Abstract
Purpose
We determined the prevalence of risk factors for the development of acute respiratory distress syndrome (ARDS), outcomes of critical illness, and the impact of HAART in HIV-1-infected patients. We hypothesized that in an urban county hospital, HIV-1-infected patients with ARDS would have a higher mortality than their HIV-1-uninfected counterparts.
Materials and Methods
Subjects were enrolled between 2006 and 2012. Baseline patient demographics, comorbidities, illness severity, causes of ARDS and clinical outcomes were obtained. The primary endpoint was hospital mortality.
Results
178 subjects with ARDS were enrolled in the study; 40 (22%) were infected with HIV-1. The median CD4 count was 75 (15.3-198.3) and 25% were on HAART. HIV-1-infected subjects were significantly younger (44 vs. 52 years, p<0.01), and had higher rates of asthma, chronic obstructive pulmonary disease, pneumonia, history of hospital acquired infections and prior sepsis. HIV-1-infected subjects had greater illness severity by APACHE II scores [29 (24-31) vs. 24 (22-25), p < 0.01]. Hospital mortality was not higher among HIV-1-infected subjects compared to HIV-1-uninfected subjects (50.0% vs. 38.4%, p=0.19).
Conclusions
In patients with ARDS, HIV-1 infection was associated with greater illness severity, but was not associated with higher mortality in ARDS. Future studies need be done to evaluate the factors that contribute to high morbidity and mortality in medically vulnerable populations who develop ARDS.
Keywords: acute respiratory distress syndrome, human immunodeficiency virus, critical illness, antiretroviral therapy, medically vulnerable
Introduction
Since the advent of highly active antiretroviral therapy (HAART) in 1996 and the use of prophylaxis medications, HIV-1-infected patients are less likely to be hospitalized for opportunistic infections, and more often present to the hospital with sequela of chronic non-HIV-1 related illnesses [1]. In fact, complications related to neoplasms, chronic pulmonary disease, dilated cardiomyopathies, acute coronary syndromes, cirrhosis, co-infection with hepatitis B and C, and polysubstance abuse have surpassed opportunistic infections as the most common reasons for hospital admission in HIV-1-infected patients [1,2].
Although improved care for HIV-1-infected patients has decreased the total number of hospital admissions, intensive care units (ICU) admissions have remained relatively stable, with 4 to 12% of hospitalized HIV-1-infected patients requiring ICU level care, primarily for acute respiratory failure [1,3,4]. Prior to the widespread availability of HAART and prophylaxis for opportunistic infections, Pneumocystis pneumonia (PCP) was the most common cause of acute respiratory failure in HIV-1-infected patients, with reported mortality rates between 51 to 91% [1,3]. In the current era of HAART, bacterial pneumonia is the most common cause of acute respiratory failure in HIV-1-infected patients in the ICU worldwide, followed by PCP and non-infectious pulmonary disease such as obstructive lung disease and interstitial lung disease [4-6]. Often, patients have two or more antecedent causes for acute respiratory failure.
Acute respiratory failure can often manifest as acute respiratory distress syndrome (ARDS), a diagnosis which continues to challenge ICU clinicians in both HIV-1-infected and HIV-1-uninfected patients. The incidence of ARDS is 1.9 times greater in African Americans compared to white Americans, and the overall incidence of ARDS has been rising with a recent estimate of 74.1 cases/100,000 persons-per-year in the US[7]. In a recent meta-analysis, the hospital mortality rate in patients with ARDS was reported as being static since 1994, with randomized controlled trials reporting rates of 35-40% and observational studies reporting rates of 40-45%[8]. However, others have reported that both short-term and long-term outcomes are improving, with 60-day mortality rates declining from 36% in 1996-1997 to 26% in 2004-2005[9].
HIV-1 infection is independently associated with hospital mortality in critically ill patients[10]. To date, few trials have exclusively evaluated HIV-1-infected patients with ARDS and only one has directly compared HIV-1-infected with HIV-1-uninfected patients with ARDS [11,12]. Most of these studies evaluated patients in the east and west coasts of the United States, as well as in Western Europe. The HIV epidemic is shifting from the east and west coast of the United States and becoming more predominant in the southern states, particularly in African Americans with low socioeconomic status [13]. Access to medical care and lack of insurance play a part in ICU utilization; uninsured patients are more likely not to be hospitalized, to be admitted to the ICU when they are hospitalized and to die in the ICU [14].
