Abstract
α-Synemin contains a unique 312 amino acid insert near the end of its C-terminal tail. Therefore we set out to determine if the insert is a site of protein–protein interaction that regulates the sub-cellular localization of this large isoform of synemin. Yeast-two hybrid analysis indicated that this region is a binding site for the M10 region of titin. This was confirmed with GST pull-down assays. Co-immunoprecipitation of endogenous proteins indicated close association of the two proteins in vivo and immunostaining of cardiomyocytes demonstrated co-localization of the proteins at the M-band of the sarcomere.
Keywords: synemin, AKAP, PKA, intermediate filaments, signal transduction, cytoskeleton
1. Introduction
Synemin is a type IV IF protein that also functions as an AKAP [1]. Synemin contains the structural features common to IF proteins: an N-terminal head domain, a central rod domain, and a C-terminal tail. Alterative splicing gives rise to at least two isoforms of synemin in the human heart, α-synemin (High) and β-synemin (Middle) [2]. These two isoforms differ by the inclusion of a 936 base pair intron that codes a 312 residue insert between the last two exons in the long C-terminal tail. This C-terminal tail helps to group it as a type VI IF protein and is a site of many protein interactions including PKA [1, 3].
Like other type IV IF proteins synemin forms heteropolymers in combination with desmin or vimentin [4]. These IFs encircle the myofibrils at the level of the Z-disc, thus this association localizes synemin to the Z-discs. Synemin is found at costameres and intercalated disks and may link IFs to these structures via interactions with its non-IF binding partners α-actinin, dystrophin, plectin, α-dystrobrevin, vinculin and/or talin. The 312 insert unique to α-synemin in particular binds the costameric proteins talin and vinculin [5-10].
AKAPs function to anchor PKA to specific subcellular locations throughout the cell, near different PKA substrates. PKA is a holoenzyme consisting of two R subunits (either two type I or type II) and two C subunits. PKA is a serine-threonine kinase activated upon binding of cAMP to the R subunits. It is generally believed that the C subunits dissociate from the R subunits, but this idea has been disputed [11-13]. AKAP anchoring of PKA on or near substrates increases the specificity of the signaling cascade upon activation of the kinase and may accelerate and amplify the signaling pathway [14]. Additionally, AKAPs may sequester PKA near substrates to facilitate basal phosphorylation events [11]. Initially, it was thought that PKA type II, as defined by its R subunits, was anchored throughout the cell while PKA type I was predominantly cytosolic. However, it has come to light that many AKAPs can anchor both type I and type II PKA (dual AKAPs) and some AKAPs are specific for PKA type I [15, 16].
Titin, the largest protein in mammals, is a component of striated muscle that spans half a sarcomere with its N-terminal end at the Z-disc and its C-terminal end at the M-band. There is overlap of adjacent titin molecules from neighboring half-sarcomeres at both the N- and C-terminal ends of the protein. Titin is composed primarily of hundreds of Ig- and fibronectin-like domains organized into functionally discrete regions. Of importance here is the M-band region which is bound on the N-terminus by a catalytic TK domain followed by ten Ig domains (M1-M10) with seven unique interdomain sequences interspersed between the Ig domains. The M-band region is considered a hotspot for mechanical sensing and integration of signal transduction [17-20].
While it has been known for some time that α- and β-synemin are co-expressed in a variety of tissues including all classes of muscle [21], the functional role of each isoform is unclear. We anticipate that any distinct functional roles for each will likely be due to differences in location and/or binding partners; and, that one obvious source for differences between α- and β-synemin is the region unique to α-synemin. Therefore, we set out to identify binding partners for this region using yeast two-hybrid analysis. We discovered that last Ig domain of titin, M10, bound to this region. Thus, our data adds to the list of binding partners specific for α-synemin and expands the subcellular localization sites to the M-band of the sarcomere. These data help to elucidate the difference between two nearly identical proteins that are co-expressed in different tissue including cardiac muscle.
2. Materials and methods
2.1 Yeast strains, media, and cell culture
Yeast strains and media used for the yeast two-hybrid assays were as described by the manufacturer (Matchmaker System 3, Clontech, Mountain View, CA, USA).
HL-1 cardiomyocytes were kindly provided by Dr. Claycomb (Department of Biochemistry and Molecular Biology, Louisiana State University, New Orleans, LA, USA [22]). Cells were maintained as described in the Online Supplementary Data.
