Abstract
Flavoprotein autofluorescence signals attributed to neuronal metabolism have been used to assess synaptic function. Here, we characterized flavoprotein autofluorescence responses in the molecular layer of rat cerebellar slices. High frequency stimulation elicited a transient fluorescence increase (peak phase) that was followed by a longer-lasting fluorescence decrease (valley phase). The peak phase was restricted to the molecular layer, whereas the valley phase extended into the Purkinje cell layer and a portion of the granule cell layer. Responses were abolished by either the Na+ channel antagonist, tetrodotoxin, or a combination of the AMPA receptor antagonists, NBQX and GIKI-53655, and were also reduced by a flavoprotein inhibitor (diphenyleneiodonium). These findings are consistent with responses being mediated by an increase in mitochondrial activity triggered by increased energy demands evoked by AMPA receptor-mediated synaptic transmission. The GABAA receptor antagonist picrotoxin did not significantly influence evoked responses. Likewise, exogenous application of ethanol, at concentrations known to increase GABAA receptor-mediated synaptic transmission at Purkinje cells, did not modify peak responses. These observations indicate that flavoprotein autofluorescence imaging could be useful to assess the coupling between glutamatergic synaptic transmission and neuronal metabolism in cerebellar slices.
Keywords: Cerebellum, Purkinje neurons, Synaptic, Mitochondria, Flavoproteins, Intrinsic imaging, Ethanol
Introduction
Autofluorescence imaging has been used to investigate regional activation in rodent brain tissues [15, 17]. While much prior work has focused on nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate autofluorescence transients seen following ultraviolet excitation [1], a complementary approach with longer wavelengths has proven very useful for in vivo brain imaging studies. In a number of brain regions, excitation with blue light (420-480 nm) generates fluorescence transients following synaptic activation that are mainly due to changes in redox potential of flavin adenine mononucleotide- and dinucleotide-linked enzymes involved in the mitochondrial electron transport chain [15]. Mechanical skin stimulation evokes a flavoprotein autofluorescence signal in the primary somatosensory cortex of anesthetized rats [14]. Odor-evoked activity in the olfactory bulb [2] and nociceptive responses in the spinal cord [6] were also visualized in anesthetized rodents using this technique. Electrical stimulation of the cerebellar cortex evoked a biphasic beam-like flavoprotein autofluorescence signal in anesthetized mice consisting of a brief increase in fluorescence, followed by a longer lasting reduction in fluorescence [11-13].
Flavoprotein autofluorescence imaging studies have also been obtained using acute brain slices [17]. Electrical stimulation of the Schaffer collaterals evokes biphasic flavoprotein autofluorescence responses in the hippocampal CA1 pyramidal region of coronal brain slices from mice [16]. This technique was also used to characterize hippocampal spreading depression induced by hypoxia in brain slices [4]. Tetanic stimulation of layer V evokes stable flavoprotein autofluorescence responses in layer II/III of slices from the rat auditory cortex [14]. These responses were abolished by tetrodotoxin (TTX) and partially blocked by 6-cyano-7-nitroquinoxaline-2,3-dione, indicating that both presynaptic and postsynaptic activity contributes to the responses. Using thick slices from the cerebellar cortex of mice, Coutinho et al. [3] showed that electrical stimulation of the molecular layer (ML) elicited biphasic responses that followed the beam-like path of the parallel fibers. Stimulation of these fibers triggered activity of multiple units in the Purkinje cell (PC) layer with presynaptic and postsynaptic components, suggesting that fluorescence signals are correlated with PC firing. These studies demonstrate the utility of flavoprotein autofluorescence imaging with brain slices to map the activity of neuronal ensembles with good spatial and temporal resolution.
Several laboratories, including our own, have demonstrated that the cerebellum is an important target of ethanol [5, 10, 18]. Acute ethanol exposure has been shown to have significant effects on PC synaptic transmission, including increased GABA release onto these neurons and potentiation of GABAA receptor function [7-9].