Hence, in this study, we sought to primarily compare clinical outcomes including hospital mortality, ICU length of stay, hospital length of stay and ICU ventilator-days between HIV-1-infected and HIV-1-uninfected patients with ARDS at a large, urban, safety-net county hospital that provides medical care to low-income, uninsured and vulnerable populations in Atlanta, Georgia. In addition, as our secondary outcomes, we sought to determine and compare the characteristics and baseline co-morbidities between the groups. We hypothesized that in a setting plagued with multiple socioeconomic challenges, HIV-1-infected patients with ARDS would have a higher mortality than their HIV-1-uninfected counterparts.
Material and Methods
Study Population
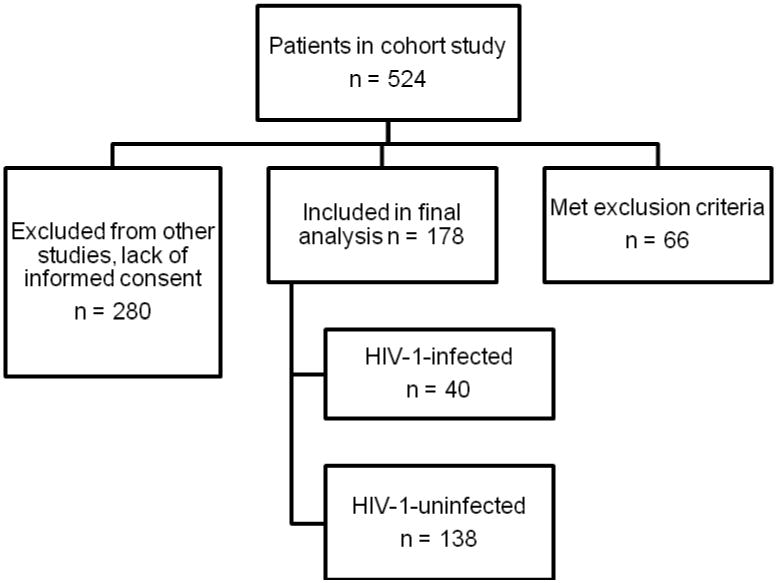
We performed a secondary analysis of a prospective observational study in three adult ICUs (Medical ICU, Surgical ICU and Neurologic ICU) at Grady Memorial Hospital, an affiliate of Emory University, in Atlanta, Georgia between 2006 and 2012. The observational study used a standardized screening protocol to identify patients who met the American-European Consensus Conference (AECC) definition of ALI/ARDS [15]. The three ICU's together comprise 60 beds, and patients were screened on a daily basis by designated screeners. The cohort included 524 patients. Inclusion criteria included patients meeting the AECC definition of ARDS, with the following at-risk diagnoses: severe pneumonia, aspiration pneumonia, trauma, multiple transfusions, pancreatitis and non-pulmonary sepsis which included non-pulmonary infections (bloodstream, genitourinary, gastrointestinal, central nervous system, skin and soft tissue). Exclusion criteria included history of congestive heart failure with an ejection fraction less than 35% and greater than seven days between documentation of at-risk diagnosis for ARDS and development of ARDS. Patients without documented PaO2/FiO2 ratios and without chest x-rays or chest CT scans within 7 days of onset of ARDS were also excluded. Of 524 subjects in the cohort, 66 met exclusion criteria. Outcomes data were not collected on 280 subjects who had failed screening for other studies. Exclusion criteria for these other studies included renal insufficiency, increased bleeding risk (defined by elevated PTT/INR and platelets < 50,000), or traumatic brain injury. HIV infection was not an exclusion criterion for these other studies. The final analysis included 178 subjects, of whom 40 were infected with HIV-1 (Figure 1).
Figure 1. Flow Diagram of Study Design for All Study Subjects.