2.2 Plasmid construction
The constructs for yeast two-hybrid studies and GST pull-down assays expressing ASI, ASIa, ASIb, ASIc (figure 1) and M10 titin along with control constructs were generated as described in the Online Supplementary Data.
Figure 1. Schematic of α-synemin and constructs used in in vitro assays.
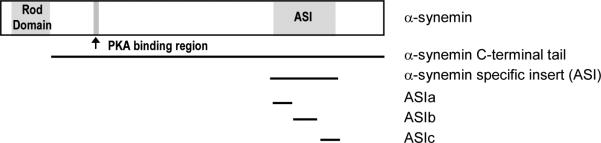
α-Synemin contains a very short head domain (10 amino acids), a rod domain (310 amino acids) allowing it to form heteropolymers with type III IF proteins, and a very long C-terminal tail classifying it as a type IV IF protein. It also contains a PKA RII binding domain classifying it as an AKAP. The 312 amino acid insert in the C-terminal tail (aa 1151-1462, ASI), absent in β-synemin, is the only difference between the two isoforms. For use in the yeast two-hybrid and GST pull down assays, the ASI peptide was subdivided into ASIa, (aa 1151-1243), ASIb (aa 1244-1358), and ASIc (aa 1359-1462).
2.3 Animal care and primary cell isolation
Animals were housed at an animal care facility at Kent State University (Kent, Ohio) that is accredited by the American Association for Accreditation of Laboratory Animal Care and is under the full-time supervision of a veterinarian. Animals were treated in accordance with institutional guidelines and approval by the Institutional Animal Care and Use Committee. Adult mouse cardiomyocytes were isolated [23] and immunostained [24] as previously described.
2.4 Yeast two-hybrid screening of a human heart library
The bait construct expressing ASI was used to screen a human heart cDNA library. Yeast two-hybrid screening was performed using the Matchmaker Pretransformed libraries kit, following manufacturer's protocol (Clontech). Details can be found in the Online Supplementary Data.
2.5 Yeast two-hybrid mating analysis of protein-protein interactions between α-synemin and titin
The prey plasmid selected for use in additional experiments, pGADT7-M10titin, was purified and used in a second round of yeast two-hybrid assays. Matings were performed using yeast expressing the bait (ASI) and prey (M10titin) proteins per manufacturer's protocol (Clontech). Assays were also carried out with either ASIa, ASIb or ASIc as the bait and M10titin as the prey in similar yeast two-hybrid experiments. Additionally, negative control experiments were carried out with ASI as the bait and either M-titin Ig 1,2; Z-titin Ig 1,2; or Z-titin Ig 4,5 as prey.
2.6 In vitro GST pull-down assays
Extracts were made from E. coli expressing GST-M10titin and used in GST pull-down assays in conjunction with MBP-ASI, MBP-ASIa, MBP-ASIb, or MBP-ASIc as described in the Online Supplementary Data.
2.7 Co-immunoprecipitation of endogenous titin and α-synemin
HL-1 cell lysates, generated as described in the Online Supplemental Data, were incubated with 5 μg of anti-α-synemin antibody R238 (a generous gift from Dr. Bloch, University of Maryland, School of Medicine, Baltimore, MD) [25] and the antibody-antigen complex was then added to Protein A/G PureProteome magnetic beads (EMD Millipore) and incubated, washed and eluted per manufacturer's protocol. The eluates were subjected to electrophoresis and western blot analysis as described in Online Supplementary Data using the anti-titin antibody, M10-1 (a kind gift from Dr. Bjarne Udd [26]). Reciprocal experiments were done using anti-titin antibody for IP and anti-α-synemin antibody for western blot analysis.
2.8 Confocal analysis of α-synemin in cardiomyocytes
Adult mouse cardiomyocytes were double stained with anti-α-synemin antibody R238 and monoclonal titin antibody T50 (a generous gift from Dr. van der Ven, Institute of Cell Biology, Bonn, Germany,[27]) both at 1:100. Alexa Fluor 488-conjugated donkey anti-rabbit and Alexa Fluor 568-conjugated donkey anti-mouse secondary antibodies (Life technologies) were used at 1:1000. Cells were examined using an Olympus Fluoview 1000 confocal laser scanning microscope with an X63 objective lens.