In this study, characterized the flavoprotein autofluorescence responses mediated by synaptic transmission between granule cell axons (both ascending segments and parallel fibers) and PCs in parasagittal slices from the cerebellar vermis of juvenile rats. Having established these as robust and reproducible, we tested their sensitivity to pharmacological agents that affect synaptic transmission, including ethanol.
Methods
All chemicals were from Sigma (St. Louis, MO) or Tocris Bioscience (Ellisville, MO). All experiments were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee. Male Sprague Dawley rats (21-25 day-old) from Harlan Laboratories (Indianapolis, IN) were used for this study. Animals were euthanized by rapid decapitation under deep anesthesia with ketamine (250 mg/kg, I.P.). Brains were rapidly removed and held for two minutes in an ice-cold solution containing (in mM): 220 sucrose, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 12 MgSO4, 10 glucose, 0.2 CaCl2 and 0.43 ketamine pre-equilibrated with 95% O2/5% CO2. Cerebellar vermis parasagittal slices (200μm) were cut in the same sucrose-containing solution using a vibrating tissue slicer (Leica Microsystems, Bannockburn, IL, USA). Slices were then transferred into artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 1 MgSO4, 2 CaCl2 and 0.4 ascorbic acid, equilibrated with 95% O2/5% CO2, and held for 30 minutes at 34–35oC. Subsequently, slices were allowed to recover at 21oC for at least 30 min before being transferred to a recording chamber perfused with ACSF (2 ml/min) at 32°C.
Concentric bipolar electrodes (25μm inner pole diameter and 125μm outer pole diameter; FHC, Bowdoin, ME) connected to an ISO-Flex stimulus isolator and a Master 8 stimulator (A.M.P.I, Jerusalem, Israel) were used to stimulate granule cell axons in the outer third of the ML. Stimulus trains (50Hz, 100μs duration, 0.1-1mA) were applied at 5 min intervals. Flavoprotein autofluorescence was monitored following excitation at 470nm using a Polychrome V system (Till Photonics, Grafeling, Germany), as previously described [16]. The fluorescence emission was monitored using a 520–540 nm band pass filter and a 10x water-immersion objective (0.3 NA, Olympus, Center Valley, PA). Optical responses were acquired using a cooled interline transfer charged-coupled device camera (IMAGO Camera; Till Photonics). The frame acquisition rate of the optical response was 3 Hz with an exposure time of 100ms. Fluorescence data were collected after 4 × 4 binning of the 344 × 260 line image. Individual pixels after binning correspond to an area of 0.27μm2. To quantify optical responses, a series of images consisting of 300 sequential frames was acquired. The first 49 pre-stimulus frames of each response were fitted to a double exponential decay phase curve (R2 = 0.89 ± 0.03; n=7 slices from 4 animals), which was then used to correct evoked flavoprotein autofluorescence responses for bleaching effects. Results are presented as the percent change in fluorescence intensity divided by pre-stimulus fluorescence (ΔF/F0 (%)). For figure presentation, images were processed using TILLvisION software (Till Photonics) or Image J 1.46r (National Institutes of Health, Bethesda, MD).
Statistical analyses were performed with Prizm 5 (GraphPad, San Diego, CA). Data were initially analyzed with the Kolmogorov-Smirnov normality test. In all cases, data followed a normal distribution and, therefore, these were analyzed using paired student’s t-tests or repeated measures one-way ANOVA followed by Dunnett’s post hoc test; a p< 0.05 was considered to be statistically significant. For all statistical analyses, the experimental unit was a slice. The results are expressed as the mean ± SEM.