Data Elements
The primary outcome was hospital mortality. Other clinical outcomes including hospital length of stay, ICU length of stay and ICU ventilator-days were also obtained. We collected 28-day mortality data on subjects whose hospital length of stay was 28 days or more. In addition, baseline patient demographics, co-morbidities, causes of ARDS, physiologic information including Acute Physiology and Chronic Health Evaluation (APACHE) II and Sepsis-related Organ Failure Assessment (SOFA) scores were collected. Information on HIV-1 status, including CD4 count and use of HAART was also obtained through review of the medical chart and review of charts from the infectious disease department clinic. HIV serologies were used when available to identify HIV-1 infected subjects who did not have documentation of HIV status in the medical chart. If HIV-1 status was not evident by HIV serology or documentation in the medical chart, they were presumed HIV-1-uninfected. HAART was defined as a combination of three or more antiretroviral drugs, belonging to at least two classes among the following: nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors. CD4 count and viral loads were determined by reviewing laboratory data in the three months prior to admission.
Statistical analysis
Univariate comparisons between HIV-1 subjects and HIV-1-uninfected subjects were calculated and evaluated for statistical significance at an alpha of 0.05 using a chi-squared test for categorical variables and a two-sample t-test for continuous variables. Results were reported as the mean (SD) or as a percentage. The data was log-transformed or a Wilcoxon Rank-Sum Test was used when the data was not normally distributed. To examine the association of HIV-1 and hospital mortality, a multivariate logistic model was created with hospital mortality as the dependent variable, the variable of interest and potential known confounders as independent variables which included age, APACHE II, Asthma, COPD, history of pneumonia, history of hospital acquired infection and history of sepsis. Statistical analysis was performed through Number Crunching Statistical Software (NCSS).
Results
Baseline Demographics between HIV-1-Infected and Uninfected Subjects
Over the seven years of this cohort study, there were 138 HIV-1-uninfected and 40 HIV-1 subjects with ALI/ARDS. Within the HIV-1-uninfected cohort, 54.3% had documented negative HIV serologies during the admission (n=75). Both groups were predominantly African-American (85.0% in HIV-1-infected vs. 73.7.5% in the HIV-1-uninfected group, p=0.14) and male (67.5% vs. 58.7%, p=0.31). The HIV-1-infected subjects were significantly younger compared to HIV-1-uninfected subjects (44(12) vs. 52(16) years old respectively, p<0.01) and had lower body mass index (BMI) (22.1(19-25) vs. 26.3(23-30) respectively, p < 0.01). With regards to baseline co-morbidities, HIV-1-infected subjects had significantly higher rates of asthma (17.5% vs. 7.2%, p=0.05), chronic obstructive pulmonary disease (COPD) (17.5% vs. 5.8%, p=0.02), history of hospital acquired infection (65.0% vs. 11.0%, p < 0.01), history of pneumonia (45.0% vs. 6.5%, p < 0.01) and history of sepsis (22.5% vs. 5.1%, p< 0.01) compared to HIV-1-uninfected subjects. Rates of chronic kidney disease, end-stage renal disease on hemodialysis, cirrhosis, hepatitis C infection, diabetes mellitus, coronary atherosclerotic heart disease and malignancy were similar between the groups. Within the HIV-1-infected cohort, 52.5% of subjects had CD4 counts less than 100, 22.5% subjects had CD4 counts between 100 and 200 and 25.0% subjects had CD4 counts greater than 200. 25.0% of HIV-1-infected subjects were on HAART prior to hospital admission. 75.0% were either not on HAART at all or HAART was initiated on admission. Given that HAART started in the hospital likely did not have time to reach full effect, these subjects were included in the “No HAART” group. There were no differences in PaO2/FiO2 ratio between the HIV-1-infected and HIV-1-uninfected subjects [142.7 (84.3-201) vs. 157.6 (108.3-209.2), p=0.33 respectively]. Although the SOFA scores were similar between the groups, the HIV-1-infected cohort had significantly higher APACHE II scores [29 (24-31) vs. 24 (22-25), p < 0.01] (Table 1).