3. Results
3.1 The ASI region of α-synemin binds to the M10 region of titin
To identify proteins interacting specifically with α-synemin, a human heart cDNA library was screened using the ASI region as bait in yeast two-hybrid experiments. Screening of 5.5 × 106 colonies yielded 45 prey clones encoding peptides capable of interacting with the bait. Of these 45 clones, 17 of them encoded 8 variations of M10 titin differing slightly in length ranging from final 83 residues to the final 37 residues. A table listing all of the ASI interacting proteins identified in the yeast two-hybrid screen can be found in the Online Supplemental Data (table S1). A prey plasmid which encoded the final 69 amino acids of titin (pADT7-M10titin) was selected for use in all further experiments. This plasmid was purified and used directly as prey in yeast two-hybrid experiments with ASI as bait in order to confirm interaction between these two peptides (figure 2, A).
Figure 2. Yeast two hybrid analysis reveals interaction between the ASI region of α-synemin and M10 titin.
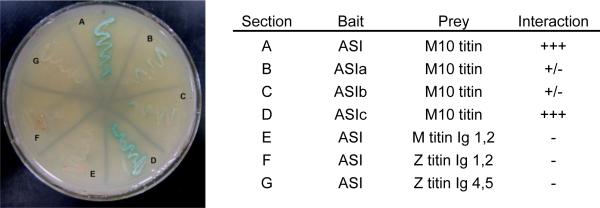
Plasmids encoding full length ASI or ASI fragments were used as bait with plasmids encoding the final sixty nine amino acids of M10 region of cardiac titin that was identified in a screen of a human heart cDNA library as described in Materials and Methods. Strong interaction was observed (heavy growth and blue color) between ASI and M10 titin and ASIc and M10 titin on QDO/X-α-gal. Weak interaction was observed for both ASIa and ASIb with titin M10. As a negative control, plasmids expressing ASI were used as bait with plasmids expressing other regions of titin structurally similar to M10; no interaction between these pairs of peptides was observed.
3.2 M10 Titin interacts with the final 103 amino acids of ASI
To precisely locate the binding region within α-synemin for M10 titin, additional yeast two-hybrid studies were performed with three bait plasmids spanning ASI (pGBKT7-ASIa, pGBKT7-ASIb, and pGBKT7-ASIc; figure 1). Although some low affinity biding between M10titin and ASIa and ASIb is apparent (figure 2, B, C), the strongest interaction was obtained between ASIc and M10titin (figure 2, D). Additionally, in order to ensure specificity of the interaction between α-synemin with M10 titin, three control constructs that contain other Ig domains of titin were also used in yeast matings as prey with the ASI as bait. No interaction was detected (figure 2; E-G).
To corroborate the yeast two-hybrid results, GST pull-down assays were carried out. ASI and its smaller fragments were cloned to form GST fusion proteins and M10titin was cloned to generate a MBP fusion protein. The full-length peptide ASI and the smaller C-terminal peptide ASIc displayed strong interaction with M10titin (figure 3). These results are consistent with the yeast two-hybrid results and confirm that there is specific interaction between the final 103 residues of the ASI region of α-synemin and the C-terminal 69 residues of M10 titin.
Figure 3. GST-pull down assays confirm ASI interacts with M10 titin.
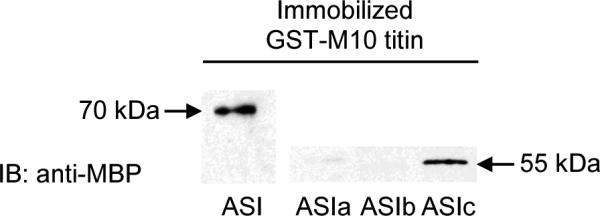
GST-M10titin fusion protein was immobilized on glutathione agarose followed by addition of protein extracts made from E. coli expressing either MBP-ASI, MBP-ASIa, MBP-ASIb, or MBPASIc. Protein complexes were eluted and separated by SDS-PAGE and visualized using anti-MBP antibody. Strong interaction was detected between the M10 region of titin and ASI. That interaction is further localized to the final 103 amino acids encoded by ASIc. Very weak interaction between the first two fragments of ASI and M10titin was detected.