Results
Electrical stimulation in the ML evokes a biphasic change in intrinsic fluorescence
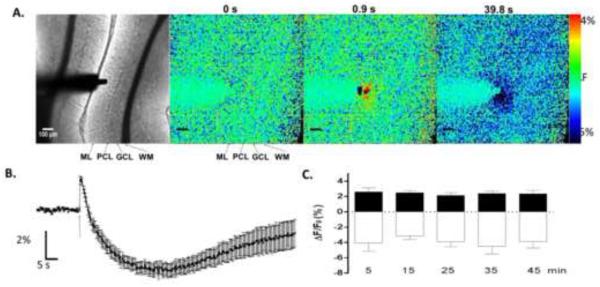
To evoke intrinsic fluorescence changes in cerebellar slices, we placed a concentric bipolar stimulating electrode in the outer third of the ML and monitored changes in optical responses (Fig 1A). Stimulation (600 μA) with a train of 10 pulses at 50 Hz induced an increase in intrinsic fluorescence that peaked 0.92±0.09 s after stimulation (n=11 slices from 6 animals). This phase of the response, denoted as the peak, spread 64.52±9.45 μm radially and 148.9±10.63 μm perpendicularly from the stimulation point (n= 8 slices from 6 animals; Fig 1A). The peak was followed by a decrease in fluorescence that reached a maximum 39.87±2.61 s after stimulation (n=11 slices from 6 animals) and spread 194.3±45.1 μm radially and 190.6±31.15 μm perpendicularly (n=8 slices from 6 animals; Fig 1A). This phase of the response will be denoted as the valley. Fig 1A shows that the peak phase was localized within the ML, whereas the valley phase extended into the PC layer and a portion of the granule cell layer.
Figure 1. Characterization of flavoprotein autofluorescence responses in cerebellar slices.
A. Shown in the left panel is a bright-field image of a cerebellar slice showing the molecular layer (ML), granule cell layer (GCL), Purkinje cell layer (PCL), white matter bundle (WM) and the stimulating electrode placement (scale bar=100 μm). The panels on the right show fluorescence images obtained from the same slice before stimulation (t=0 s) and t = 0.92 s corresponding to the peak phase and t = 39.8 s corresponding to the valley phase. B. Average flavoprotein autofluorescence responses illustrating the peak and valley phases (n=11 slices from 6 animals). The arrow indicates time of stimulation. Data are presented as the mean ΔF/F0 (%) ± SEM. C. Average amplitude of the peak (black bars) and valley (white bars) phases as a function of time (n=5 slices from 3 animals). The responses shown in all panels were evoked by a train of 10 pulses (pulse duration = 100 μs) at 50 Hz (stimulation intensity = 600 μA).
Fig 1B illustrates that relatively consistent responses could be evoked in slices (n=11 slices from 6 animals). The maximum change in ΔF/F0 was 2.44±0.23% for the peak phase and −4.70±0.46% for the valley phase, indicating that the variance of the peak amplitudes for the two components was similar. Fig 1C shows that the intrinsic autofluorescence responses were also relatively stable over a 45 min recording period (peak: F(4,16)=0.3491; p=0.6955; valley: F(4,6)=0.4626; p=0.6705 by repeated measures one-way ANOVA; n=5 slices from 3 animals).
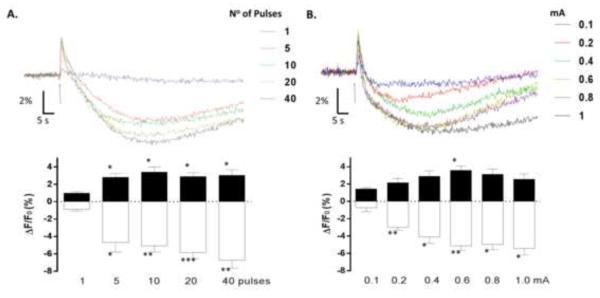
Fig 2A (upper panel) shows optical signals evoked by an increasing number of stimuli. One stimulus was not sufficient to elicit a stable biphasic response, with only a small increase in fluorescence being evident under this condition. The typical biphasic response was clearly observed with 5 or more stimuli. One-way ANOVA with repeated measures detected a significant effect of the number of pulses on both the peak [F(6,24)=4.626; p=0.0310] and the valley phases [F(6,24)=20.5; p=0.0001] (n=7 slices from 5 animals). Analysis with the Dunnett’s post hoc test detected a significant difference between the responses induced by 5 or more stimuli vs.1 stimulus (Fig 2A, lower panel). Fig 2B (upper panel) illustrates that the optimal stimulation intensity to evoke the peak phase was 0.6 mA. One-way ANOVA with repeated measures showed a significant effect of stimulation intensity on the amplitude of the peak phase [F (4,20)=6.085; p= 0.0257]. Post hoc analysis with Dunnett’s multiple comparison test revealed a statistically significant difference between the peak amplitude of responses evoked by 0.1 mA vs. 0.6 mA (Fig 2B, lower panel; n=5 slices from 5 animals). Regarding the amplitude of the valley phase, one-way ANOVA with repeated measures showed a significant effect of stimulation intensity [F(4,20)=13.35; p=0.0017]. Post hoc analysis with Dunnett’s multiple comparison test revealed that the amplitude of the valley phase induced at a stimulation intensity of 0.1 mA was significantly different from those induced by higher stimulation intensities (n=5 slices from 5 animals; Fig 2B, lower panel). For subsequent experiments, we chose to stimulate with a train of 10 pulses at 50 Hz with an intensity of 0.6 mA.