Table 1. Characteristics of HIV-1-infected and HIV-1-uninfected patients with ARDS.
| Variables | HIV-1-infected (n=40) | HIV-1-uninfected (n=138) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 44 (12) | 52 (16) | <0.01 |
| BMI, median (IQR) | 22.1 (19-25) | 26.3 (23-30) | <0.01 |
| Male, n (%) | 27 (67.5) | 81 (58.7) | 0.31 |
| Race, n (%) | |||
| White | 4 (10.0) | 34 (24.6) | |
| Black | 34 (85.0) | 100 (72.5) | |
| Baseline Comorbidities, n(%) | |||
| Asthma | 7 (17.5) | 10 (7.2) | 0.05 |
| Chronic Kidney Disease | 3 (7.5) | 11 (8.0) | 0.9 |
| ESRD on dialysis | 0 (0.0) | 3 (2.2) | 0.35 |
| Cirrhosis | 1 (2.5) | 7 (5.1) | 0.49 |
| Hepatitis C infection | 8 (20.0) | 15 (10.9) | 0.78 |
| COPD | 7 (17.5) | 8 (5.8) | 0.02 |
| History of Hospital Acquired Infection | 26 (65.0) | 15 (11.0) | <0.01 |
| Diabetes Mellitus | 6 (15.0) | 35 (25.4) | 0.17 |
| Coronary Artery Disease | 1 (2.5) | 9 (6.5) | 0.33 |
| History of Pneumonia | 18 (45.0) | 9 (6.5) | <0.01 |
| History of Sepsis | 9 (22.5) | 7 (5.1) | <0.01 |
| History of Cancer | 3 (7.5) | 21 (17.4) | 0.21 |
| Prognostic Indices | |||
| P/F ratio, median (IQR) | 142.7 (84.3-201.0) | 157.6 (108-.3-209.2) | 0.33 |
| SOFA, median (IQR) | 11.0 (8.0-13.0) | 9.0 (6.5-12.0) | 0.11 |
| APACHE II, median (IQR) | 29 (24-31) | 24 (22-25) | <0.01 |
| CD4 > 200, n (%) | 10 (25.0%) | - | - |
| CD4 100-200, n (%) | 9 (22.5%) | - | - |
| CD4 < 100, n (%) | 21 (52.5%) | - | - |
| Clinical Outcomes | |||
| Hospital LOS, median (IQR) | 26 (16-42) | 25 (14-39) | 0.96 |
| ICU LOS, median (IQR) | 14 (9-24) | 15 (10-24) | 0.78 |
| ICU Ventilator Days, median (IQR) | 12 (6-22) | 12 (7-23) | 0.68 |
| Hospital Mortality, n (%) | 20 (50.0%) | 53 (38.4%) | 0.19 |
At-Risk Diagnoses for ARDS between HIV-1 Infected and Uninfected Subjects
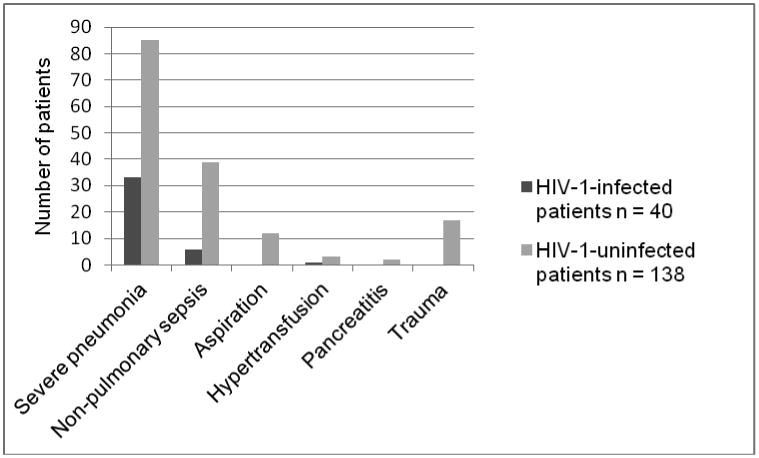
The most common at-risk diagnosis for the development of ARDS in both groups was severe pneumonia, 82.5% in HIV-1-infected vs. 61.6% in HIV-1-uninfected followed by non-pulmonary sepsis, 12.5% in HIV-1-infected vs. 28.3% in HIV-1-uninfected. In the HIV-1-uninfected group, there were 17 cases of trauma, 12 cases of aspiration, 3 cases of multiple transfusion and 2 cases of pancreatitis. In the HIV-1-infected group, one subject had multiple transfusions as a risk factor for ARDS; the remainder had severe pneumonia or non-pulmonary sepsis (Figure 2).