3.3 Endogenous α-synemin binds titin in HL-1 cells
To examine the interactions of α-synemin and titin inside cells, we performed co-IP of endogenous proteins expressed in HL-1 cells. α-Synemin was detected on western blots when anti-titin antibody was used to IP (figure 4A, lane E). Additionally, titin was detected when anti-α-synemin antibody was used to IP (figure 4B, lane E), the doublet in lane E is likely due to the fact that there are two major isoforms in cardiac muscle and that there is a significantly higher concentration of the detected protein in the eluate than in the lysate fractions allowing both to be visualized [18]. Neither α-synemin nor titin were detected when the negative control antibody anti-myc was used for IP (figure 4C, lane E). These findings indicate that endogenous α-synemin interacts with endogenous titin in intact cells.
Figure 4. Endogenous α-synemin and titin interact in vivo.
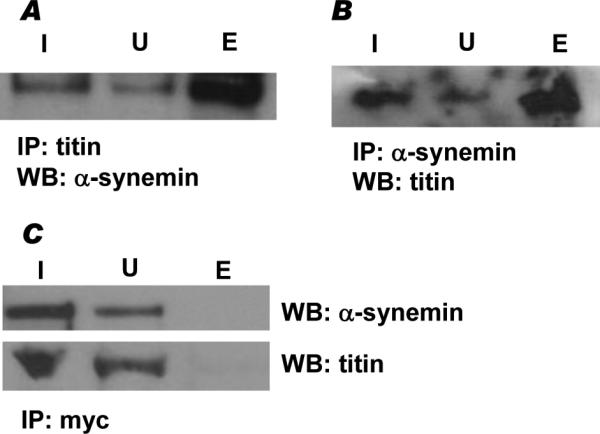
A, Endogenous α-synemin and titin were co-immunoprecipitated from HL-1 cells using anti-titin antibody and visualized using anti-α-synemin antibody (E). B, In reciprocal experiments anti-α-synemin antibody was used to immunoprecipitate endogenous proteins and anti-titin antibody was used to visualize the co-immunoprecipitated titin protein (E). C, In control experiments, α-synemin or titin did not co-immunoprecipitate with anti-myc antibody. In each experiment, the entire volume of the eluate was loaded on the gel along with equivalent volumes of input (I) and unbound (U) fractions: I, input; U, unbound, E, eluate.
3.4 α-Synemin is localized at the M-band in cardiomyocytes
Confocal microscopy of adult mouse cardiomyocytes revealed that α-synemin is localized to the Z-discs as expected (figure 5) [1, 25]. Z-discs define the borders of sarcomeres and are sites of attachment for the actin thin filament and the N-terminal end of titin. Costameres, analogues to focal adhesion complexes, are found in register with peripheral Z-discs (just under the sarcolemma) and are sites of communication between the extracellular matrix and the myofibrils [28, 29]. IFs ring Z-discs and link together adjacent myofibrils and myofibrils to costameres and organelles [29-31].These linkages are important for sarcomere, organelle and costamere organization.
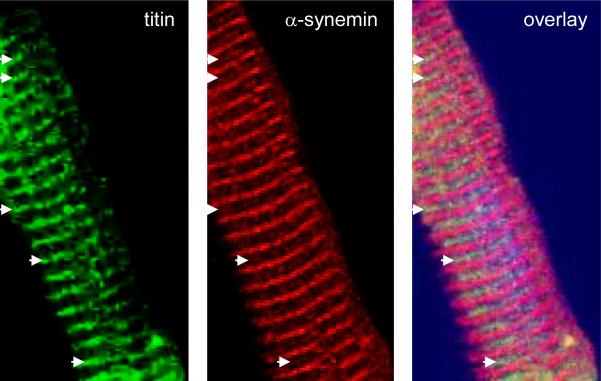
Figure 5. Confocal analysis reveals α-synemin is localized to the M-band in cardiomyocytes.
Isolated adult mouse cardiomyocytes were double stained with antibody recognizing the M-band region of titin (green) and α-synemin (red). Confocal analysis revealed that α-synemin localizes to the Z-line as expected and to the M-band where it co-localizes with M-band titin (arrows).
The isoform specific synemin antibody also revealed that α-synemin co-localizes to the M-band of the sarcomere as the titin antibody used was raised against a peptide corresponding to the M9 domain which lies adjacent to the M10 domain within the M-band region of titin (figure 5, arrows) [27]. The M-band, found in the center of the sarcomere in the middle of the H-band, is a region of crosslinking that allows for regular packing of the myosin thick filaments and for interconnecting the C-terminal ends of titin molecules from adjacent sarcomeres. This is accomplished by a lattice structure that is formed by three proteins, myomesin, M-protein and myomesin 3 [29, 32-34].