Figure 2. Flavoprotein autofluorescence responses evoked under different stimulation conditions.
A. The upper panel shows representative flavoprotein autofluorescence responses evoked by the indicated number of pulses (pulse duration = 100 μs; pulse frequency=50 Hz; stimulation intensity=600 μA). The lower panel illustrates the average amplitudes of the peak (black bars) and valley (white bars) phases as a function of the number of pulses in the stimulus train (n=7 slices from 5 rats; * p□0.05, ** p□0.01, *** p□0.001 by repeated measures one-way ANOVA followed by Dunnett’s multiple comparison post-hoc test). B. The upper panel shows representative flavoprotein autofluorescence responses evoked by the indicated stimulus intensities. Other stimulation parameters were held constant (10 pulses, pulse duration=100 μs; pulse frequency=50 Hz). The lower panel illustrates the average amplitudes of the peak (black bars) and valley (white bars) phases as a function of the stimulation intensity (n=5 slices from 5 rats; * p?0.05, ** p□0.01 by repeated measures one-way ANOVA followed by Dunnett’s multiple comparison post-hoc test). Data are presented as the mean ΔF/F0 (%)±SEM. The arrows indicate time of stimulation.
Autofluorescence signals are mediated by flavoprotein oxidation triggered by AMPA receptor-dependent synaptic transmission
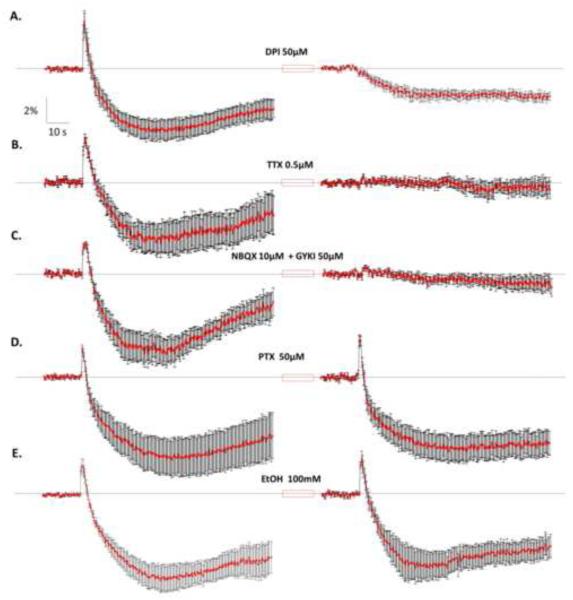
To confirm the metabolic origin of the intrinsic fluorescence signals evoked by electrical stimulation, we tested the effect of diphenyleneiodonium (DPI), a specific inhibitor of flavoproteins. Application of DPI for 20 min abolished the peak phase and significantly reduced the valley phase (Fig 3A). A paired t-test demonstrated a significant reduction in the maximum amplitude of both the peak phase (Control ΔF/F0= 3.67 ±0.88%; DPI ΔF/F0=0.06±0.33%; t(6)=4.171; p=0.0059; n=7 slices from 4 animals) and the valley phase (Control ΔF/F0 =-4.89 0.81%; DPI ΔF/F0=-2.30±0.45%; t(6)=3.290; p=0.0166; n=7 slices from 4 animals).
