a measure of premorbid functioning. This neuropsychological battery was adapted from that used in the Northeast AIDS Dementia (NEAD) cohort (The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders 1996). Depression symptomatology was assessed using the Beck Depression Inventory (Beck and Mendelson 1961). Published age- and education-adjusted normative data were used to calculate z scores (NPZ), and NPZ composite scores were calculated by taking the arithmetic mean of z scores within the cognitive domain as follows: NPZglobal (Trail Making Test (TMT), Grooved Pegboard, WAIS-R Digit Symbol, RAVLT Total, RAVLT Delayed Recall, Rey Complex Figure Test-Delayed Recall, Timed Gait, CalCap, FAS, Animals, DKEFS Color-Word Interference); psychomotor speed and attention (NPZpma) (TMT, Grooved Pegboard, WAIS-R Digit Symbol, Timed Gait, CalCap), learning and memory (NPZlrn_mem) (RAVLT Total, RAVLT Delayed Recall, Rey Complex Figure Test-Delayed Recall), and executive functions (NPZef) (TMT-Part B, FAS, DKEFS color-word interference).
Blood specimens
Blood from study visits were processed within 2 h with plasma collected by centrifugation and cryo-preserved in 1 ml aliquots. Peripheral blood mononuclear cells (PBMC) were obtained by using Ficoll Histopaque (Sigma) density gradient centrifugation and washed three times with RPMI 1640 culture media (Life Technologies) containing 2 % heat-inactivated fetal bovine serum (FBS) (Gibco) and 1 % Pen/Strep (Gibco) before being cryopreserved in aliquots in liquid nitrogen.
Cell staining and flow cytometric analysis
Cryopreserved PBMC were thawed in warm media (RPMI 1640 supplemented with 10 % fetal bovine serum, 1 % penicillin-streptomycin, 10 mM HEPES, 2 mM L-glutamine (all Hyclone), and 10 μg/ml DNAse I (Sigma)), washed, and stained for viability with a Yellow Amine Reactive Dye (YARD) for 15 min at room temperature. To identify monocytes, the cells were then surface-stained for 30 min with V500-conjugated anti-CD3, Qdot605-conjugated anti-CD14, Alexa700-conjugated anti-CD16, PE-Cy7-conjugated anti-CD56, PE-Cy7-conjugated anti-CD19, PE-Cy7-conjugated anti-CD20, and APC-H7-conjugated HLA-DR monoclonal antibodies (mAbs). All antibodies were from BD Biosciences except for Q605-conjugated anti-CD14 and yellow Live/Dead (Life Technologies). Alexa700-conjugated anti-CD3, APCH7-conjugated anti-CD8, V450-conjugated anti-CD38, and APC-conjugated anti-HLA-DR all mAbs (all from BD Biosciences) were used to define T cell activation. Fluorescence minus one samples were prepared for each fluorochrome to facilitate gating. All cells were fixed with 1 % PFA in PBS, and analyzed by flow cytometry using a four-laser custom BD-Fortessa instrument (Becton Dickinson). A total of 100,000 cells was collected and analyzed with FlowJo software (TreeStar).
CD14+ monocyte isolation and HIV DNA quantification
Frozen PBMC were thawed in warm media (RPMI 1640 supplemented with 20 % fetal bovine serum), washed once, and resus-pended in RoboSep buffer (StemCell Technologies). Samples were placed in a RoboSep automated cell separator (StemCell Technologies), and CD14+ cells were purified through magnetic separation using the EasySep human monocyte enrichment kit without CD16 depletion (StemCell Technologies). DNA was extracted from CD14+ monocytes or total PBMC using the QIAamp DNA Micro Extraction kit (Qiagen) and quantified using the ND-2000 spectrophotometer (NanoDrop Technologies) as previously described [19]. Determination of HIV DNA content was assessed using multiplex real-time PCR with HIV gag and β-globin primer pairs to amplify respective regions that were detected with FAM-labeled HIV gag and VIC-labeled β-globin probes. Using standard reference plasmids with one copy of the β-globin housekeeping gene and one copy of the HIV gag gene and appropriate positive/negative controls, samples were run in triplicate on StepOnePlus Real-Time PCR System and analyzed using the StepOne software (Applied Biosystems). The copy numbers of each sample gene were analyzed against the standard curves and used to calculate HIV DNA copy number per 1×106 cells.
sCD163 ELISA
Soluble CD163 (sCD163) was quantified by ELISA according to the manufacturer's protocol (Trillium Diagnostics).
Statistical Analyses
The demographic and clinical information of participants were listed, and continuous variables summarized by median and interquartile range (IQR). The changes in immune parameters and NPZ score from week 0 to each indicated week were compared by Wilcoxon signed-rank test. Statistical analyses were performed using R version 3.0.1. A two-sided p value <0.05 was considered statistically significant.
Results
Study subjects
Fifteen subjects were enrolled into the study and the study results were based on 12 subjects who were judged to be evaluable following successful completion of a 24-week course of MVC intensification given in addition to their baseline cART regimen. Of the initial 15 patients, 3 were excluded from the study. One subject dropped out of the study early due to the development of pancreatitis judged to be unrelated to study medications. Two others were eliminated prior to study analyses, one due to relapse of alcohol abuse several months prior to week 24 with intoxication during the week 24 neuropsychological testing, and the other due to incomplete week 24 neuropsychological testing. The demographics of the 12 individual subjects are shown in Table 1 and can be summarized as follows: age (median, 56 years; IQR [49, 61]) and viral load (median, 2.1 log10 copies/ml, IQR [1.3, 2.4]) with a median duration of cART of 7.5 years (4.5, 13.3).