4. Discussion
Our work expands the known location of synemin in muscle cells and adds to the body of knowledge differentiating α- and β-synemin, as we show here for the first time that α-synemin is localized to the M-band of sarcomeres in cardiomyocytes due to interaction with M10 titin. Yeast two-hybrid exploratory and confirmatory assays were supported by GST pull-down experiments that demonstrated specific interaction between the region of α-synemin that is unique to the larger isoform (ASI) and the last domain of titin, M10. Additionally, yeast two-hybrid and GST pull-down assays were used to further refine the region of interaction within α-synemin to the final 103 amino acids of ASI. The presence of α-synemin and titin in the same complex in intact cells was demonstrated by co-immunoprecipitation of the endogenous proteins from HL-1 cells. Furthermore, close association of these two proteins was observed in cardiomyocytes using antibodies specific for α-synemin and the M-band region of titin via confocal microscopy. Taken together, these provide strong evidence that α-synemin binds to titin at the M10 domain, thus localizing α-synemin to the M-band of the sarcomere in cardiomyocytes. This finding is supported by earlier studies that showed PKA type II localizes to the M-band in neonatal cardiomyocytes via anchoring by AKAP(s), however the AKAP(s) involved were not identified [35].
Eight tandem repeats have been identified in the C-terminal tail of α-synemin, seven of which span ASI [9]. Each contains a unique sequence motif of S/T-X-R-H/Q where the X represents any of the five amino acids V, G, I, F, or L. While the function of the repeats and sequence motifs is currently unknown, they have been conserved in birds and mammals thus implicating functional importance. The titin binding region within ASI was more precisely localized to ASIc, the last 103 residues of ASI, via yeast two-hybrid and GST pull-down assays. ASIc contains the final two and a half tandem repeats; and, interestingly, the two longest stretches of identity conserved between human, mouse and chicken synemin (residues 1400-1404 and 1458-1465 in human α-synemin). Therefore, it is possible that one or more of these regions is important in protein-protein interaction between α-synemin and titin. This same fragment bound to vinculin in blot overlay assays and also co-localized with vinculin in cultured cells emphasizing its importance in protein-protein interaction [10].
The fact that synemin is a structural IF protein and an AKAP is already known [1, 36]; however the individual role of each isoform is less established. As discussed earlier, functional differences between the isoforms are likely based on different subcellular locations and/or binding proteins. Towards this end, Lund et. al. [25] discovered β-synemin was targeted to the Z-discs in neonatal cardiomyocytes and α-synemin to the sites of cell-cell contact (intercalated disks). They also showed that knocking down synemin resulted in disruption of Z-discs proving the necessity of synemin for IF formation in cardiomyocytes. We show α-synemin localizes to the M-band. Since down regulation of synemin in cardiomyocytes in the study described above [25] did not result in disruption of M-band organization as evidenced by the normal distribution of M-band titin and obscurin, we believe the function of α-synemin at the M-band is not structural and may be related to its role as an AKAP.
The M10 region of titin is the final domain of this very large protein and resides in the M-band. This region is found in all full-length titin isoforms, including the cardiac isoforms N2B and N2BA [37]. Even though positioning at M10 titin places α-synemin near several sarcomeric PKA substrates, some are less likely than others to be the substrates of this pool of PKA due to the distance between the pool of PKA and the potential substrates. This is important to consider in light of the fact that the C subunits may not dissociate from the R subunits upon activation of the kinase and thus the holoenzyme (2R:2C) would remain associated with the AKAP, as was seen for AKAP18δ [11]. The two known PKA substrates closest to α-synemin anchored PKA at the M-band are myomesin and M-protein. Phosphorylation of myomesin regulates binding to titin and may play a role in sarcomere formation and maintenance and phosphorylation of M-protein regulates its binding to myosin [38, 39]. While these phosphorylation events may not play a role in the β-adrenergic pathway, the primary PKA pathway in cardiomyocytes, they may be important during muscle development and/or upkeep (i.e. protein turnover). Approximately 30% of PKA activity is believed to be cAMP-independent and sequestering PKA near these substrates is critical in maintaining selectivity and specificity of the kinase [13]; perhaps α-synemin anchored PKA regulating myomesin and/or M-protein is an example of this type of PKA activity.
