Abstract
Rats with lesions of the pedunculopontine tegmental nucleus (PPTg) reliably overconsume high concentration sucrose solution. This effect is thought to be indicative of response-perseveration or loss of behavioral control in conditions of high excitement. While these theories have anatomical and behavioral support, they have never been explicitly tested. Here, we used a contact lickometer to examine the microstructure of drinking behavior to gain insight into the behavioral changes during overconsumption. Rats received either excitotoxic (ibotenic acid) damage to all PPTg neuronal subpopulations or selective depletion of the cholinergic neuronal sub-population (Dtx-UII lesions). We offered rats a variety of pleasant, neutral and aversive tastants to assess the generalizability and specificity of the overconsumption effect. Ibotenic lesioned rats consumed significantly more 20% sucrose than sham controls, and did so through licking significantly more times. However, the behavioral microstructure during overconsumption was unaffected by the lesion and showed no indications of response-perseveration. Furthermore, the overconsumption effect did not generalize to highly consumed saccharin. In contrast, while only consuming small amounts of quinine solution, ibotenic lesioned rats had significantly more licks and bursts for this tastant. Selective depletion of cholinergic PPTg neurons had no effect on consumption of any tastant. We then assessed whether it is the salience of the solution which determines overconsumption by ibotenic lesioned rats. While maintained on free-food, ibotenic lesioned rats had normal consumption of sucrose and hypertonic saline. After mild food deprivation ibotenic PPTg lesioned rats overconsumed 20% sucrose. Subsequently, after dietary induced sodium deficiency, lesioned rats consumed significantly more saline than controls. These results establish that it is the salience of the solution which is the determining factor leading to overconsumption following excitotoxic PPTg lesion. They also find no support for response-perseveration contributing to this effect. Results are discussed in terms of altered DA and salience signaling.
Keywords: pedunculopontine, salience, sucrose, overconsumption, Dtx-UII
Introduction
Located in the upper brainstem, the pedunculopontine tegmental nucleus (PPTg) is an interface between basal ganglia, thalamus and the reticular formation (Inglis & Winn, 1995; Winn, 2006; Wilson et al., 2009b). Comprised of glutamatergic, cholinergic and GABAergic neurons the PPTg is highly reciprocally interconnected with the entire basal ganglia complex (to the extent that it has been suggested it could be considered a functional part of basal ganglia) (Mena-Segovia et al., 2004; Wang & Morales, 2009; Kita & Kita, 2011). In addition, PPTg sends both cholinergic and non-cholinergic efferent connections to midbrain dopamine (DA) systems, the thalamus, the collicluli and motor output sides in the medulla and spinal cord (Semba & Fibiger, 1992; Winn, 2006; Mena-Segovia et al., 2008). Consistent with connections to midbrain DA and basal ganglia, lesion and inactivation studies affecting all neuronal populations within PPTg have shown persistent impairment in spatial learning tasks (Keating & Winn, 2002; Taylor et al., 2004), associative operant learning for food and drug reward (Alderson et al., 2004; Wilson et al., 2009a) and a block of the updating of goal directed action-outcome learning (Maclaren et al., 2013). These impairments are in the absence of a change in baseline levels of locomotion (Olmstead & Franklin, 1994; Alderson et al., 2003; MacLaren et al., 2014). However, while causing no reduction in baseline spontaneous locomotion, PPTg lesions alter the sensitized locomotor response to drugs of abuse including amphetamine (Alderson et al., 2003) and nicotine (Alderson et al., 2008), again consistent with altered basal ganglia function. Rats bearing bilateral lesions of all neuronal types within PPTg also consume significantly more of high concentration sucrose solutions (>12% sucrose) than sham controls (Olmstead et al., 1999; Alderson et al., 2001; Ainge et al., 2006). This overconsumption effect is not observed for low concentration (4%) sucrose solution, drinking water or lab chow (Keating et al., 2002). Standard interpretations of the sucrose overconsumption consider the effect as being indicative of a loss of behavioral control during conditions of high excitement. For example overconsumption may be the result of response-perseveration (continued execution of an ongoing behavior beyond a normal stopping point (Chambers & Self, 2002)) or alternatively general behavioral disorganization (Keating et al., 2002; Ainge et al., 2006; Winn, 2006). Both of these effects could be the result of disrupted basal ganglia function, particularly the late, early, or ignored generation of a ‘stop’ signal in the subthalamic nucleus (Baunez et al., 2002; Schmidt et al., 2013) which has reciprocal connections with PPTg (Semba & Fibiger, 1992; Mena-Segovia et al., 2004) involving both the cholinergic and non-cholinergic neuronal sub-populations (Kita & Kita, 2011). To the best of our knowledge, these hypotheses have not been explicitly tested.
In order to test the hypothesis that the overconsumption effect is due to response-perseveration or loss of behavioral control, we utilized a lickometer system to examine the behavioral microstructure during overconsumption. We hypothesized that response-preservation would be revealed by an increase in burst duration (the number of licks in each individual drinking episode) which would indicate the continuation of an ongoing behavior past a normal stopping point (Chambers & Self, 2002). Alternatively, if overconsumption were due to loss of behavioral control, we predicted this would be revealed by a more general change in drinking patterns. For example, an increase in number of drinking episodes (bursts) and a decrease in length of drinking episodes (number of licks per burst). In the first set of experiments we assessed the specificity and generalizability of the overconsumption effect by offering rats with ether global (ibotenic acid) or cholinergic-neuron specific (Dtx-UII) lesions of the PPTg a variety of pleasant, aversive and neutral tastants. In the second set of experiments we further probed the conditions leading to overconsumption, assessing whether the salience of the solution is the determining factor leading to this behavioral effect.
Experimental procedures
Subjects and housing
Male Sprague Dawley rats weighing 320–350g at time of surgery were used in all experiments. Except where described below, rats were single housed in plastic cages in a temperature and humidity controlled room. Lights were on a 12 hour light dark cycle (lights on 07:00). Details of dietary manipulations are described below. Other than where stated, rats had free access to food (Harlan diet 2018 [Harlan Laboratories; Houston, TX, USA]) and water in the homecage. Animals’ weights were monitored to ensure they never fell below 85% of either their free-food or pre-surgery weight at any point during these experiments. Rats in experiment 1 had previously undergone motor testing as part of another experiment not described here (see (MacLaren et al., 2014) for details). At least 7 days separated the completion of any motor test and the commencement of lickometer testing. Rats in experiment 2 were experimentally naïve. All experiments were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory animals.
Surgery
Rats were anaesthetized with 65 mg/kg i.p. sodium pentobarbital (Sigma-Aldrich, St. Louis, MO, USA, prepared in 10% ethanol) and also administered 0.05 mg/kg of the non-centrally acting anti-muscarinic glycopyrrolate (West Ward, Eatontown, NJ, USA) to reduce pharyngeal secretions and 5mg/kg of the anti-inflammatory carprofen (Pfizer, New York, NY, USA) as an analgesic. Once anaesthetized, the scalp was clipped and rats were secured in a stereotaxic frame (Stoelting Co, Wood Dale, IL, USA), a midline incision was made and craniotomies were drilled to allow toxin infusion at the appropriate coordinates (below). Once all infusions were complete, the wound was closed with surgical clips. Post-operatively rats were administered 3 mL saline (s.c.) and maintained in a heated environment until recovery was complete.