Table 1.
Participants characteristics
| Patient ID | Age | Gender | Ethnicity | CD4 Nadir cells/mm3 | CD4 count cells/mm3 | PBMC HIV DNA copies/106 ave at entry/screen | HAART regimen | Duration of suppressive HAART (years) |
|---|---|---|---|---|---|---|---|---|
| 5 | 62 | M | Caucasian | 8 | 210 | 1,582 | RAL, ETR, 3TC | 12 |
| 18 | 64 | M | Caucasian | 34 | 438 | 379 | SQV/r, ABC, 3TC | 24 |
| 21 | 49 | F | Caucasian | 181 | 878 | 213 | EFV/FTC/TDF | 8 |
| 26 | 55 | M | Hawaiian | 128 | 1,380 | >400 | ATV, ABC/3TC | 18 |
| 28 | 44 | M | Caucasian | 423 | 972 | 266 | FTC/TDF, ATV/r | 3 |
| 37 | 60 | M | Asian | 110 | 320 | 1,525 | FPV, EFV, ABC | 5 |
| 41 | 59 | M | Caucasian | 450 | 892 | 102 | EFV/FTC/TDF | 12 |
| 58 | 48 | M | Caucasian | 0 | 373 | 322 | RAL, DRV/r, FTC/ TDF |
7 |
| 65 | 31 | M | Asian/American Indian |
280 | 539 | >500 | EFV/FTC/TDF | 2 |
| 92 | 57 | M | Caucasian | 29 | 509 | 496 | DRV/r | 17 |
| 199 | 50 | M | Caucasian | 3 | 131 | 900 | DRV/r, FTC/TDF | 2 |
| 291 | 73 | M | Caucasian/American Indian |
250 | 1,598 | 84 | EFV/FTC/TDF | 5 |
| Median (IQR) |
56 (49, 61) | 119 (29, 250) | 524 (360, 912) | 7.5 (4.5, 13.3) |
Change in CD4+ T cell counts after MVC intensification
At entry, the median CD4+ T cell count was 524 cells/mm3 (IQR [355, 912]). At week 24 after MVC-intensification, the CD4+ T cell count was 586 (402, 983) and the associated change from entry was 6 (−45, 47)cells/ml and not significantly different.
Declines in frequency of CD16+ monocytes subsets after MVC intensification
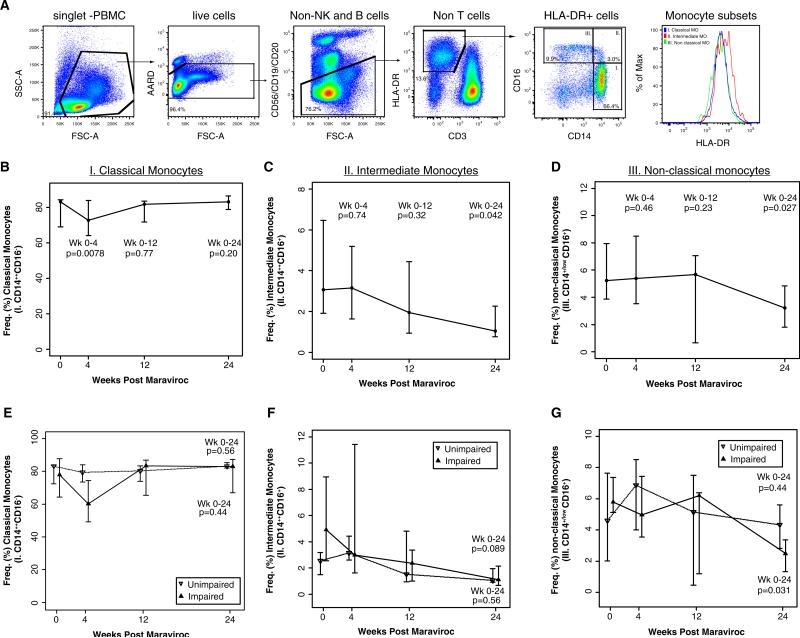
Using a multiparametric flow cytometry panel that excludes nonmonocyte populations (Fig. 1a) that we and others have adopted (Abeles et al. 2012; Barbour et al. 2014; Jalbert et al. 2013; Shikuma et al. 2014), there was a transient decrease in the frequency of classical monocytes between weeks 0 and 4 (Fig. 1b; p=0.0078) but there was no difference from weeks 0 to 12 or to week 24 among all subjects in the study. By 24 weeks, however, we observed a significant decline in the frequency of “intermediate” (CD14++CD16+) monocyte subset from median 3.06 % (1.93, 6.45) to 1.05 % (0.77, 2.26) (p =0.042; Fig. 1c) and a significant decline of the nonclassical (CD14+/lowCD16+) monocyte subset in 11 of 12 subjects, from 5.2 % (3.8, 7.9) to 3.2 % (1.8, 4.8) (p=0.027; Fig. 1d). When the results were stratified by cognitive status, the impaired group showed a significant drop at week 24 in non-classical monocytes from baseline (p=0.031; Fig. 1g). However, no significant differences in the changes in nonclassical, intermediate, or classical monocyte subsets from baseline to week 24 between the impaired and unimpaired groups (Fig. 1e, g) were observed.
Fig. 1.