Figure 2. Causes of ARDS in All Study Subjects.
Outcomes among HIV-1 Infected and Uninfected Subjects
There were no statistically significant differences between HIV-1-infected and HIV-1-uninfected subjects in hospital mortality (50.0% vs. 38.4%, p=0.19), ICU ventilator-days [12 (6-22) vs. 12 (7-23), p=0.68], ICU length of stay [14 (9-24) vs. 15 (10-24) days, p=0.78] or hospital length of stay [26 (16-42) vs. 25 (14-39) days, p=0.96]. The rates of withdrawal of life support and Do not resuscitate/Do not intubate statuses were not statistically different between the two groups; HIV-1-infected group had 70% rate of withdrawal life support (n=14 out of 20 deaths) and HIV-1-uninfected group had 69.8% rate of withdrawal (n=37 out of 53 deaths) (p=0.35). After logistic regression analysis was performed with age, APACHE II, Asthma, COPD, history of pneumonia, history of hospital acquired infection and history of sepsis entered as potential confounders into the model, HIV infection was associated with an odds ratio (OR) of 1.64 for increased hospital mortality, but this was not statistically significant [95%CI (0.58-4.63), p=0.35]. APACHE II was associated with increased hospital mortality with an OR of 1.10 [95%CI (1.04-1.15), p<0.01] (Table 2). There were a total of 10 HIV-1-infected subjects on HAART with a hospital mortality of 60.0% (n=6) and 28-day mortality of 40.0% (n=4). Two patients on HAART died after 28 days in the hospital. There were 30 HIV-1-infected subjects not on HAART with a hospital mortality of 50.0% (n=14) and 28-day mortality of 23.3% (n=7). The differences between HIV-1-infected subjects off and on HAART were not statistically significant.
Table 2. Multivariable Logistic Regression Model for Hospital Mortality.
| Variable | β-coefficient (SE) | Odds Ratio | 95% CI | p-value |
|---|---|---|---|---|
| Age | 0.0257 (0.01) | 1.03 | (1.000, 1.053) | 0.05 |
| APACHE II | 0.0907 (0.03) | 1.09 | (1.040, 1.153) | 0.01 |
| Asthma | 0.0035 (0.66) | 1.00 | (0.277, 3.640) | 1.00 |
| COPD | -1.9255 (0.85) | 0.15 | (0.028, 0.771) | 0.01 |
| History of Pneumonia | 0.1533 (0.62) | 1.17 | (0.347, 3.919) | 0.80 |
| History of Hospital Acquired Infection | -0.2361 (0.63) | 0.79 | (0.231,2.697) | 0.71 |
| History of Sepsis | 0.3670 (0.75) | 1.44 | (0.328, 6.343) | 0.63 |
| HIV | 0.4949 (0.53) | 1.64 | (0.581, 4.634) | 0.35 |
Discussion
In summary, this study showed there was a trend toward higher hospital mortality in HIV-1-infected subjects with ARDS, although not statistically significant. In comparison, all-comers with ARDS in large prospective trials report mortality rates of 35-45% [8]. In addition, there were no statistically significant differences in ventilator-days, hospital length of stay or ICU length of stay. Although this was a small number, even patients already on HAART had a median CD4 count of 85. This group might reflect a sub-set of HIV-1-infected individuals known as ‘immunological non-responders’, as defined by a persistently low CD4 count (<350/μl) despite adequate virologic control [16]. These patients have worse outcomes including a greater progression to AIDS and a greater risk of lung infections [17]. We do not have data on how long these patients were on HAART prior to hospital admission. Nevertheless, further research needs to be done to address novel therapies and preventative strategies to improve clinical outcomes in patients with advanced HIV disease and concomitant ARDS.