Excitotoxic lesions
A zero dead volume 0.5μl Hamilton syringe (Hamilton Company, Reno, NV, USA) with a 25 gauge needle was mounted in a syringe holder attached to the stereotaxic frame. 200nl of ibotenic acid (0.1M in PB; pH 7.4; Tocris Bioscience, Bristol, UK) was delivered at two sites along the anterior-posterior plane of the PPTg at +0.3 from interaural line (IAL); +-1.8 from midline (ML); -6.3 from dura and IAL+1.2, ML+-1.9, dura-6.7. Toxin was infused in a step down manner with 25μL being unfused every 15 seconds. The needle was left insitu for a further 3 minutes before being removed. Bilateral surgeries were performed in two separate unilateral procedures separated by 7 days, which is the standard procedure for reducing the post-surgery mortality associated with this procedure (Gut & Winn, 2011). The order of hemisphere targeted first was counterbalanced across animals. During the immediate post-operative recovery period, rats receiving ibotenic acid infusions displayed signs of seizure related activities, barrel rolling, circling behavior and a Cheyne–Stokes pattern of breathing. Due to the use of pentobarbital based anesthetic, the post-operative treatment with diazepam to reduce seizures (typically used after isoflurane anesthesia (Wilson et al., 2009a)) was not required. These symptoms generally subsided within 10 hours after toxin infusion, at which point rats were returned to their home-cages. All rats had returned to pre-surgery bodyweight within 3 days of surgery and no lesion versus sham differences in bodyweight were observed throughout the remainder of the experiment. Sham rats underwent the identical procedure with the exception of receiving only the vehicle solution (sterile PB).
Dtx-UII lesions
Dtx-UII was created by the fusion of diphtheria toxin (Dtx) and the peptide Urotensin II (UII), as previously described (Clark et al., 2007). 1.2μL of 3% Dtx-UII was infused from a drawn glass pipette with a tip diameter of ~40 microns at each of the PPTg co-ordinates: +0.3 from interaural line (IAL); +-1.8 from midline (ML); -6.3 from dura and IAL+1.2, ML+-1.9, dura-6.7. Six infusions, with 60 seconds between infusion, of 200nL of Dtx-UII were placed equally spaced along the dorsal ventral plane, beginning 0.2mm ventral to the final co-ordinate and continuing to 0.2mm dorsal to the final co-ordinate. Both hemispheres were targeted in the same surgical procedure, with order of hemisphere targeted being counterbalanced across animals. Prior experience has shown that, unlike excitotoxic lesions, bilateral Dtx-UII infusions can be performed in the same procedure without adverse health effects. During the immediate post-operative recovery period, there were no observable differences between Dtx-UII and sham infused rats. The neurotoxic effects of Dtx-UII are through protein synthesis inhibition with cell death occurring 4–21 days post-surgery (Clark et al., 2007). During this lesion formation period there were no overt signs of ill health or distress, with the only noticeable effect being mild bodyweight instability during the first 10 days post-surgery in the Dtx-UII group. Bodyweights stabilized within 14 days post-surgery and no lesion versus sham difference in bodyweight was observed throughout the remainder of the experiment. Sham rats underwent the identical procedure with the exception of receiving only the vehicle solution (sterile PBS). It has previously been shown that Dtx-UII lesions lead cause a progressive loss of cholinergic PPTg over the 21 days post-surgery (Clark et al., 2007), behavioral testing therefore began 28 days post-surgery.
Behavioral testing
Fluid intake and licking behavior
Lickometer system
The lickometer system consisted of metal wire hanging cages equipped with two bottles mounted on the front of the cage and a food hopper at the rear. The spigots of the water bottles were electrically isolated from the homecage. When the lickometer system was running the rats tongue contacting the spigot of the water bottle temporarily completed an electrical circuit. This system was interfaced by a digital I/O device (National Instruments, Austin, TX) to a computer system running a custom MATLAB program (MathWorks, Natick, MA). Each individual lick was recorded and time-stamped by the system. Bottles were weighed before and after each test to establish total amount of solution consumed. The entire system was designed and constructed by the Psychology Electronics Shop, University of Pennsylvania, PA, USA.
Experimental procedures
Experiment 1: assessing the specificity and generalizability of the overconsumption effect
The aim of this experiment was to assess the generalizability and specificity of the overconsumption effect in PPTg lesioned rats. We also wished to assess any behavioral changes in drinking microstructure during overconsumption. We hypothesized that response-perseveration in a highly motivated or excited behavior would be revealed by an increase in the burst size (mean number of licks per burst, with a burst defined as a group of consecutive licks where any two licks are not more than 1 s apart) during overconsumption. In contrast, behavioral disorganization would be revealed by more general changes in the behavioral pattern during consumption (for example, an increase in number of bursts (frequency at which rats engage in drinking behavior and reduction in number of licks per burst)). We also hypothesized that if overconsumption occurs due to response-perseveration or loss of behavioral control in a highly motivated or excited behavior, that the overconsumption should also generalize to other highly consumed tastants. However, if overconsumption is driven by another mechanism not dependent on the motivation or excitement during consumption, then changes in consumption patterns may also be observed for solutions not consumed in large amounts. To assess this, in addition to assessing consumption of 20% sucrose solution we also assessed consumption of the sweet tasting but nutrient devoid saccharin solution (at a concentration shown to be consumed in equal amounts as 20% sucrose), physiological saline (which is palatable and consumed in approximately equal quantities as tap water) and quinine (which is an aversive tastant generally avoided by rats).
Prior to the start of lickometer testing, daily food intake was reduced such that rats were maintained at ~90% of free food weight through the remainder of the experiment. Rats were acclimatized to the lickometer cages for 5 days prior to behavioral testing. Water was available in both bottles and on the final day of acclimatization consumption and licking behavior was collected to establish a baseline and ensure rats were drinking from both bottles. Subsequently, rats were offered each of the following tastants in one bottle with standard tap water in the second: 20% sucrose, 0.3% saccharin, 0.01% quinine, 0.05% saccharin, 0.9% saline. Between tastants there was a 24 hour wash-out period where tap water was available in both bottles. All solutions were prepared in tap water immediately prior to testing. Solutions were offered to the rats in the early afternoon (8 hours into the light phase) and rats were fed ~2 hours later.
Experiment 2: Salience manipulation
A separate batch of animals were used in these experiments. Subject type, housing and surgical procedures were identical to experiment 1. Due to finding no significant effects in the Dtx-UII lesioned rats (see results) in experiment 1, in experiment 2 rats received only ibotenic acid lesions. Based on the results of experiment 1, in this experiment we aimed to assess whether it is the salience of the solution which is the determining factor leading to overconsumption in PPTg lesioned rats. To test this we manipulated the salience of the sucrose solution and saline solution through food restriction and dietary sodium deprivation, respectively.