Flow cytometric gating strategy to assess changes in monocyte subset frequencies (a). Representative gating strategy of single-cell multiparametric flow cytometry assessment of monocytes subsets based on CD14 and CD16 expression among live HLA-DR-expressing PBMC that excluded Tcells, B cells, and NK cells. HLA-DR expression intensity on the three monocyte subsets is represented in the histogram (a). The plots depict changes in frequency of classical (b, e), intermediate (c, f), and nonclassical (d, g) monocyte subsets in all study subjects (b–d) or stratified by cognitive status (e–g). The data were analyzed in 12 subjects before and during 24 weeks of MVC intensification. Graphs depict the change in CD14 and CD16 expression on HLA-DR monocytes, plotted with the medians and interquartile ranges for weeks 0, 4, 12, and 24. The p values are provided for the changes in monocyte subsets from week 0 to each indicated week. Median levels at weeks 0, 4, 12, and 24 are plotted with interquartile ranges, and p values are provided Wilcoxon signed-rank tests for the changes from week 0 to each indicated week
Modest decline in CD8+ T cell activation after MVC intensification
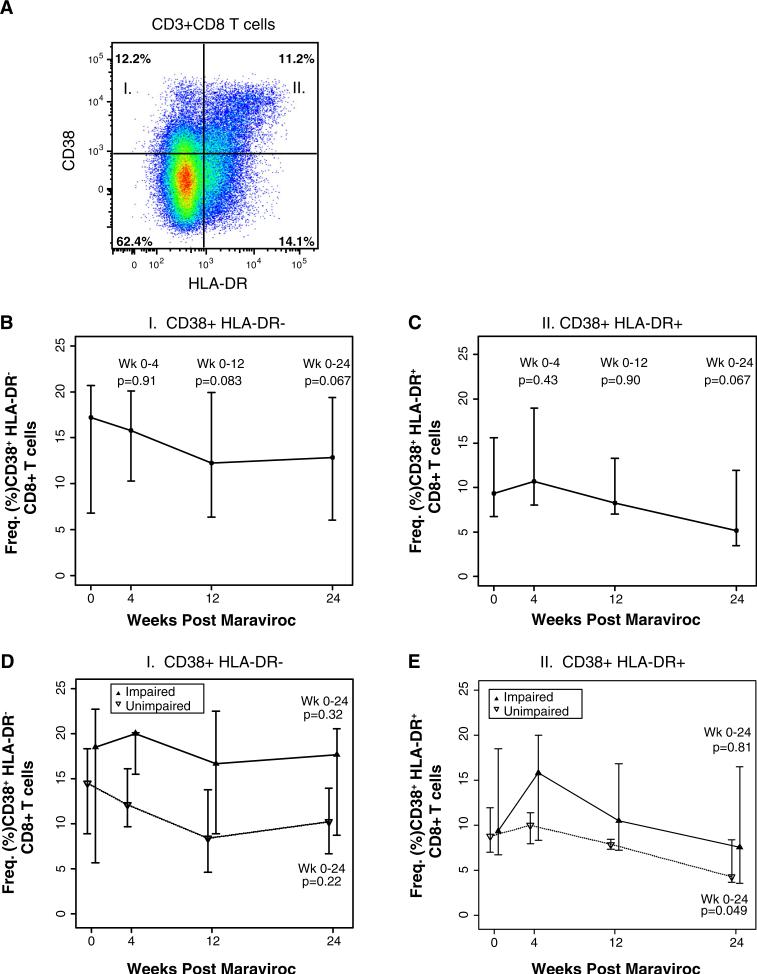
By flow cytometry, we measured and compared the frequency of HLA-DR and CD38 expression and co-expression of CD8+ T cells at entry up to week 24 (Fig. 2a). Among all the T subsets, we observed a modest decline in the frequency of CD38+ HLA-DR− CD8+ T cells from a median 17.2 % (6.7, 20.7) at entry compared to 12.8 % (6.0, 13.9) at week 24 (p=0.06) (Fig. 2b) and CD38+ HLA-DR+ CD8+ T cells median (IQR) 9.3 % (6.7, 15.6) at entry compared to 5.1 % (3.4, 11.9) at week 24 (p=0.06; Fig. 2c) in all subjects. When stratified by cognitive status, there were no significant differences in the changes in CD38+ HLA-DR+ or CD38+ HLA-DR− CD8+ T cells from baseline to week 24 between impaired and unim-paired groups (Fig. 3d, e). However, the unimpaired group showed a significantly drop in the frequency of CD38+ HLADR+ CD8+ T cells at week 24 from baseline (p=0.049; Fig. 2e) to levels that are observed in healthy HIV-uninfected subjects (Crawford et al. 2011; Zheng et al. 2014).
Fig. 2.
Flow cytometric gating strategy to assess changes in Tcell subset frequencies Representative gating strategy of single-cell flow cytometry assessment of live CD3+ CD8+ T subsets based on a CD38 and HLA-DR expression. The data were analyzed in 12 subjects before and during 24 weeks of MVC intensification. Graphs depict the change in CD38 and HLA-DR expression on CD8+ T cells plotted with the medians and interquartile ranges for weeks 0, 4, 12, and 24 in the group as a whole (b, c) or stratified based on cognitive status (n=6 each) (d, e). The p values are provided for the changes in T cell activation from week 0 to each indicated week. Median levels at weeks 0, 4, 12, and 24 are plotted with interquartile ranges, and p values are provided Wilcoxon-signed rank tests for the changes from week 0 to each indicated week
Fig. 3.