In regard to the organization of titin at the M-band, there is another possible function of α-synemin at this location. The overlapping ends of titin at this location place α-synemin very close to the TK domain in the titin protein from the adjacent sarcomere [38]. This region acts as a scaffolding site for the formation of a signalosome that participates in protein turnover through autophagy [40, 41]. Autophagosomal adaptor proteins NBR1 and p62 bind to TK. Both NBR1 and p62 recruit the LC3 autophagosomal membrane to ubiquitinated targets which promotes their degradation through fusion with a lysosome [42]. Additionally, it has been shown that PKA phosphorylation of LC3 prevents its recruitment into the autophagosomes [43]. In this way, α-synemin anchored PKA at the M-band may regulate LC3, and by extension, basal turnover of organelles and cellular response to stress such as ischemia, reperfusion, hypertrophy, and heart failure [44].
Similar to the Z1 and Z2 domain of titin [19], the M10 domain of titin is a site of multiple interactions. This region has been shown to interact with obscurin, obscurin-like 1, and myospryn [45-47] in addition toα-synemin. Interestingly, myospryn has also been identified as an AKAP capable of binding RIIα. While the substrate(s) of myospryn-anchored PKA is not currently known, it has been shown that this pool of PKA is disrupted in Duchenne muscular dystrophy and that this disruption appears to contribute to the pathogenesis of the disease [48]. It is not unusual for two AKAPs to localize to the same subcellular location/substrate in the heart. Both AKAP18α/15 and AKAP79/150 associate with L-type Ca2+ channels and enhance the open probability upon β-adrenergic stimulation; and, gravin and AKAP79/150 associate with the β-adrenergic receptor and act to switch the receptor between the PKA pathway and the MAP kinase cascade [49, 50]. Additional studies are needed to determine if these two M-band AKAPs have any overlapping functions.
In conclusion, these studies have increased our understanding of the differences between α- and β-synemin by revealing a novel location for the larger isoform at the M-band of sarcomeres. We believe that at this location α-synemin functions as an AKAP localizing PKA near substrates such as myomesin and M-protein. This may be relevant during myofibrillogenesis and sarcomere maintenance. Moreover, there may be substrates regulated by this pool of PKA, such as LC3, which has yet to be explored in cardiomyocytes.
Supplementary Material
Highlights.
The 312 amino acid insert unique to α-synemin interacts with the M10 domain of titin
Endogenous α-synemin and titin co-immunoprecipitate from HL-1 cells
Endogenous α-synemin localizes to the M-band in cardiomyocytes
Acknowledgements
This work was supported by grants from the NHLBI HL65701 to D.D.
Abbreviations
- IF
intermediate filament
- AKAP
A-kinase anchoring protein
- PKA
protein kinase A
- R
regulatory subunits of PKA
- C
catalytic subunits of PKA
- Ig
immunoglobulin
- TK
titin kinase
- ASI
α-synemin specific insert
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Refer to Web version on PebMed Central for Online Supplementary Data.
References
- 1.Russell MA, Lund LM, Haber R, McKeegan K, Cianciola N, Bond M. The intermediate filament protein, synemin, is an AKAP in the heart. Arch Biochem Biophys. 2006;456:204–15. doi: 10.1016/j.abb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Titeux M, Brocheriou V, Xue Z, Gao J, Pellissier JF, Guicheney P, Paulin D, Li Z. Human synemin gene generates splice variants encoding two distinct intermediate filament proteins. Eur J Biochem. 2001;268:6435–49. doi: 10.1046/j.0014-2956.2001.02594.x. [DOI] [PubMed] [Google Scholar]
- 3.Guerette D, Khan PA, Savard PE, Vincent M. Molecular evolution of type VI intermediate filament proteins. BMC Evol Biol. 2007;7:164. doi: 10.1186/1471-2148-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellin RM, Sernett SW, Becker B, Ip W, Huiatt TW, Robson RM. Molecular characteristics and interactions of the intermediate filament protein synemin. Interactions with alpha-actinin may anchor synemin-containing heterofilaments. J Biol Chem. 1999;274:29493–9. doi: 10.1074/jbc.274.41.29493. [DOI] [PubMed] [Google Scholar]