Manipulation of sucrose salience through food deprivation
While maintained on free feeding conditions, rats were given access to tap water and 20% sucrose solution and consumption was monitored for 24 hours. Subsequently, rats were placed on food deprivation of 10–12g/day/rat of their standard lab chow (Harlan diet 2018 [Harlan Laboratories; Houston, TX, USA]). Water was freely available at all times and weights were monitored to ensure they did not fall below 85% free food weight at any point. On the eleventh day of food control, rats were again given access to bottles containing tap water and 20% sucrose solution for 24 hours. Daily food was given ~2 hours after the bottles were placed in the cage. Following completion of this test, rats were returned to free feeding conditions.
Sodium deprivation
Ten days after being returned to free feeding conditions rats had 24 hour access to 1.8% saline beside standard tap water and consumption was monitored. Subsequently, rats were placed in clean cages and maintained on free access to a sodium deficient (<0.01% sodium) diet for 10 days (Teklad TD.90228, Harlan Laboratories). On the eleventh day of sodium deficiency rats had access to 1.8% saline and tap water for 24 hours and consumption was monitored.
Histology
Rats were deeply anaesthetized with sodium pentobarbital (Fatal Plus; Vortech Pharmaceuticals Ltd.; Dearborn, MI, USA) and transcardially perfused with phosphate buffered saline followed by >300mL of 4% paraformaldehyde in 0.1M phosphate buffer. Brains were removed and stored in 4% paraformaldehyde at 4°C for 24 hours before being transferred to 20% sucrose solution in 0.1M phosphate buffer. Brains were mounted in a freezing cryostat (Leica; Watertown, MA, USA) and coronal 50 μM sections were cut through the mesopontine tegmentum. Parallel 1:4 series of sections were immunohistochemically processed (free-floating) for: (i) neuron-specific nuclear protein using a mouse-derived anti-neuronal nuclear protein (NeuN) monoclonal antibody (EMD Millipore Cat# MAB377, RRID:AB_2298772, Millipore Corporation, Billerica, MA, USA), a horse raised anti-mouse IgG secondary antibody and avidin-biotin complex, both from an anti-mouse Vector Laboratories Elite ABC kit (Vector Laboratories Inc, Burlingame, CA, USA) and DAB peroxidase substrate (Vector Laboratories) and (ii) choline acetyltransferase (ChAT), using goat anti-ChAT polyclonal antibody (EM Millipore Cat# AB144P-1ML, RRID:AB_262156, Millipore Corporation), a rabbit raised anti-goat IgG secondary antibody and avidin-biotin complex, both from an anti-goat Vector Laboratories Elite ABC kit (Vector Laboratories) and DAB peroxidase substrate. Sections were mounted onto Superfrost Plus slides and cover-slipped with DPX.
Data analysis
Consumption and licking microstructure was analyzed via several dependent variables: Total amount consumed (volume of solution consumed, measured in g); Total licks (the total number of licks); Total bursts (the total number of separable drinking bursts. A burst is defined as a group of consecutive licks where any two licks are not more than 1 s apart); Average burst duration (mean number of licks per burst); Inter-lick interval (the mean interval between licks within a burst, measured in s); Maximum burst length (the number of licks in the longest burst); Number of short and number of long bursts (a short burst being a burst with less licks than the modal number of licks per burst, a long burst being the number of bursts with more than the modal number of licks per burst). Statistical analysis was conducted in SPSS v21 (IBM Corporation, Armonk, NY, USA). Each dependent variable was analyzed by univariate ANOVA. Group means were considered significantly different when p < 0.05. Where graphs are displayed, these depict group means ± SEM. On graphs, * indicates significant difference (sham v lesion) at the p = 0.01 – 0.05 confidence level, ** indicates significance at p ≤ 0.01.
Lesion analysis
Slides were viewed under a light microscope. Lesion extent on the NeuN sections was judged by lack of cell bodies. Lesioned areas on these sections were drawn onto schematic sections from an electronic stereotaxic atlas (Paxinos & Watson, 2005). Each lesion was drawn as a transparent gray shape and overlaid onto a set of schematics along the posterior-anterior plane of the PPTg, this formed a composite image representing the distribution of cell loss in all ibotenic lesioned rats. To estimate the degree of damage to cholinergic cells, software assisted counts of ChAT positive neurons within the PPTg were taken. A series of 1:4 sections was photographed and subsequently loaded into the ImageJ program (ImageJ; U. S. National Institutes of Health, Bethesda, Maryland, USA). Individual ChAT positive cells were then manually tagged using the cell counter plugin. Values from each section were aligned to a standardized set of 13 sections along the posterior-anterior plane of the PPTg, with section 1 corresponding to the most posterior section where ChAT+ neurons would be expected (based on sham animals) and section 13 being the most anterior section with expected ChAT+ neurons.
Results
Lesion results
Histological results
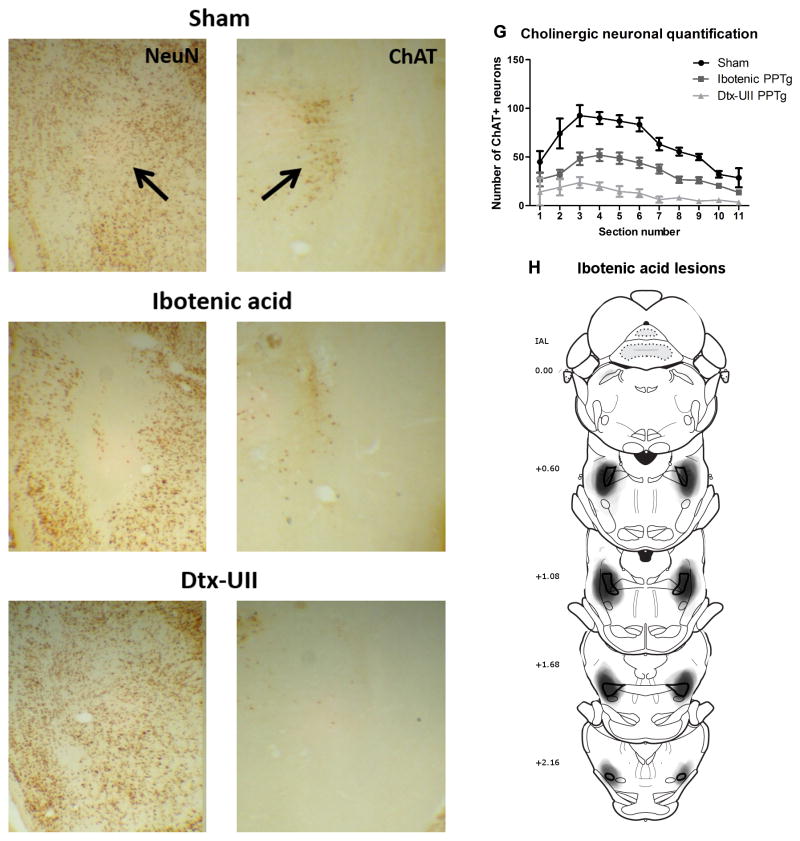
Photomicrographs of sham (A,B), ibotenic acid (C,D) and Dtx-UII lesions (E,F) lesions of the PPTg are shown in figure 1.