Figure 3. Pharmacological characterization of the flavoprotein autofluorescence responses.
A. average responses obtained before and after a 20 min exposure to diphenyleneiodonium (DPI; 50μM), a flavoprotein inhibitor (n=7 slices from 4 rats). B. Responses obtained before and after a 10 min application of tetrodotoxin (TTX; 0.5μM) (n=7 slices from 5 rats). C. Responses obtained before and after a 10 min application of the AMPA receptor antagonists NBQX (10μM) and GYKI-53655 (50μM) (n=6 slices from 5 rats). D. Application of picrotoxin (PTX; 50μM for 10 min) did not affect the amplitude of the peak and valley phases (n=6 slices from 3 rats). E. Application of ethanol (EtOH; 100mM for 10 min) did not affect the amplitude of the peak and valley phases of the responses (n=7 slices from 6 rats). Data are the mean ΔF/F0 (%)±SEM. In all cases, responses were evoked by stimulating at 600μA (10 pulses; pulse duration = 100μs; pulse frequency= 50Hz). For results of statistical analysis, see text.
We next determined whether glutamatergic and/or GABAergic synaptic transmission at the ML mediates the intrinsic fluorescence signal evoked by electrical stimulation. To block action potential-dependent transmitter release, we used the voltage-gated Na+ channel blocker, TTX (0.5μM), which significantly reduced the responses. A paired t-test showed a significant decrease in the amplitude of both the peak phase (Control ΔF/F0 =2.60±0.27%; TTX ΔF/F0=0.57±0.11%; t(6)=7.430; p=0.0003; n=7 slices from 5 animals) and valley phase (Control ΔF/F0=-4.04±0.77%; TTX ΔF/F0=-0.06±0.08%; t(6)=4.028; p=0.0069; n=7 slices from 5 animals) (Fig 3B). A similar effect was observed with a combination of competitive (NBQX, 10μM) and non-competitive (GYKI-53655; 50μM) antagonists of AMPA receptors, which also significantly decreased the amplitude of the peak phase (Control ΔF/F0=1.93±0.14%; NBQX+GYKI-53655 ΔF/F0=0.78±0.02%; paired t-test: t(5)=6.656; p=0.0012; n=6 slices from 5 animals), and the valley phase (Control ΔF/F0=-5.06±0.83%; NBQX+GYKI-53655 ΔF/F0=− 0.32±0.28%; paired t-test: t(5)=4.782; p=0.0050; n=6 slices from 5 animals) (Fig 3C). Application of the GABAA receptor antagonist, picrotoxin (PTX; 50μM), neither significantly changed the peak phase (Control ΔF/F0=2.58±0.27%; PTX ΔF/F0=2.72±0.37 %; paired t-test: t (5)=0.4548; p=0.6684; n=6 from 3 animals) nor the valley phase (Control ΔF/F0=-7.92±1.62%; PTX ΔF/F0=-5.66±0.78%; paired t-test: t(5)=1.725; p=0.145; n=6 slices from 3 animals) (Fig 3D). The duration of the peak phase under control conditions was 1.99±0.28 s and in presence of PTX 2.72±0.49 s (t(5)=1.698, p=0.1502; n=6 slices from 3 animals). The duration of the valley phase under control conditions was 81.2±0.33 s and in presence of PTX 81.03±0.31 s (t(5)=0.3612, p=0.7327; n=6 slices from 3 animals).