In addition, this study showed that critically ill HIV-1-infected subjects in a large urban teaching hospital have several different characteristics compared to HIV-1-uninfected subjects with respect to demographics, co-morbidities, and illness severity. HIV-1-infected subjects in this cohort were younger with lower BMIs and had higher rates of pulmonary co-morbidities including asthma, COPD and history of pneumonia. HIV-1-infected subjects were also more likely to have prior bouts of sepsis and hospital-acquired infections. As shown in other studies, the most common cause of ARDS in this cohort was severe pneumonia [1,6,18]. HIV-1-infected subjects had significantly higher APACHE II scores due to their lower serum albumin levels, sodium levels and white blood counts, consistent with previous studies [19].
Currently, there is a paucity of literature regarding critically ill HIV-1-infected patients as we approach the end of the second decade of HAART. Recent studies cite that clinical outcomes for HIV-1-infected patients admitted to the ICU are comparable to that for critically ill HIV-1-uninfected patients [12,20]. The majority of hospital survival rates of critically ill HIV-1-infected patients in the HAART era range between 61 to 81% [4,6,20-22]. Our study is unique in that we compared HIV-1-infected subjects with HIV-1-uninfected subjects admitted to the ICU with the same diagnoses, and examined the epidemiology and outcomes of ARDS in a southern, urban, safety net hospital. Only one other study by Mendez-Tellez et al. specifically evaluated HIV-1-infected subjects with ARDS in the ICU [12]. Similar to our study, Mendez-Tellez et al. did not show statistically significant differences in hospital mortality between the groups possibly because of the low sample size of HIV-1-infected patients in both studies [12].
The crude mortality rate of 50% among the HIV-1-infected patients in this cohort is much higher than has been reported in other studies of critically ill HIV-1-infected patients [4-6,12,20-22]. This mortality rate may be unique to medically vulnerable populations with poor access to health care, high rates of non-adherence to HAART and higher burden of co-morbid conditions. In a New York study with a similar demographic as Grady Memorial Hospital, HIV-1-infected patients requiring mechanical ventilation had significantly higher mortality (44% vs. 22%) and incidence of ventilator associated pneumonias (71% vs 25%) compared to HIV-1-uninfected patients [23]. Most of our patient population is African American, and evidence suggests that African Americans with ARDS have a higher risk of death compared with Caucasians [24]. This is likely multi-factorial including genetic polymorphisms regarding the regulation of chemokines and differences in biochemical markers such as interleukin-8 that may explain the reported 17% increase in 60-day mortality in African Americans enrolled in ARDS Network clinical trials [25]. Our predominant African American population in both groups may explain the high mortality rate seen even in our HIV-1-uninfected subjects when compared to other studies of ARDS patients [4-6,12,22].
There is also noteworthy underutilization of HAART in our HIV-1-infected cohort which was also shown in another study from our institution of HIV-1-infected patients with severe sepsis; HAART use in prior to admission was 22.5% [26]. In other studies, HAART use prior to ICU admission ranges from 21% to 52% [4,12]. Several studies have looked at the utility of HAART in the ICU and have shown mixed results. Currently, there is only expert opinion to guide clinicians [2]. HAART use has been associated with improved survival rates. Meybeck et al. reported lower six-month mortality rates in patients taking HAART during their ICU stay [27]. In the series of studies from San Francisco General Hospital, mortality rate in HIV-1-infected patients admitted with AIDS-related illness was significantly lower in patients on HAART (25% vs. 63%)[28]. In the Greenberg study published from our institution using the same cohort, there was a trend towards increased survival in HIV-1-infected patients with severe sepsis on HAART. This benefit was non-significant, likely because of the overall low rates of HAART usage and the low incidence of acute-AIDS defining illnesses, which is the sub-population thought to benefit the most from HAART in the ICU [26]. Particularly in our institution where HAART is underutilized, it is important to understand the implications of HAART in a critical care setting. Patients with opportunistic infections may benefit from initiation of HAART while in other patients it may be detrimental by precipitating immune reconstitution inflammatory syndromes or drug toxicity issues [29].