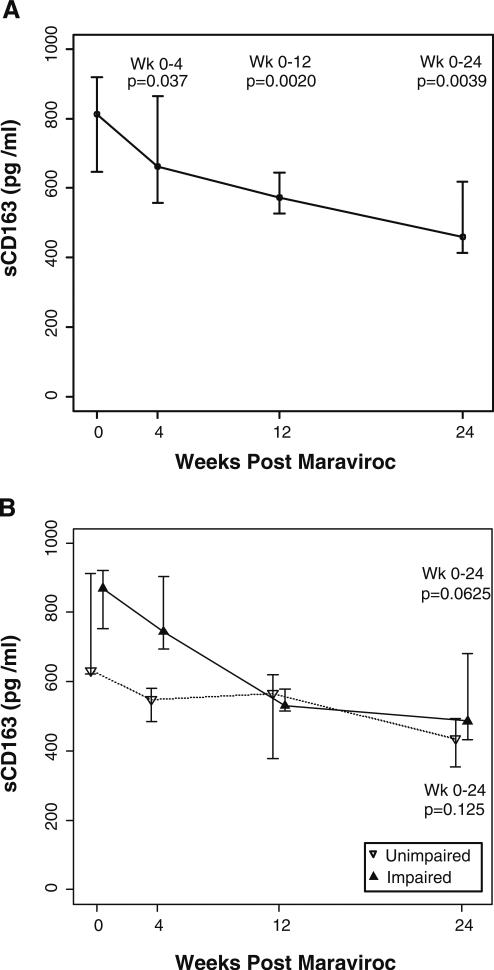
Change in plasma levels of sCD163 at entry (week 0) and weeks 4, 12, and 24 of MVC intensification plasma levels of sCD163 were analyzed using the Wilcoxon signed-rank test and are plotted showing the median and interquartile ranges in the group (a) or separated based on cognitive status. The p values are depicted for the changes in sCD163 levels from week 0 to each indicated week
Marked declines in plasma sCD163 after MVC intensification
As MVC led to declines in proinflammatory monocyte subsets, we also assessed whether these changes affected systemic monocyte activation markers. We examined sCD163 levels in patients upon entry and at weeks 4, 12, and 24. At entry in all patients, the median (IQR) sCD163 levels were 811 pg/ml (646.9, 918.9). We observed a decline in sCD163 as early as week 4 with a difference of 149 pg/ml (p=0.037). By week 24, sCD163 had significantly declined by half to 460.6 pg/ml (412.9, 617.8) compared to entry levels (p=0.0039; Fig. 3a). When subjects were segregated by cognitive status, the impaired group had significantly higher levels of sCD163 at week 4 compared to the unimpaired group (p=0.0015; Fig. 3b); however, no significant difference in the change from entry to week 4 between impaired versus the unimpaired subjects was observed. From weeks 0 to 24, the levels of sCD163 in both groups declined to similar levels though the impaired group had a greater trend in decline (p=0.06) compared to the unimpaired group (p=0.125; Fig. 3b).
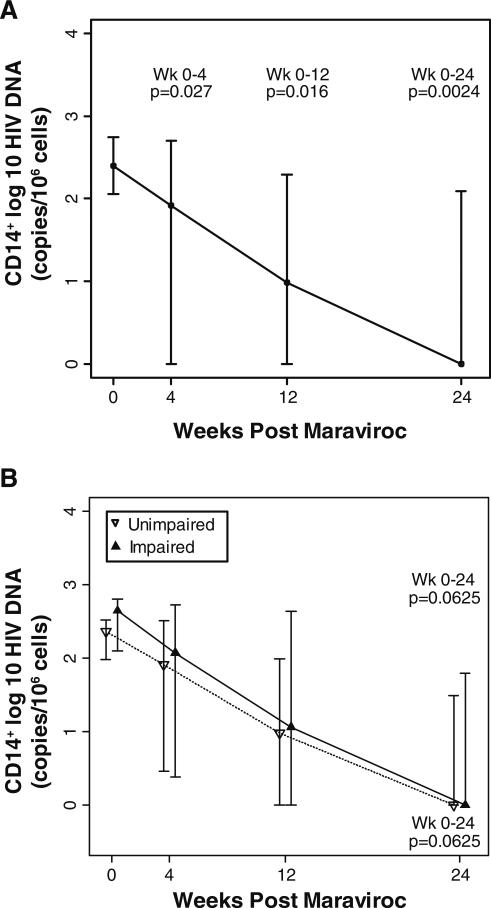
HIV DNA content in monocytes declines following MVC intensification
Specimens from 12 subjects were compared at week 24 and entry. A decrease in HIV DNA was observed in the monocyte (CD14+) subset in 10 of the 12 subjects. The HIV DNA log10 copies/106 cells within monocytes significantly declined from a median (IQR) of 2.4 (2.1, 2.7) on entry to 0.0 (0.0, 2.1) at week 24 (p=0.0024; Fig. 4) in the group as a whole. When separated by cognitive status, both groups trended to decline in HIV DNA levels (p=0.06; Fig. 4). No significant differences in the changes in HIV DNA levels from baseline to week 24 between the impaired and unimpaired groups were observed.
Fig. 4.
Change in monocyte HIV DNA content at entry (week 0) and weeks 4, 12, and 24 of MVC intensification. This graph depicts the change in DNA content on a log10 scale, plotted with the medians and interquartile ranges for weeks 0, 4, 12, and 24 in all study subjects (a) or separated based on cognitive status (b). The p values are provided for the changes in HIV DNA from week 0 to each indicated week
Improvement in neuropsychological test performance following MVC intensification among cognitively impaired subjects
Although there was no significant change from entry (week 0) to week 24 in global composite scores (NPZglobal) or any NP subdomain scores, there was an improvement in executive function (median change of NPZef, 0.37) that showed a trend toward significance (p=0.08). The median change in NPZglobal in the entire group from weeks 0 to 24 was 0.13 (p=0.27). However, some significant neuropsychological improvement was evident when the six subjects who entered the study with impairment (NPZglobal≤−0.5) were analyzed separately. Table 2 presents NPZ global test results at entry, at week 24, and the weeks 0–24 changes for all 12 subjects, as well as NPZ subdomain scores (executive function, psycho-motor speed and attention, learning and memory) for the cognitively impaired group only. Between weeks 0 and 24, impaired subjects showed significant improvements in global functioning (median change in NPZglobal, 0.57; p=0.03), learning and memory (median change in NPZlrn_mem, 0.66; p=0.03), and executive function (median change in NPZef, 0.89; p=0.046).