- 5.Bellin RM, Huiatt TW, Critchley DR, Robson RM. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J Biol Chem. 2001;276:32330–7. doi: 10.1074/jbc.M104005200. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM. Desmuslin, an intermediate filament protein that interacts with alpha -dystrobrevin and desmin. Proc Natl Acad Sci U S A. 2001;98:6156–61. doi: 10.1073/pnas.111153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hijikata T, Nakamura A, Isokawa K, Imamura M, Yuasa K, Ishikawa R, Kohama K, Takeda S, Yorifuji H. Plectin 1 links intermediate filaments to costameric sarcolemma through {beta}-synemin, {alpha}-dystrobrevin and actin. J Cell Sci. 2008;121:2062–74. doi: 10.1242/jcs.021634. [DOI] [PubMed] [Google Scholar]
- 8.Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM. Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem Biophys Res Commun. 2006;346:768–77. doi: 10.1016/j.bbrc.2006.05.192. [DOI] [PubMed] [Google Scholar]
- 9.Sun N, Critchley DR, Paulin D, Li Z, Robson RM. Identification of a repeated domain within mammalian alpha-synemin that interacts directly with talin. Exp Cell Res. 2008;314:1839–49. doi: 10.1016/j.yexcr.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Sun N, Critchley DR, Paulin D, Li Z, Robson RM. Human alpha-synemin interacts directly with vinculin and metavinculin. Biochem J. 2008;409:657–67. doi: 10.1042/BJ20071188. [DOI] [PubMed] [Google Scholar]
- 11.Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, Gonen T. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. eLife. 2013;2:e01319. doi: 10.7554/eLife.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, Fletcher WH, Johnson DA. Regulation of cAMP-dependent protein kinase: enzyme activation without dissociation. Biochemistry. 1995;34:6267–71. doi: 10.1021/bi00019a002. [DOI] [PubMed] [Google Scholar]
- 13.Scott JD, Dessauer CW, Tasken K. Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol. 2013;53:187–210. doi: 10.1146/annurev-pharmtox-011112-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwald EC, Saucerman JJ. Bigger, better, faster: principles and models of AKAP anchoring protein signaling. Journal of cardiovascular pharmacology. 2011;58:462–9. doi: 10.1097/FJC.0b013e31822001e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Molecular interventions. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aye TT, Mohammed S, van den Toorn HW, van Veen TA, van der Heyden MA, Scholten A, Heck AJ. Selectivity in enrichment of cAMP-dependent protein kinase regulatory subunits type I and type II and their interactors using modified cAMP affinity resins. Molecular & cellular proteomics : MCP. 2009;8:1016–28. doi: 10.1074/mcp.M800226-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontrogianni-Konstantopoulos A, Ackermann MA, Bowman AL, Yap SV, Bloch RJ. Muscle giants: molecular scaffolds in sarcomerogenesis. Physiological reviews. 2009;89:1217–67. doi: 10.1152/physrev.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Bharmal SJ, Esbona K, Greaser ML. Titin diversity--alternative splicing gone wild. Journal of biomedicine & biotechnology. 2010;2010:753675. doi: 10.1155/2010/753675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linke WA, Kruger M. The giant protein titin as an integrator of myocyte signaling pathways. Physiology (Bethesda) 2010;25:186–98. doi: 10.1152/physiol.00005.2010. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BR, Granzier HL. Titin-based tension in the cardiac sarcomere: molecular origin and physiological adaptations. Progress in biophysics and molecular biology. 2012;110:204–17. doi: 10.1016/j.pbiomolbio.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirako Y, Yamakawa H, Tsujimura Y, Nishizawa Y, Okumura M, Usukura J, Matsumoto H, Jackson KW, Owaribe K, Ohara O. Characterization of mammalian synemin, an intermediate filament protein present in all four classes of muscle cells and some neuroglial cells: co-localization and interaction with type III intermediate filament proteins and keratins. Cell Tissue Res. 2003;313:195–207. doi: 10.1007/s00441-003-0732-2. [DOI] [PubMed] [Google Scholar]
- 22.Claycomb WC, Lanson NA, Jr., Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damron DS, Bond M. Modulation of Ca2+ cycling in cardiac myocytes by arachidonic acid. Circ Res. 1993;72:376–86. doi: 10.1161/01.res.72.2.376. [DOI] [PubMed] [Google Scholar]