Our study had several limitations. It was a secondary analysis of a prospective and ongoing cohort study. Given that our cohort study began prior to the widespread use of the Berlin Definition for ARDS, the cohort may have failed to include certain patients, particularly those without documented pulmonary capillary wedge pressures. The retrospective nature of the study was itself a limitation; co-morbidities were defined by history, not formal diagnostic testing. Within the HIV-1-uninfected cohort, 54.3% had documented negative HIV serologies during the admission (n=75); the remainder of the patients were presumed HIV-1-uninfected by history from the medical chart. Viral loads were not available for the majority of HIV-1-infected patients. In addition, this study retrospectively studied ARDS subjects who were enrolled into other ongoing studies. Therefore, it is unknown whether or not a significant number of HIV-1-infected patients were excluded from this current study as a result of not meeting criteria for the other ongoing projects. Our sample size of HIV-1-infected patients was small, particularly when comparing patients on and off HAART. The study may have been underpowered to find subtle differences in mortality within these groups. We did not have data on long term outcomes, so we could not gather information for 28-day mortality on patients whose hospital length of stays were less than 28 days. Furthermore, this is a single center study, subject to local ICU admission criteria and ICU practices which may not necessarily be generalized. However, data on an urban inner city hospital in the current HAART era is lacking and therefore informative of the nature of critically ill HIV-1-infected patients.
Conclusion
Our study showed that HIV-1-infection was not associated with increased mortality in patients with ARDS. This could be due to advances in critical care medicine including the adoption of low tidal volume ventilation during the years of our study. However, HIV-1-infected patients did have higher comorbid respiratory conditions and a higher illness severity compared to their HIV-1-uninfected counterparts. In addition, the crude mortality rate of 50% among the HIV-1-infected patients was much higher than has been reported in other studies of critically ill HIV-1-infected patients [4-6,12,20-22] which could be due to the underutilization of HAART and their higher illness severity at presentation. We suspect that this crude mortality may also be related to the safety-net hospital population; further clinical trials need to evaluate these socioeconomic factors and their role in outcomes of critically ill HIV-1-infected and HIV-1-uninfected patients.
Acknowledgments
The authors thank Celeste Sarmiento, Kavitha Srinivasan, and the Grady Research Team for their assistance with patient enrollment and data collection. We also thank Neeta Shenvi for her assistance with database management.
Sources of funding: NIH P50 AA-013757 and FDA R01 FD-003440
Footnotes
Conflicts of interest: No conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akgun KM, Pisani M, Crothers K. The changing epidemiology of HIV-infected patients in the intensive care unit. J Intensive Care Med. 2011;26:151–164. doi: 10.1177/0885066610387996. [DOI] [PubMed] [Google Scholar]
- 2.Akgun KM, Huang L, Morris A, Justice AC, Pisani M, Crothers K. Critical illness in HIV-infected patients in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8:301–307. doi: 10.1513/pats.201009-060WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khouli H, Afrasiabi A, Shibli M, Hajal R, Barrett CR, Homel P. Outcome of critically ill human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. J Intensive Care Med. 2005;20:327–333. doi: 10.1177/0885066605281087. [DOI] [PubMed] [Google Scholar]
- 4.Powell K, Davis JL, Morris AM, Chi A, Bensley MR, Huang L. Survival for patients With HIV admitted to the ICU continues to improve in the current era of combination antiretroviral therapy. Chest. 2009;135:11–17. doi: 10.1378/chest.08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afessa B, Green B. Clinical course, prognostic factors, and outcome prediction for HIV patients in the ICU. The PIP (Pulmonary complications, ICU support, and prognostic factors in hospitalized patients with HIV) study. Chest. 2000;118:138–145. doi: 10.1378/chest.118.1.138. [DOI] [PubMed] [Google Scholar]
- 6.De Palo VA, Millstein BH, Mayo PH, Salzman SH, Rosen MJ. Outcome of intensive care in patients with HIV infection. Chest. 1995;107:506–510. doi: 10.1378/chest.107.2.506. [DOI] [PubMed] [Google Scholar]