Figure 1.
Histological results. Panels A–F show photographs of sham (A,B), ibotenic acid (C,D,) and Dtx-UII (E,F) lesioned rats, with neuronal nuclei (NeuN) staining on the left (A,C,E) and ChAT staining on the right (B,D,E). The black arrow indicates the location of the PPTg. 1G shows results of quantification of ChAT+ neurons within the PPTg for sham, ibotenic acid and Dtx–UII UII-lesioned rats. 1H shows areas of lesion damage in the ibotenic acid lesion group, drawn from the NeuN staining onto schematics from the atlas of Paxinos and Watson (2005). The PPTg is outlined in black and each individual lesion was drawn as a semi-transparent grey shape corresponding to lesioned area observed on the NeuN section, forming a composite image representing all lesions. Numbers on the left show distance from the interaural line (IAL).
Histological results - Ibotenic acid lesions
Twenty-eight rats had successful bilateral ibotenic acid lesions of the PPTg (experiment 1, n = 15; experiment 2, n = 13). Four additional rats were excluded from all analyses due to having predominantly unilateral lesions (n=2) or mis-placed lesions (n=2). A composite figure showing overlays of lesion size and location in all rats drawn onto schematics from the atlas of Paxinos and Watson (2005) is shown in Figure 1H. Analysis of the ChAT+ cell quantification showed that ibotenic acid lesions destroyed a mean of 46% of ChAT+ PPTg neurons. The distribution of ChAT+ cell loss along the posterior-anterior plane within PPTg is shown in figure 1G. These results are in line with previous studies reporting lesions with a clear boundary and cholinergic cell survival within the lesioned area following ibotenic acid infusion into the PPTg (Inglis et al., 2001; Rostron et al., 2008; Wilson et al., 2009a). Where track damage was visible on the NeuN sections, it was seen as a clean line with no sign of damage immediately beside the track area. On all visible tracks, this was through the areas above the PPTg (principally the colliculi and cuniform) and there was no difference in amount of track damage between sham and lesioned rats. There was no indication of lesion in any sham treated rat (n = 27).
Histological results - Dtx-UII lesions
Fourteen rats had successful selective bilateral Dtx-UII-mediated lesions of cholinergic PPTg neurons. Four additional rats were excluded from all analysis due to: small or unilateral lesions (n=2) or no clear indication of lesion (n=2). Analysis of ChAT+ cell quantification revealed that the successful lesions destroyed a mean of 81% of ChAT+ PPTg neurons. The distribution of ChAT cell loss along the posterior-anterior plane of the PPTg is shown in figure 1G. Examination of the NeuN staining at the site of toxin infusion and throughout the posterior-anterior plane of the PPTg showed extensive NeuN+ staining throughout the region with no areas of visibly depleted neurons in or around PPTg. Combined with the extensive ChAT+ cell loss and in line with previous studies (Clark et al., 2007) this indicates that the toxin maintained high selectivity for the UII-R expressing cholinergic PPTg neurons. Cholinergic structures near the PPTg (the laterodorsal tegmental nucleus (LDTg) and the parabigeminal nucleus) appeared to be intact when analyzed by visual inspection. As we did not quantify the number of ChAT neurons present in these regions, we cannot conclusively exclude the possibility of damage outside the boundaries of the PPTg. However, the relatively slow infusion of toxin and the presence of a protease cleavage site between the Dtx and the UII reduce the likelihood of spread of active toxin. Our infusion volume and rate is also similar to another group using Dtx-UII in the PPTg who do not report damage to the LDTg (Cyr et al., 2014). There was no indication of a lesion in any sham treated rat (n=10). Track damage resulting from glass pipette insertion (Dtx-UII lesions and shams) was more difficult to locate than after the syringe surgeries (ibotenic acid lesions and shams). Where visible, there was no sign of a difference in track size between Dtx-UII and sham infused rats. As all statistical analysis was performed within each experimental group (eg Dtx-UII lesions compared to Dtx-UII shams) the slight difference in track damage and other surgical procedures between the syringe and pipette surgeries cannot contribute to any significant effects observed.
Behavioral results
Experiment 1: assessing the specificity and generalizability of the overconsumption effect
The aim of this experiment was to assess the specificity and generalizability of the overconsumption effect and assess any changes in behavioral microstructure during overconsumption.
Ibotenic acid (non-selective) PPTg lesions
Tap water alone
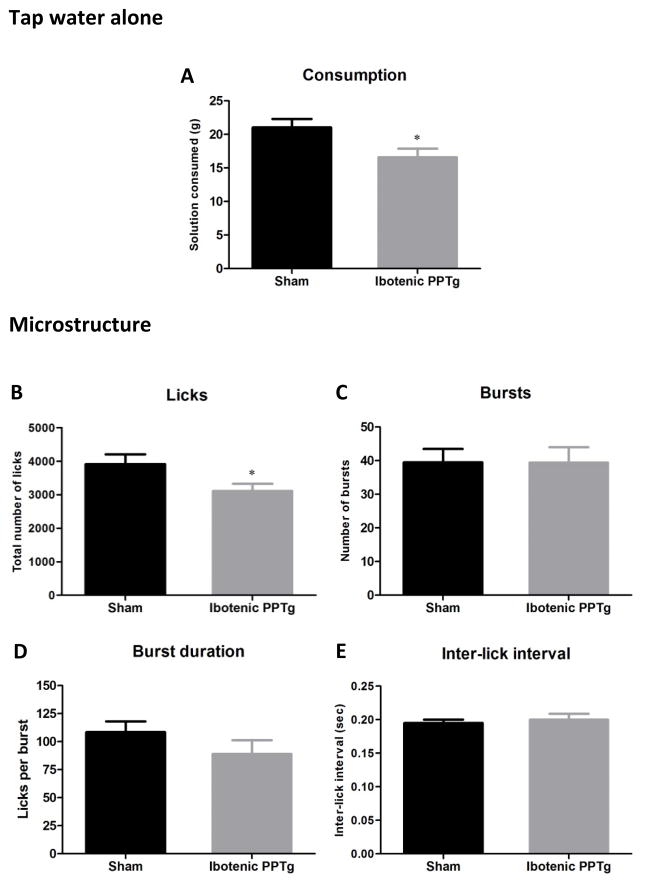
Prior to offering rats any tastants, we assessed consumption of tap water alone. Consumption and lick analysis for tap water is shown in figure 2. Rats with ibotenic acid lesions of the PPTg consumed significantly less tap water than sham controls (F(1,30) = 5.97, p = 0.021) and had significantly fewer licks for tap water than sham controls (F(1,30) = 4.79, p = 0.037). No other measure of drinking microstructure of tap water was altered by PPTg lesion (p > 0.05 in all cases). The significant reduction in tap water consumption is in line with a previous study showing a modest yet significant decrease in water intake following excitotoxic PPTg lesion (Allen & Winn, 1995). This is a curious effect and to the best of our knowledge there are no other reports of studies investigating it. While the reduction in tap water is significant, it is also modest (mean 4.4ml reduction in the lesion group) and does not appear to impact overall health (after the surgery recovery period, PPTg lesioned rats gain normal amounts of weight and show no visible signs of stress or suffering). Food consumption is unaltered by PPTg lesion which also supports the view that the reduction in water is not large enough to impact overall health (Dunbar et al., 1992; Keating et al., 2002). Our results extend the previous finding by showing that despite significant reductions in consumption and corresponding reductions in licking, all other measures of the microstructure of water consumption, including the inter-lick-interval, are unaffected by the lesion (figure 2). This suggests that the reduced water intake is not due to motor impairment (which is likely to change inter-lick-interval, eg (Lydall et al., 2010)). One speculative possibility would be that loss of the PPTg impairs the flow of information between areas in the brainstem and forebrain which are known to form a network controlling homeostatic intake of water (including the median preoptic nucleus, lateral hypothalamus, bed nucleus of the stria terminalis, nucleus acumbens, various thalamic nuclei, and the parabrachial nucleus (PBN), for full review see (Menani et al., 2014)). This possibility is supported by the anatomical connections between PPTg and many of these regions (Winn, 2006; Martinez-Gonzalez et al., 2011). The important point to consider here is whether reduction in tap water intake alters the interpretation of intake patterns of other tastants. While the mechanism behind reduced tap water intake remains unknown, as the effects of PPTg lesions on consumption of other tastants are specific enhancement of consumption under certain circumstances (see remainder of results section) the reduction in tap water intake is unlikely to be a major contributing factor.