Table 2.
Entry (week 0), week 24, and weeks 0–24 change in NPZglobal scores following Maraviroc (MVC) intensification in each of the 12 subjects identified by patient ID (PID). Median change Δ (weeks 24–week 0) in global NP function (NPZglobal) is shown for all participants. Changes in executive function (NPZef), psychomotor speed and attention (NPZpma), and learning and memory (NPZlrn_mem) are presented for those identified as cognitively impaired at entry (NPZglobal ≤–0.5). P values were produced by Wilcoxon signed-rank test
| PID (Cognitively Unimpaired, n=6) | NPZglobal (Wk 0) | NPZglobal (Wk 24) | NPZglobal Δ | |||
| 5 | –0.14 | –0.15 | –0.01 | |||
| 18 | 1.13 | 0.42 | –0.71 | |||
| 37 | 1.06 | 1.08 | 0.02 | |||
| 58 | 0.23 | 0.28 | 0.05 | |||
| 92 | 0.45 | 0.01 | –0.44 | |||
| 291 | 0.42 | –0.25 | –0.67 | |||
| PID (Cognitively Impaired, n=6) | NPZglobal (Wk 0) | NPZglobal (Wk 24) | NPZglobal Δ | NPZef Δ | NPZpma Δ | NPZlrn_mem Δ |
| 21 | –1.15 | 0.66 | 1.81 | 1.44 | 2.63 | 0.77 |
| 26 | –1.43 | –0.25 | 1.18 | 0.95 | 1.77 | 0.11 |
| 28 | –1.17 | –0.42 | 0.75 | 1.08 | 0.68 | 0.58 |
| 41 | –1.17 | 0.97 | 0.20 | –0.20 | –0.19 | 1.14 |
| 65 | –0.90 | 0.57 | 0.33 | 0.82 | 0.11 | 0.52 |
| 199 | 0.55 | 0.16 | 0.39 | 0.60 | 0.07 | 0.74 |
| NPZglobal Median Δ (IQR) (All Subjects, n=12) | 0.13 (–0.12, 0.48) | |||||
| Median Δ (IQR) (Cognitively Impaired, n=6) | 0.57 (0.35, 1.07) | 0.89 (0.60, 1.08) | 0.40 (0.08, 1.50) | 0.66 (0.54, 0.76) | ||
| P-value for median Δ (Cognitively Impaired, n=6) | 0.028 | 0.046 | 0.116 | 0.028 |
P-values produced by Wilcoxon signed-rank test
Discussion
This small study provides data that suggests that a single CCR5 antagonist (maraviroc, MVC) favorably alters monocyte activation, lowers the HIV DNA burden in CD14+ monocytes, and is associated with evidence of improvement in neuropsychological (NP) performance among subjects who initiated the study with some cognitive impairment. The findings of this study serve to argue for therapy-directed against monocytes/macrophages as a therapeutic modality to reduce the frequency or severity of HAND and for the development of HIV antiretroviral medications with efficacy against monocytes.
Our rationale for embarking on a pilot study of MVC for HAND was prompted by our understanding that monocytes are central players in its pathogenesis, our past work demonstrating an association between levels of HIV-infected MO and HAND, and the fact that CCR5 serves as the principal co-receptor for entry of HIV into monocytes. We have further published that efficacy of HIV antiretroviral therapy in preventing HIV infection of monocytes can be correlated to better cognition among HIV-infected subjects on suppressive cART (Shikuma et al. 2012), and we noted with interest that published data on MVC monocyte EC50 suggested a high level of potential efficacy against preventing HIV infection of MO (Aquaro et al. 2006). We hypothesized that assessment of the impact of MVC on monocyte subsets and on HIVinfection of monocytes may allow an understanding of whether monocyte directed therapy may have potential efficacy against HAND.
In our study, we observed nonsignificant but sustained modest declines in CD38+HLA-DR+ and CD38+ HLA-DR− CD8+ T cell activation after MVC intensification. Several reports have revealed that MVC-treated patients demonstrated significant decreases in CD4+ and CD8+ T cell immune activation (Psomas et al. 2013; Rusconi et al. 2013; Stefano et al. 2013; Westrop et al. 2012). We are aware that contradictory results have been reported in studies looking at MVC intensification and CD8+ T cell immune activation (Funderburg et al. 2010; Gutierrez et al. 2010; Hunt et al. 2011; Rusconi et al. 2010; Wilkin et al. 2010); in particular, one small placebo-controlled MVC intensification study found an increase in CD8+ T cell immune activation in HIV subjects whose ART was intensified with MVC and modest increases in sCD163 and sCD14 (Hunt et al. 2011). The population studied in this trial focused on HIV subjects with very low nadir CD4+ T cell counts, extensive pre-existing immune depletion, and poor CD4+ Tcell recovery and is therefore unlikely to be applicable to our population studied here who were good immunological responders. It has also been suggested that starting baseline levels of T cell activation may also be relevant in interpreting the differing effects of MVC intensification on T cell immune activation (Psomas et al. 2013). Taken together, the cellular immune effect of MVC intensification against T cell immune activation remains unclear; it may depend on a patient's immunological status or suppressive therapy regimen among other factors.