- 7.Cooke CR, Erickson SE, Eisner MD, Martin GS. Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40:1532–1538. doi: 10.1097/CCM.0b013e31824518f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 9.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palepu A, Khan NA, Norena M, Wong H, Chittock DR, Dodek PM. The role of HIV infection and drug and alcohol dependence in hospital mortality among critically ill patients. J Crit Care. 2008;23:275–280. doi: 10.1016/j.jcrc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Barbier F, Coquet I, Legriel S, Pavie J, Darmon M, Mayaux J, et al. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med. 2009;35:1678–1686. doi: 10.1007/s00134-009-1559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez-Tellez PA, Damluji A, Ammerman D, Colantuoni E, Fan E, Sevransky JE, et al. Human immunodeficiency virus infection and hospital mortality in acute lung injury patients. Crit Care Med. 2010;38:1530–1535. doi: 10.1097/CCM.0b013e3181e2a44b. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. [Accessed July 2, 2013];HIV Surveillance Report, 2011. 2013 23 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published February 2013. Ref Type: Generic. [Google Scholar]
- 14.Danis M, Linde-Zwirble WT, Astor A, Lidicker JR, Angus DC. How does lack of insurance affect use of intensive care? A population-based study. Crit Care Med. 2006;34:2043–2048. doi: 10.1097/01.CCM.0000227657.75270.C4. [DOI] [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 16.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol. 2012;2012:670957. doi: 10.1155/2012/670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicastri E, Chiesi A, Angeletti C, Sarmati L, Palmisano L, Geraci A, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–160. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- 18.Schein RM, Fischl MA, Pitchenik AE, Sprung CL. ICU survival of patients with the acquired immunodeficiency syndrome. Crit Care Med. 1986;14:1026–1027. doi: 10.1097/00003246-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg AL, Seneff MG, Atiyeh L, Wagner R, Bojanowski L, Zimmerman JE. The importance of bacterial sepsis in intensive care unit patients with acquired immunodeficiency syndrome: implications for future care in the age of increasing antiretroviral resistance. Crit Care Med. 2001;29:548–556. doi: 10.1097/00003246-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Dickson SJ, Batson S, Copas AJ, Edwards SG, Singer M, Miller RF. Survival of HIV-infected patients in the intensive care unit in the era of highly active antiretroviral therapy. Thorax. 2007;62:964–968. doi: 10.1136/thx.2006.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akgun KM, Tate JP, Pisani M, Fried T, Butt AA, Gibert CL, et al. Medical ICU admission diagnoses and outcomes in human immunodeficiency virus-infected and virus-uninfected veterans in the combination antiretroviral era. Crit Care Med. 2013;41:1458–1467. doi: 10.1097/CCM.0b013e31827caa46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narasimhan M, Posner AJ, DePalo VA, Mayo PH, Rosen MJ. Intensive care in patients with HIV infection in the era of highly active antiretroviral therapy. Chest. 2004;125:1800–1804. doi: 10.1378/chest.125.5.1800. [DOI] [PubMed] [Google Scholar]
- 23.Pathak V, Rendon IS, Atrash S, Gagadam VP, Bhunia K, Mallampalli SP, et al. Comparing outcomes of HIV versus non-HIV patients requiring mechanical ventilation. Clin Med Res. 2012;10:57–64. doi: 10.3121/cmr.2011.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177:279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kangelaris KN, Sapru A, Calfee CS, Liu KD, Pawlikowska L, Witte JS, et al. The association between a Darc gene polymorphism and clinical outcomes in African American patients with acute lung injury. Chest. 2012;141:1160–1169. doi: 10.1378/chest.11-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg JA, Lennox JL, Martin GS. Outcomes for critically ill patients with HIV and severe sepsis in the era of highly active antiretroviral therapy. J Crit Care. 2012;27:51–57. doi: 10.1016/j.jcrc.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meybeck A, Lecomte L, Valette M, Van GN, Boussekey N, Chiche A, et al. Should highly active antiretroviral therapy be prescribed in critically ill HIV-infected patients during the ICU stay? A retrospective cohort study. AIDS Res Ther. 2012;9:27. doi: 10.1186/1742-6405-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris A, Wachter RM, Luce J, Turner J, Huang L. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS. 2003;17:73–80. doi: 10.1097/00002030-200301030-00010. [DOI] [PubMed] [Google Scholar]
- 29.Goldsack NR, Allen S, Lipman MC. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect. 2003;79:337–338. doi: 10.1136/sti.79.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]