Figure 2.
Consumption patterns of ibotenic PPTg lesioned rats drinking tap water presented alone. Excitotoxic PPTg lesions reduced the total volume of water consumed (2A) and the total number of licks (2B) but had no effect on any other measure of microstructure (C–E).
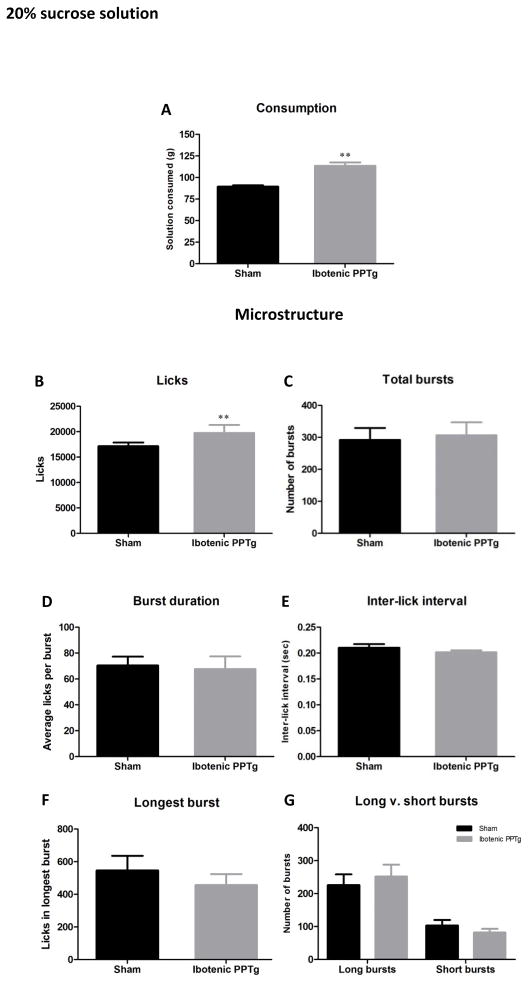
20% sucrose
Consumption and lick analysis for sucrose solution is shown in figure 3. Rats with excitotoxic PPTg lesions consumed significantly more 20% sucrose than sham controls (F(1,29) = 37.20, p < 0.001). Analysis of drinking microstructure revealed a significant increase in the number of licks for sucrose solution in lesioned rats (F(1,29) = 10.64, p = 0.003) but that no other measure of drinking microstructure was altered (p > 0.05 in all cases). Of particular interest, the burst duration showed no sign of being increased (which appears inconsistent with the hypothesis of response-perseveration – see discussion) and the number of bursts, while higher in the lesion group, was not significantly increased. The vast majority of licks occurred within bursts (99.7% for sham and 99.8% for ibotenic lesion) with no significant difference between the groups (p > 0.05).
Figure 3.
Consumption patterns of ibotenic PPTg lesioned rats drinking 20% sucrose solution. Excitotoxic PPTg lesions increased the volume of sucrose solution consumed (3A) and the total number of licks for sucrose solution (3B) but had no effect on any other measure of licking microstructure (C–G).