Our study uniquely focused on the impact of MVC against monocytes rather than T cells. We found that MVC intensification led to decreases in monocyte HIV DNA levels and in immune activation in these cells. Of interest in our findings is that when compared to the unimpaired group, MVC lead only to a significant decline in nonclassical monocytes in the impaired group. While it is tempting to conclude that decrease in the level of HIV-infected monocytes lead to decrease in immune activation in these cells, caution is warranted as declines in total HIV DNA in monocytes were similar between the two groups. The patients who entered into our single-arm MVC intensification study had plasma HIV RNA <48 copies/ml. Although convincing studies in the literature have demonstrated that treatment intensification does not reduce residual HIV viremia in patients already on highly active antiretroviral therapy (Dinoso et al. 2009; Havlir 2003), a direct MVC antiviral effect on lymphocytes due to intensification of the ART regimen cannot be excluded. Alternatively, the beneficial clinical effect of MVC may have been mediated not by MVC's direct antiviral effect (Rossi et al. 2011) but by MVC's ability to block or alter the ligation of major human inflammatory agents, the chemokines MIP-1α, and RANTES to its CCR5 chemokine receptor. CCR5 has been shown to be critical for lymphocyte recruitment to tissues (Reshef et al. 2012; Rossi et al. 2010). Serum from patients receiving MVC prevented CCR5 internalization by CCL5 and blocked T-cell chemotaxis in vitro, providing evidence of antichemotactic activity. Blockade of CCR5 may prevent CCR5-bearing T cells as well as monocytes from stimulation and may also inhibit the migration of CCR5-expressing immune cells.
We found improvement in neuropsychological test performance among subjects who started the study with evidence of mild to moderate cognitive impairment. These results are reinforced by seeing significant improvement or trend toward improvement in learning and memory and executive function, two subdomains that are typically impacted by HIV, and which can directly impact the overall quality of life and functionality of those living with HIV. Only limited studies are available regarding the potential CNS effects of MVC. MVC monotherapy for 5 months in a SIV-infected macaque model demonstrated a reduction of SIV RNA and proviral DNA levels, and a reduction in CD68 immunostaining and in TNF-α and CCL2 RNA expression in brain (Kelly et al. 2013). A decrease in plasma CD163, a marker of monocyte activation, was also demonstrated. A small improvement in the cerebral metabolite marker of neuronal integrity N-acetyl aspartate/creatine (NAA/Cr) was observed in 12 patients after 14 days of treatment intensification with MVC (Vera et al. 2012). Our results of reduction in monocyte HIV DNA levels and lowering of monocyte immune activation, including a decrease in plasma sCD163 levels and in a modest improvement in cognitively impaired subjects, is consistent with these prior studies.
Our study was limited by the small sample size and not being placebo controlled. Examination of CSF HIV RNA or brain metabolites by MRS may have been helpful in collaborating the conclusions drawn from changes observed in immune activation parameters and in neuropsychological test performance. Nevertheless, the data is suggestive that MVC intensification of suppressive ART leads to a decrease in circulating HIV-infected monocytes and in monocyte immune activation, and post hoc analysis show an improvement in cognition among individuals who initiated the study with evidence of neurocognitive impairment. These results have implications for the management of HIV cognitive impairment in the era of suppressive ART because it provides proof of concept that targeting the hypothesized pathogenesis of HAND-involving monocytes may have efficacy in preventing or treating HIV-associated cognitive impairment. Development of HIV antiretroviral medication with potent activity against peripheral monocytes may be warranted.
Acknowledgments
We thank our study participants and community physicians for their roles in this study, Dr. Mark S. Shaefer (ViiV Healthcare) for the donation of Maraviroc and Mr. Kawakahi Amina for assistance with ELISA studies. This work was presented in part at the 19th and 20th Conference on Retroviruses and Opportunistic Infections (CROI) in March 5–8, 2012 at Seattle, WA, USA and March 3–6, 2013 at Atlanta, GA, USA. This work was supported in part by NIH/NIMHD grant U54MD007584, G12MD007601, and U19MH081835 McGrath, M (PI) [UCSF].
Footnotes
No conflicts of interest are declared by all co-authors.
Contributor Information
Lishomwa C. Ndhlovu, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA Department of Tropical Medicine, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Tracie Umaki, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Glen M. Chew, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA
Dominic C. Chow, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA
Melissa Agsalda, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA; Department of Tropical Medicine, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Kalpana J. Kallianpur, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA
Robert Paul, Department of Psychology, University of Missouri, St. Louis, MO, USA.
Guangxiang Zhang, Biostatistics and Data Management Core, John A. Burns School of Medicine, University of Hawai’i, Honolulu, HI, USA.
Erika Ho, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Nancy Hanks, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Beau Nakamoto, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Bruce T. Shiramizu, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA Department of Tropical Medicine, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA.