We also tested the acute effect of ethanol (EtOH; 100 mM), which has been shown to increase GABA release at PCs [7, 9]. This agent did not significantly affect either the peak phase (Control ΔF/F0=3.29±0.42%; EtOH ΔF/F0=3.37±0.52; paired t-test: t(6)=0.7557; p=0.9198; n=7 slices from 6 animals) or the valley phase (Control ΔF/F0=-6.50±1.84; EtOH ΔF/F0 =-6.67±1.43; paired t-test: t(6)=0.2025; p=0.8462; n= 7 slices from 6 animals) (Fig 3E). The duration of the peak phase under control conditions was 2.56±0.40 s and in presence of EtOH 3.85±0.54 s (t(6)=1.554, p=0.1713; n=7 from 6 animals). The duration of the valley phase under control conditions was 80.78±0.42 s and in presence of EtOH 79.59±0.75 s (t(6)=1.290, p=0.2444; n=7 from 6 animals).
Discussion
This study demonstrates that flavoprotein autofluorescence responses can be electrically evoked by trains of high frequency stimulation delivered to the ML of thin parasagittal slices from the rat cerebellar vermis. The responses were composed of a brief initial increase in fluorescence followed by a longer-lasting decrease in fluorescence. The responses were similar to those previously reported in thin hippocampal and cortical slices [14, 16], as well as thick cerebellar [3]. The range of stimulation intensities and number of pulses required to evoke the responses was in general agreement with those used in those studies. Consistent with the findings of Shuttleworth et al. [16] with hippocampal slices, the spatial distribution of the two components did not match. The peak phase was spatially restricted to the ML, near the site of stimulation. In contrast, the valley phase spread significantly more, involving the PC layer and a portion of the granule cell layer. The amplitudes of both phases were similar to those evoked by electrical stimulation in thick cerebellar [3]. Under our experimental conditions, responses were relatively stable over time and reproducible across slices from different animals, indicating that this preparation could be useful to characterize the effect of physiological, pharmacological, and/or genetic manipulations on the function of cerebellar cortical circuits.
The fluorescence responses could be blocked with DPI, indicating that these originate at flavoproteins, likely located in mitochondria. In addition, the responses were reduced by antagonists of Na+ channels and AMPA receptors, suggesting that they are mediated by action potential-dependent glutamate release from granule cell axons onto dendrites of PCs, and perhaps also dendrites of ML interneurons and Golgi cells. Activation of AMPA receptors in these neurons triggers Na+ influx, leading to activation of the Na+/K+ ATPase and other energy-dependent pumps that restore ionic concentrations inside the cell [3, 16]. This increase in the activity of membrane-bound pumps increases ATP demand, leading to activation of the electron transport chain in mitochondria and the oxidation of FADH2 into fluorescent FAD+ by complex II [15]. Another mechanism that could play a role in the generation of these signals involves AMPA receptor-mediated activation of voltage-dependent Ca2+ channels, leading to an increase in [Ca2+]i which is then taken into mitochondria, causing activation of the tricarboxylic acid cycle and FADH2 oxidation [11]. However, studies with hippocampal slices suggest that the latter mechanism may play a more secondary role in the generation of intrinsic autofluorescence signals generated by glutamate receptor stimulation [15].
An unexpected finding of our study is that blockade of GABAA receptors did not have a significant effect on the flavoprotein autofluorescence responses. Moreover, we did not detect an effect of acute ethanol, using a concentration that is known to increase GABA release at PCs [7, 9]. In hippocampal slices, Shuttleworth et al. [16] detected a large increase in both components of the response after application of bicuculline. Using thick cerebellar, Coutinho et al. [3] also demonstrated a large increase in the responses after exposure to PTX. One possibility is that in thin parasagittal cerebellar slices, GABAA receptor-dependent inhibition is not sufficiently strong to significantly inhibit AMPA receptor-mediated responses evoked by high frequency stimulation. Moreover, high frequency stimulation could elicit synaptic plasticity (e.g., long-term depression) of GABAA receptor-mediated transmission or a long-lasting decrease in GABA release.
Conclusion
We provide further evidence indicating that flavoprotein autofluorescence imaging is a useful technique to characterize the function of neuronal circuits in thin cerebellar slices. Advantages over electrophysiological analysis include reproducibility and spatial resolution. In addition, this imaging technique does not require the use of potentially toxic exogenous fluorophores or stimulation with damaging ultraviolet light. The technique could be useful to characterize coupling between synaptic transmission and energy metabolism under physiological and pathophysiological conditions.