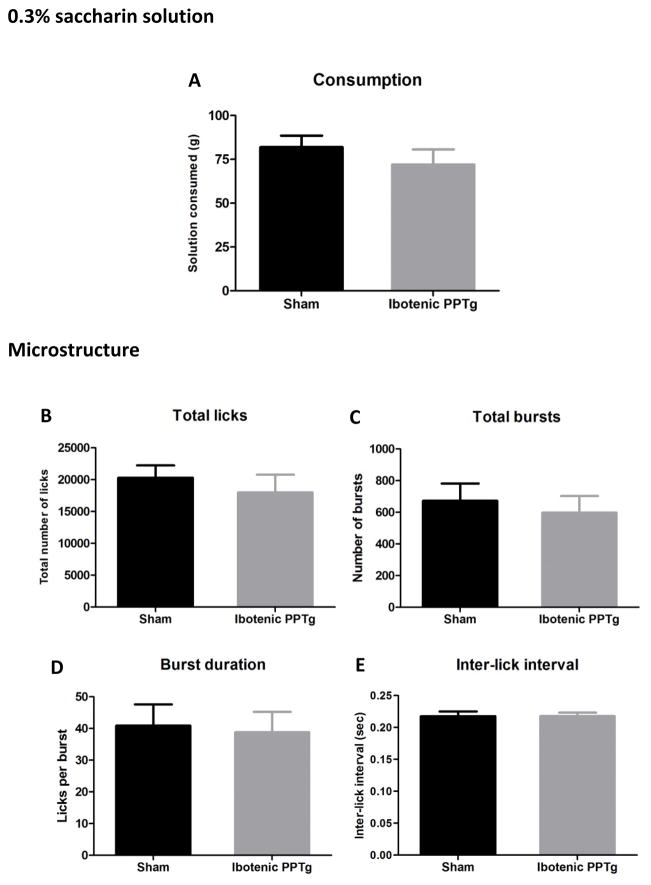
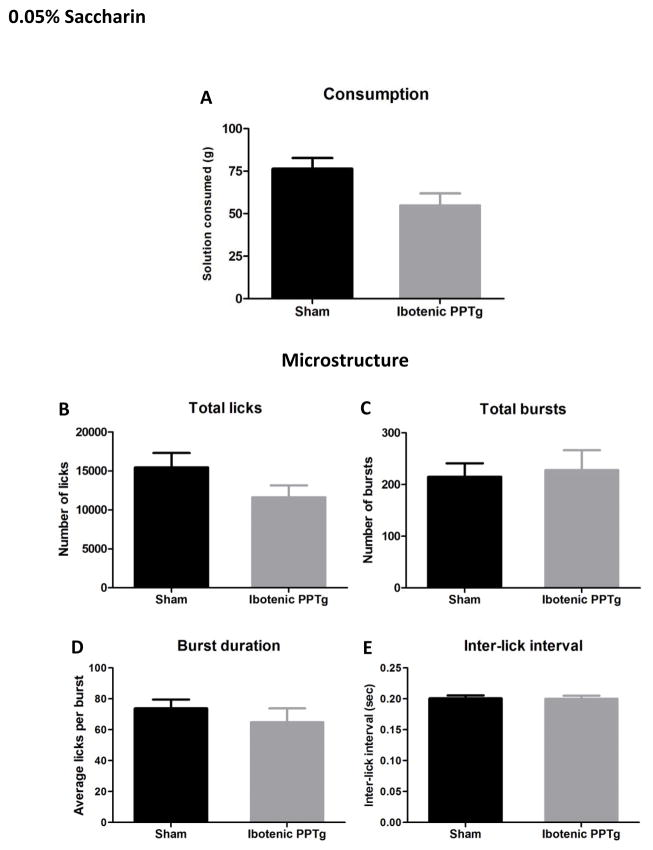
0.3% and 0.05% saccharin
Consumption and lick analysis for 0.3% saccharin and 0.05% saccharin are shown in figures 4 and 6. Despite drinking large quantities of saccharin solution (sham rats drank equal amounts of 0.3% saccharin and 20% sucrose solution) rats with excitotoxic PPTg lesions did not have altered consumption of either concentration of saccharin (p > 0.05 in all cases). Likewise, no aspect of drinking microstructure was significantly different in the lesion group (p > 0.05 in all cases). Contrary to the predictions that overconsumption would generalize to other highly consumed tastants, PPTg lesioned rats did not overconsume either concentration of saccharin solution.
Figure 4.
Consumption patterns of ibotenic PPTg lesioned rats drinking 0.3% saccharin solution. Excitotoxic PPTg lesions had no effect on the amount of solution consumed (4A) or any measure of licking microstructure (B–E).
Figure 6.
Consumption patterns of ibotenic PPTg lesioned rats drinking 0.05% saccharin solution. Excitotoxic PPTg lesions did change the amount of 0.05% quinine consumed (6A) or any measure of drinking microstructure (B–E).
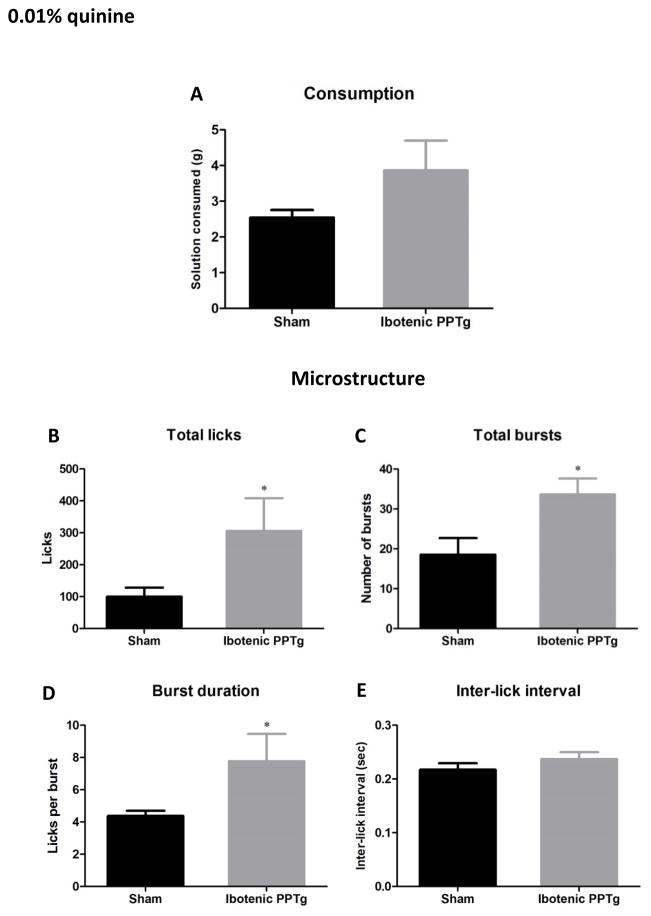
0.01% quinine
Consumption and lick analysis for quinine is shown in figure 5. Both sham and lesion groups consumed very small amounts of quinine solution and there was no significant difference between groups in amount consumed. However, analysis of drinking microstructure revealed that rats with PPTg lesions made significantly more licks (F(1,28) = 4.22, p = 0.049), significantly more bursts (F(1,28) = 6.70, p =0.015). and significantly more licks per burst (F(1,28) = 4.40, p =0.045) than sham controls. This altered consumption of quinine by PPTg lesioned rats is in line with a previous study showing that when quinine solution is the only liquid available, PPTg lesioned rats have higher consumption than sham controls (Walker & Winn, 2007).
Figure 5.
Consumption patterns of ibotenic PPTg lesioned rats drinking 0.01% quinine solution. Excitotoxic PPTg lesions did not significantly increase the amount of solution consumed (5A), but did increase the total number of licks (5B), number of bursts (5C) and burst duration (5D).
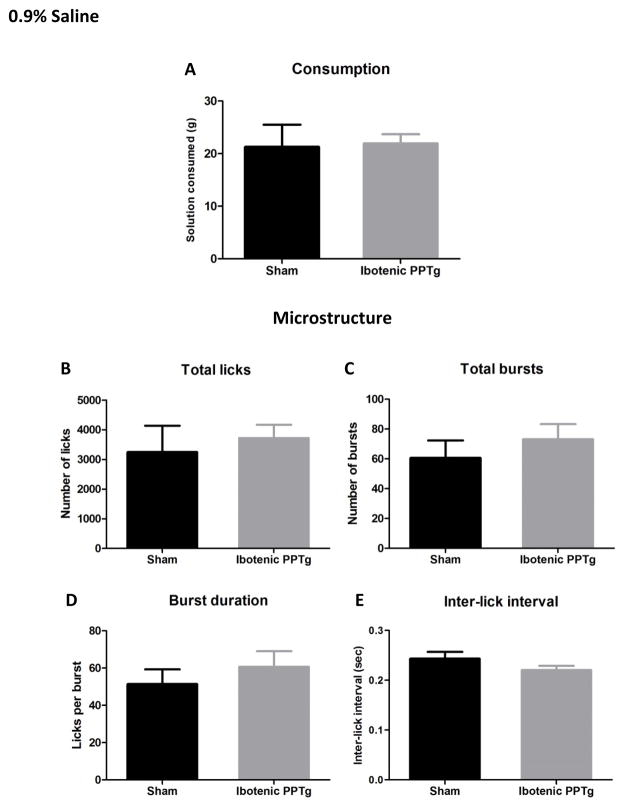
0.9% saline
See figure 7 for consumption and lick analysis of 0.9% saline. Excitotoxic PPTg lesions had no effect on the amount of 0.9% saline solution consumed and did not alter any measure of drinking microstructure (p > 0.05 in all cases).