Cecilia M. Shikuma, Hawaii Center for AIDS, University of Hawai’i, 651 Ilalo St, BSB 325C, Honolulu, HI 96815, USA
References
- Abdulle S, Hagberg L, Svennerholm B, Fuchs D, Gisslen M. Continuing intrathecal immunoactivation despite two years of effective antiretroviral therapy against HIV-1 infection. AIDS. 2002;16:2145–2149. doi: 10.1097/00002030-200211080-00006. [DOI] [PubMed] [Google Scholar]
- Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, Xystrakis E, Khamri W, Shawcross DL, Ma Y, Wendon JA, Vergani D. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi)/CD16(neg) monocytes: expansion of CD14(hi)/CD16(pos) and contraction of CD14(lo)/CD16(pos) monocytes in acute liver failure. Cytometry A. 2012;81:823–834. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. A RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Andrade AS, Deutsch R, AC S, Duarte NA, Marcotte TD, Umlauf A, Atkinson JH, McCutchan JA, Franklin D, Alexander TJ, McArthur JC, Marra C, Grant I, Collier AC. Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. J Acquir Immune Defic Syndr. 2013;62:282–92. doi: 10.1097/QAI.0b013e31827ed678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Aquaro S, Svicher V, Schols D, Pollicita M, Antinori A, Balzarini J, Perno CF. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80:1103–1110. doi: 10.1189/jlb.0606376. [DOI] [PubMed] [Google Scholar]
- Barbour JD, Jalbert EC, Chow DC, Gangcuangco LM, Norris PJ, Keating SM, Heitman J, Nagamine L, Seto T, Ndhlovu LC, Nakamoto BK, Hodis HN, Parikh NI, Shikuma CM. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis. 2014;232:52–58. doi: 10.1016/j.atherosclerosis.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck ATWC, Mendelson M. Beck Depression Inventory (BDI). Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Mezhir JJ, Walsh K, Hewitt RG. Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch Clin Neuropsychol. 2000;15:535–544. [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 made by monocyte/macrophages is a novel marker of HIVactivity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. Aids. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology. 1996;47:1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Crawford TQ, Ndhlovu LC, Tan A, Carvidi A, Hecht FM, Sinclair E, Barbour JD. HIV-1 infection abrogates CD8+ T cell mitogen-activated protein kinase signaling responses. J Virol. 2011;85:12343–12350. doi: 10.1128/JVI.05682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuro-psychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, O'Shea A, Callender M, Spivak A, Brennan T, Kearney MF, Proschan MA, Mican JM, Rehm CA, Coffin JM, Mellors JW, Siliciano RF, Maldarelli F. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste A, Sorg C, Hogger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem Biophys Res Commun. 1999;256:110–113. doi: 10.1006/bbrc.1999.0294. [DOI] [PubMed] [Google Scholar]
- Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- Farina C, Theil D, Semlinger B, Hohlfeld R, Meinl E. Distinct responses of monocytes to toll-like receptor ligands and inflammatory cytokines. Int Immunol. 2004;16:799–809. doi: 10.1093/intimm/dxh083. [DOI] [PubMed] [Google Scholar]
- Funderburg N, Kalinowska M, Eason J, Goodrich J, Heera J, Mayer H, Rajicic N, Valdez H, Lederman MM. Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLoS One. 2010;5:e13188. doi: 10.1371/journal.pone.0013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Giancola ML, Lorenzini P, Balestra P, Larussa D, Baldini F, Corpolongo A, Narciso P, Bellagamba R, Tozzi V, Antinori A. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active anti-retroviral therapy. J Acquir Immune Defic Syndr. 2006;41:332–337. doi: 10.1097/01.qai.0000197077.64021.07. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsycho-logical impairment. Neuropsychol Rev. 2009;19:186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C DL, Hernandez-Novoa B, et al. Effect of the intensification with a CCR5 antagonist on the decay of the HIV-1 latent reservoir and residual viremia.. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlir DV. Strategic approaches to antiretroviral treatment. Top HIV Med. 2003;11:145–149. [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW SN, Hayes T, Dahl V, Somsouk M, Funderburg N, Landay AL, Adeyemi O, Shafer R, Clagett B, Rodriquez B, Martin JN, Shacklett B, Palmer S, Lederman MM, Deeks SG. The immunomodulatory effects of Maraviroc intensification among ART-suppressed patients with incomplete CD4 recovery.. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- Jalbert E, Crawford TQ, D'Antoni ML, Keating SM, Norris PJ, Nakamoto BK, Seto T, Parikh NI, Shikuma CM, Ndhlovu LC, Barbour JD. IL-1beta enriched monocytes mount massive IL-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable anti-retroviral therapy at risk for cardiovascular disease. PLoS One. 2013;8:e75500. doi: 10.1371/journal.pone.0075500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, Shikuma C. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex. 2012;22:2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur KJ, Shikuma C, Kirk GR, Shiramizu B, Valcour V, Chow D, Souza S, Nakamoto B, Sailasuta N. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80:1792–1799. doi: 10.1212/WNL.0b013e318291903f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Beck SE, Pate KA, Queen SE, Dorsey JL, Adams RJ, Avery LB, Hubbard W, Tarwater PM, Mankowski JL. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. Aids. 2013 doi: 10.1097/QAD.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavy O. Immunotherapy: stopping monocytes in their tracks. Nat Rev Immunol. 2011;11:715. doi: 10.1038/nri3096. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervoussystem. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, Pruett JE, Cohn ZA. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci U S A. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell NA, Kadow JF. Maraviroc, a chemokine CCR5 receptor antagonist for the treatment of HIV infection and AIDS. Curr Opin Investig Drugs. 2007;8:669–681. [PubMed] [Google Scholar]