Highlights.
We characterized flavoprotein autofluorescence responses in rat cerebellar slices
Molecular layer stimulation initially increased fluorescence, which then decreased
Responses were abolished by Na+ channel or AMPA receptor antagonists
Neither a GABAA receptor antagonist nor ethanol affected the responses
This technique could be used to study cerebellar effects of pharmacological agents
Acknowledgements
Supported by NIH grant R01-AA014973. We thank Drs. Diaz and Morton for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chance B. Mitochondrial NADH redox state, monitoring discovery and deployment in tissue. Methods Enzymol. 2004;385:361–370. doi: 10.1016/S0076-6879(04)85020-1. [DOI] [PubMed] [Google Scholar]
- [2].Chery R, L'Heureux B, Bendahmane M, Renaud R, Martin C, Pain F, Gurden H. Imaging odor-evoked activities in the mouse olfactory bulb using optical reflectance and autofluorescence signals. Journal of visualized experiments : JoVE. 2011:e3336. doi: 10.3791/3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Coutinho V, Mutoh H, Knopfel T. Functional topology of the mossy fibre-granule cell--Purkinje cell system revealed by imaging of intrinsic fluorescence in mouse cerebellum. Eur J Neurosci. 2004;20:740–748. doi: 10.1111/j.1460-9568.2004.03533.x. [DOI] [PubMed] [Google Scholar]
- [4].Hepp S, Gerich FJ, Muller M. Sulfhydryl oxidation reduces hippocampal susceptibility to hypoxia-induced spreading depression by activating BK channels. J Neurophysiol. 2005;94:1091–1103. doi: 10.1152/jn.00291.2005. [DOI] [PubMed] [Google Scholar]
- [5].Jaatinen P, Rintala J. Mechanisms of ethanol-induced degeneration in the developing, mature, and aging cerebellum. Cerebellum. 2008;7:332–347. doi: 10.1007/s12311-008-0034-z. [DOI] [PubMed] [Google Scholar]
- [6].Jongen JL, Pederzani T, Koekkoek SK, Shapiro J, van der Burg J, De Zeeuw CI, Huygen FJ, Holstege JC. Autofluorescent flavoprotein imaging of spinal nociceptive activity. J Neurosci. 2010;30:4081–4087. doi: 10.1523/JNEUROSCI.0011-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- [8].Lin AM, Freund RK, Hoffer BJ, Palmer MR. Ethanol-induced depressions of cerebellar Purkinje neurons are potentiated by beta-adrenergic mechanisms in rat brain. J Pharmacol Exp Ther. 1994;271:1175–1180. [PubMed] [Google Scholar]
- [9].Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Palmer MR, Hoffer BJ. GABAergic mechanisms in the electrophysiological actions of ethanol on cerebellar neurons. Neurochem Res. 1990;15:145–151. doi: 10.1007/BF00972204. [DOI] [PubMed] [Google Scholar]
- [11].Reinert KC, Dunbar RL, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;92:199–211. doi: 10.1152/jn.01275.2003. [DOI] [PubMed] [Google Scholar]
- [12].Reinert KC, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging in the cerebellar cortex in vivo. J Neurosci Res. 2007;85:3221–3232. doi: 10.1002/jnr.21348. [DOI] [PubMed] [Google Scholar]
- [13].Reinert KC, Gao W, Chen G, Wang X, Peng YP, Ebner TJ. Cellular and metabolic origins of flavoprotein autofluorescence in the cerebellar cortex in vivo. Cerebellum. 2011;10:585–599. doi: 10.1007/s12311-011-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, Watanabe S, Kouuchi T, Tanaka R. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J Physiol. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shuttleworth CW. Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem Int. 2010;56:379–386. doi: 10.1016/j.neuint.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Theyel BB, Llano DA, Issa NP, Mallik AK, Sherman SM. In vitro imaging using laser photostimulation with flavoprotein autofluorescence. Nature protocols. 2011;6:502–508. doi: 10.1038/nprot.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int Rev Neurobiol. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]