Figure 7.
Consumption patterns of ibotenic PPTg lesioned rats drinking physiological saline. Excitotoxic PPTg lesions did change the amount of 0.9% saline consumed (7A) or any measure of drinking microstructure (B–E).
Dtx-UII (cholinergic neuron selective) PPTg lesions
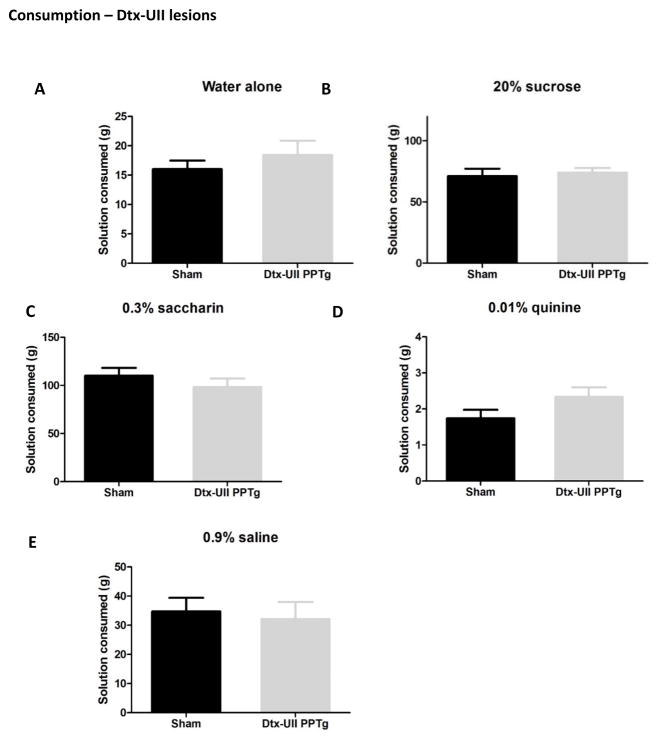
Consumption of all tastants by rats with selective lesions of cholinergic PPTg neurons is shown in figure 8. In contrast to the excitotoxic lesions, selective depletion of cholinergic PPTg neurons did not alter consumption of any tastant tested. There were no significant differences in consumption of tap water, 20% sucrose, 0.3% saccharin, 0.01% quinine or 0.9% saline (p > 0.05 in all cases). Analysis of the drinking microstructure is shown in table 1. Paralleling the consumption results, no measured aspect of drinking microstructure was altered in lesioned rats (p > 0.05 in all cases).
Figure 8.
Consumption patterns of rats with selective cholinergic (Dtx-UII) lesions of the PPTg. These lesions had no effect on the amount of tap water (A), 20% sucrose (B), 0.3% saccharin (C), 0.01% quinine (D) or physiological saline (E) consumed.
Table 1.
Licking microstructure of rats bearing bilateral lesions of cholinergic PPTg neurons. Loss of cholinergic PPTg neurons had no effect on any measured aspect of licking microstructure. Table shows group means. S.E.M. shows standard error of the mean. Sig indicates whether lesion group is significantly different to corresponding sham group.
| Total licks | Total bursts | Burst duration | Inter-lick interval | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Sham | Lesion | Sham | Lesion | Sham | Lesion | Sham | Lesion | |
|
| ||||||||
| Water alone | 2668 | 2496 | 38.3 | 37.9 | 71.7 | 72.8 | 0.19 | 0.20 |
| S.E.M. | ±231.7 | ±140.5 | ±3.7 | ±4.9 | ±7.2 | ±6.6 | ±0.06 | ±0.01 |
| Sig (vs sham) | n.s. | n.s. | n.s. | n.s. | ||||
|
| ||||||||
| 20% sucrose | 12498.7 | 12903.8 | 228.8 | 233.9 | 66.2 | 59.7 | 0.22 | 0.22 |
| S.E.M. | ±1049.3 | ±835.3 | ±45.6 | ±16.6 | ±8.5 | ±7.2 | ±0.006 | ±0.008 |
| Sig (vs sham) | n.s. | n.s. | n.s. | n.s. | ||||
|
| ||||||||
| 0.3% saccharin | 22909.1 | 19422.3 | 1333.7 | 1086 | 19.2 | 18.9 | 0.25 | 0.24 |
| S.E.M. | ±2238.6 | ±3369.6 | ±189.7 | ±155.5 | ±3.7 | ±3.1 | ±0.004 | ±0.005 |
| Sig (vs sham) | n.s. | n.s. | n.s. | n.s. | ||||
|
| ||||||||
| 0.01% quinine | 95.9 | 74.8 | 19.9 | 16.9 | 4.2 | 3.9 | 0.29 | 0.28 |
| S.E.M. | ±18.0 | ±12.3 | ±2.9 | ±2.7 | ±0.4 | ±0.2 | ±0.02 | ±0.02 |
| Sig (vs sham) | n.s. | |||||||
|
| ||||||||
| 0.9% saline | 5429.7 | 4819.2 | 153.5 | 95.4 | 48.5 | 60.8 | 0.24 | 0.24 |
| S.E.M. | ±809.6 | ±937.7 | ±34.1 | ±20.8 | ±11.0 | ±12.4 | ±0.01 | ±0.01 |
| Sig (vs sham) | n.s. | n.s. | n.s. | n.s. | ||||
n.s. = non-significant difference.
Summary of experiment 1 results
As previously shown, rats with non-selective ibotenic PPTg lesions consumed significantly more 20% sucrose than sham controls. Analysis of drinking microstructure revealed a corresponding increase in total licks, but no indications of response-perseveration or other large change in behavioral patterns. Ibotenic PPTg lesioned rats did not overconsume saccharin solution and had normal drinking microstructure of this solution. However, despite consuming only small amounts of quinine, ibotenic PPTg lesioned rats had enhanced consumption – increased licks, bursts and burst duration. Consumption of physiological saline was unaffected by lesion. Selective depletion of cholinergic PPTg neurons (Dtx-UII lesions) did not alter the consumption or drinking patterns of any solution. Based on the results of experiment 1, in experiment 2 we tested the hypothesis that the salience of the solution was the determining factor leading to overconsumption (high salience solutions with enhanced consumption: sucrose and quinine, low salience solutions with normal consumption: saccharin and saline. See discussion for full explanation).