- 24.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, Damron DS, Bond M. AKAP-mediated targeting of protein kinase a regulates contractility in cardiac myocytes. Circ Res. 2001;88:291–7. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 25.Lund LM, Kerr JP, Lupinetti J, Zhang Y, Russell MA, Bloch RJ, Bond M. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes. FASEB J. 2012;26:137–48. doi: 10.1096/fj.10-179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackman P, Marchand S, Sarparanta J, Vihola A, Penisson-Besnier I, Eymard B, Pardal-Fernandez JM, Hammouda el H, Richard I, Illa I, Udd B. Truncating mutations in C-terminal titin may cause more severe tibial muscular dystrophy (TMD) Neuromuscular disorders : NMD. 2008;18:922–8. doi: 10.1016/j.nmd.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Van der Ven PF, Ehler E, Perriard JC, Furst DO. Thick filament assembly occurs after the formation of a cytoskeletal scaffold. J Muscle Res Cell Motil. 1999;20:569–79. doi: 10.1023/a:1005569225773. [DOI] [PubMed] [Google Scholar]
- 28.Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- 29.Sequeira V, Nijenkamp LL, Regan JA, van der Velden J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochimica et biophysica acta. 2014;1838:700–22. doi: 10.1016/j.bbamem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Capetanaki Y, Bloch RJ, Kouloumenta A, Mavroidis M, Psarras S. Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res. 2007;313:2063–76. doi: 10.1016/j.yexcr.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–85. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Schoenauer R, Lange S, Hirschy A, Ehler E, Perriard JC, Agarkova I. Myomesin 3, a novel structural component of the M-band in striated muscle. J Mol Biol. 2008;376:338–51. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 34.Obermann WM, Gautel M, Steiner F, van der Ven PF, Weber K, Furst DO. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol. 1996;134:1441–53. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–44. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 36.Becker B, Bellin RM, Sernett SW, Huiatt TW, Robson RM. Synemin contains the rod domain of intermediate filaments. Biochem Biophys Res Commun. 1995;213:796–802. doi: 10.1006/bbrc.1995.2200. [DOI] [PubMed] [Google Scholar]
- 37.Kolmerer B, Olivieri N, Witt CC, Herrmann BG, Labeit S. Genomic organization of M line titin and its tissue-specific expression in two distinct isoforms. J Mol Biol. 1996;256:556–63. doi: 10.1006/jmbi.1996.0108. [DOI] [PubMed] [Google Scholar]
- 38.Obermann WM, Gautel M, Weber K, Furst DO. Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. Embo J. 1997;16:211–20. doi: 10.1093/emboj/16.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obermann WM, van der Ven PF, Steiner F, Weber K, Furst DO. Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Mol Biol Cell. 1998;9:829–40. doi: 10.1091/mbc.9.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gautel M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Archiv : European journal of physiology. 2011;462:119–34. doi: 10.1007/s00424-011-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogomolovas J, Gasch A, Simkovic F, Rigden DJ, Labeit S, Mayans O. Titin kinase is an inactive pseudokinase scaffold that supports MuRF1 recruitment to the sarcomeric M-line. Open biology. 2014;4:140041. doi: 10.1098/rsob.140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogov V, Dotsch V, Johansen T, Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014;53:167–78. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Cherra SJ, 3rd, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, Chu CT. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010;190:533–9. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell death and differentiation. 2009;16:31–8. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 45.Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol. 2001;154:123–36. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band - implications for hereditary myopathies. J Cell Sci. 2008;121:1841–51. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- 47.Sarparanta J, Blandin G, Charton K, Vihola A, Marchand S, Milic A, Hackman P, Ehler E, Richard I, Udd B. Interactions with M-band titin and calpain 3 link myospryn (CMYA5) to tibial and limb-girdle muscular dystrophies. J Biol Chem. 2010;285:30304–15. doi: 10.1074/jbc.M110.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds JG, McCalmon SA, Donaghey JA, Naya FJ. Deregulated protein kinase A signaling and myospryn expression in muscular dystrophy. J Biol Chem. 2008;283:8070–4. doi: 10.1074/jbc.C700221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kritzer MD, Li J, Dodge-Kafka K, Kapiloff MS. AKAPs: The architerctural underpinnings of local cAMP signaling. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perino A, Ghigo A, Scott JD, Hirsch E. Anchoring proteins as regulators of signaling pathways. Circ Res. 2012;111:482–92. doi: 10.1161/CIRCRESAHA.111.262899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.