- Nakasujja N, Miyahara S, Evans S, Lee A, Musisi S, Katabira E, Robertson K, Ronald A, Clifford DB, Sacktor N. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology. 2013;80:196–202. doi: 10.1212/WNL.0b013e31827b9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psomas C, Lavigne JP, Barbuat C, Trabelsi S, Ghosn J, Lascoux-Combe C, Flandre P, Cuzin L, Reynes J, Autran B, Corbeau P. Maraviroc-induced decrease in circulating bacterial products is not linked to an increase in immune activation in HIV-infected individuals. Blood. 2013;122:2282–2283. doi: 10.1182/blood-2013-06-507012. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol. 2004;157:93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, Goldstein SC, Stadtmauer EA, Smith J, Bailey S, Mick R, Heitjan DF, Emerson SG, Hoxie JA, Vonderheide RH, Porter DL. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Lichtner M, Sauzullo I, Mengoni F, Marocco R, Massetti AP, Mastroianni CM, Vullo V. Downregulation of leukocyte migration after treatment with CCR5 antagonist maraviroc. J Acquir Immune Defic Syndr. 2010;54:e13–e14. doi: 10.1097/QAI.0b013e3181ed18f6. [DOI] [PubMed] [Google Scholar]
- Rossi R, Lichtner M, De Rosa A, Sauzullo I, Mengoni F, Massetti AP, Mastroianni CM, Vullo V. In vitro effect of anti-human immunodeficiency virus CCR5 antagonist maraviroc on chemotactic activity of monocytes, macrophages and dendritic cells. Clin Exp Immunol. 2011;166:184–190. doi: 10.1111/j.1365-2249.2011.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S VP, Adorni F, et al. Maraviroc intensification for HIV-1-positive immunological non-responders despite virological suppression during HAART.. Tenth International Congress on Drug Therapy in HIV Infection; Glasgow. 2010. [Google Scholar]
- Rusconi S, Vitiello P, Adorni F, Colella E, Foca E, Capetti A, Meraviglia P, Abeli C, Bonora S, D'Annunzio M, Di Biagio A, Di Pietro M, Butini L, Orofino G, Colafigli M, d'Ettorre G, Francisci D, Parruti G, Soria A, Buonomini AR, Tommasi C, Mosti S, Bai F, Di Nardo Stuppino S, Montano M, Tau P, Merlini E, Marchetti G. Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: an Italian randomized clinical trial. PLoS One. 2013a;8:e80157. doi: 10.1371/journal.pone.0080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Zhang J, Evans SR, Sacktor N, Simpson D, Millar LL, Hung VL, Miller EN, Smith E, Ellis RJ, Valcour V, Singer E, Marra CM, Kolson D, Weihe J, Remmel R, Katzenstein D, Clifford DB. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007;69:1314–1321. doi: 10.1212/01.wnl.0000268487.78753.0f. [DOI] [PubMed] [Google Scholar]
- Shikuma CM, Nakamoto B, Shiramizu B, Liang CY, DeGruttola V, Bennett K, Paul R, Kallianpur K, Chow D, Gavegnano C, Hurwitz SJ, Schinazi RF, Valcour VG. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17:1233–1242. doi: 10.3851/IMP2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma CM, Chow DC, Gangcuangco LM, Zhang G, Keating SM, Norris PJ, Seto TB, Parikh N, Kallianpur KJ, Nakamoto BK, Nagamine LS, Ndhlovu LC, Barbour JD. Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One. 2014;9:e90330. doi: 10.1371/journal.pone.0090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Ananworanich J, Chalermchai T, Siangphoe U, Troelstrup D, Shikuma C, De Grutolla V, Sithinamsuwan P, Praihirunkit P, Rattanamanee S, Valcour V, SEARCH 001.1 Study Group Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol. 2012;18:69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S, Vitiello P, Adorni F, Colella E, Focà E, Capetti A, Meraviglia P, Abeli C, Bonora S, D'Annunzio M, Di Biagio A, Di Pietro M, Butini L, Orofino G, Colafigli M, d'Ettorre G, Francisci D, Parruti G, Soria A, Buonomini AR, Tommasi C, Mosti S, Bai F, Di Nardo Stuppino S, Morosi M, Montano M, Tau P, Merlini E, Marchetti G. Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: an Italian randomized clinical trial. PloS One. 2013 doi: 10.1371/journal.pone.0080157. DOI: 10.1371/journal.pone.0080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML, Giulianelli M, Narciso P, Antinori A. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009;52:56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- Valcour VG, Shiramizu BT, Sithinamsuwan P, Nidhinandana S, Ratto-Kim S, Ananworanich J, Siangphoe U, Kim JH, de Souza M, Degruttola V, Paul RH, Shikuma CM, Southeast Asia Research Collaboration with the University of Hawaii 001 protocol team HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 cohort study. Neurology. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, Schuetz A, Shikuma C, Liang CY, Jirajariyavej S, Sithinamsuwan P, Tipsuk S, Clifford DB, Paul R, Fletcher JL, Marovich MA, Slike BM, DeGruttola V, Shiramizu B. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One. 2013;8:e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera JH, Garvey LJ, Allsop JM, Kaye S, McClure MO, Back D, Taylor-Robinson SD, Winston A. Alterations in cerebrospinal fluid chemokines are associated with maraviroc exposure and in vivo metabolites measurable by magnetic resonance spectroscopy. HIV Clin Trials. 2012;13:222–227. doi: 10.1310/hct1304-222. [DOI] [PubMed] [Google Scholar]
- Westrop SJ, Moyle G, Jackson A, Nelson M, Mandalia S, Imami N. CCR5 antagonism impacts vaccination response and immune profile in HIV-1 infection. Mol Med. 2012;18:1240–1248. doi: 10.2119/molmed.2012.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin T LC, Tenorio A, et al. Maraviroc intensification for suboptimal CD4+ cell response despite sustained virologic suppression: ACTG 5256.. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- Zheng L, Taiwo B, Gandhi RT, Hunt PW, Collier AC, Flexner C, Bosch RJ. Factors associated with CD8+ T-cell activation in HIV-1-infected patients on long-term antiretroviral therapy. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW. Definition of human blood monocytes. J Leukoc Biol. 2000;67:603–606. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]