Results: Experiment 2 - Salience manipulation
Based on the results of experiment 1, we tested the hypothesis that the salience of the solution is the determining factor leading to overconsumption in ibotenic PPTg lesioned rats. In this experiment we systematically altered the salience of sucrose and saline. Sucrose salience was manipulated by testing rats when maintained on lifelong free-food (therefore calories have relatively low salience) and after a period of mild food deprivation (which should increase the salience of calorific sucrose solution). The salience of saline was manipulated by testing consumption of hypertonic (1.8%) saline when rats were maintained on standard lab chow (which has ample sodium and therefore saline solution has relatively low salience) and after maintenance on a diet essentially devoid of sodium. This dietary manipulation is a standard technique to induce sodium appetite and sodium seeking behavior (eg (Mietlicki & Daniels, 2011; Tandon et al., 2012)) and therefore should increase the salience of saline solution.
Food control and sucrose consumption
When maintained on standard lab chow, rats bearing bilateral excitotoxic PPTg lesions consumed normal amounts of 20% sucrose (figure 9) (F(1,17) = 0.015, p = 0.91). After 10 days maintenance on food control (10–12g/rat/day – bodyweight did not fall to below 85% free feeding weight) ibotenic PPTg lesioned rats consumed significantly more 20% sucrose solution than sham controls (figure 9) (F(1,17) = 23.46, p = < 0.001). This deprivation-dependent effect of sucrose overconsumption in ibotenic PPTg lesioned rats has been previously noted (Olmstead et al., 1999). The consumption of 20% sucrose was not significantly different between sham rats maintained on free food and on food control (p > 0.05). While initially it might be expected that sham rats would have higher sucrose consumption when food deprived, our finding is consistent with other reports showing food deprivation does not increase sucrose solution consumption in intact animals (Duclos et al., 2013). One suggested explanation is that as long as energy expenditure is not increased (for example via exercise on a running wheel) mild food control in standard homecage does not reduce calorific needs enough to substantially increase the intake of high concentration sucrose consumption when measured over 24 hours (J.P. Konsman 2014; personal communication).
Figure 9.
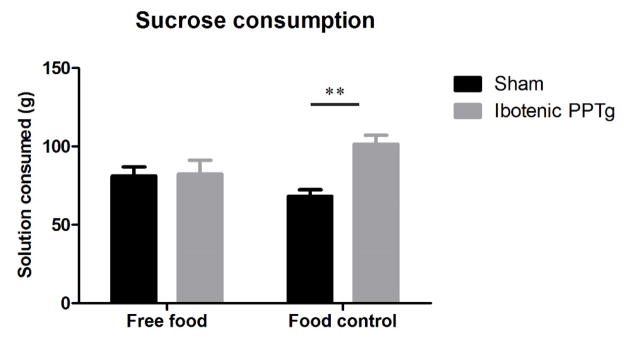
Effects of food deprivation on consumption of 20% sucrose. When maintained on free-food, rats with ibotenic PPTg lesions consumed normal amounts of 20% sucrose solution, after 10 days food control, PPTg lesioned rats consumed significantly more sucrose than sham controls.
Sodium deprivation and saline consumption
While maintained on free access to standard lab chow, rats with ibotenic PPTg lesions consumed normal amounts of 1.8% saline solution (figure 10; Also mirrors results for 0.9% saline in experiment 1 - figure 7) (F(1,16) = 0.88, p = 0.36). After dietary sodium deprivation, rats with ibotenic PPTg lesioned rats consumed significantly more 1.8% saline solution than sham controls (figure 10) (F(1,16) = 5.54, p = 0.03).
Figure 10.
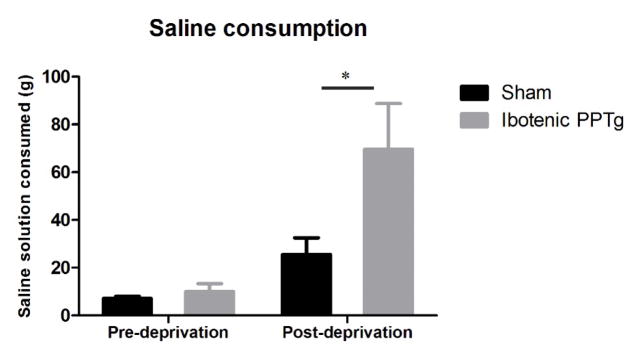
Effects of sodium deprivation on consumption of 1.8% saline. When maintained standard lab chow, rats with ibotenic PPTg lesions consumed normal amounts hypertonic saline. After 10 days dietary sodium deprivation, PPTg lesioned rats consumed significantly more saline than controls.
Summary of experiment 2 results
The results of experiment 2 show a state dependent effect of the overconsumption effect observed in PPTg lesioned rats. With free access to food, ibotenic PPTg lesioned rats consumed normal amounts of sucrose solution. With restricted access to food (and therefore also calories) ibotenic PPTg lesioned rats consumed significantly more 20% sucrose than sham controls. Likewise, under normal feeding conditions (free access to standard lab chow) rats with ibotenic PPTg lesions consumed normal amounts of 1.8% saline. However, after dietary induced sodium deprivation, ibotenic PPTg lesioned rats consumed significantly more saline than sham controls.
Discussion
These experiments were conducted to analyze the well documented effect of overconsumption of sucrose by rats with excitotoxic lesions of the PPTg. Using a contact lickometer system, we assessed the behavioral microstructure during overconsumption and by offering rats a variety of tastants we then tested the generalizability and specificity of this effect. In experiment 1 we tested the hypothesis that overconsumption is a result of response-perseveration or loss of behavioral organization in conditions of high excitement. Furthermore, it was addressed whether this effect is attributable solely to the loss of cholinergic PPTg neurons. In line with previous studies, rats with excitotoxic damage to all neuronal sub-populations within PPTg over-consumed 20% sucrose solution (Figure 3A). Analysis of the behavioral patterns leading to overconsumption (Figure 3B-G) revealed a corresponding increase in the total number of licks, but no changes in the pattern of behavior leading to overconsumption. When assessing the generalizability of over-consumption we found PPTg lesioned rats consumed large, yet entirely normal amounts of saccharin (Figure 4 and 6) and also normal amounts of physiological saline solution (Figure 7). However, despite consuming very small amounts of the aversive tastant quinine, ibotenic PPTg lesions caused an enhanced consumption of this tastant (Figure 5). Selective depletion of cholinergic neurons within PPTg, achieved using the fusion toxin Dtx-UII, had no effect on the consumption or behavioral microstructure of any tastant (Figure 8 and table 1.).
Based on these results, in experiment 2 we tested the hypothesis that the salience of the solution is the determining factor leading to increased consumption. This was achieved by altering the salience of calorific sucrose (through food deprivation) and hypertonic saline solution (through dietary sodium deprivation). When maintained on free-food, ibotenic PPTg lesioned rats consumed normal amounts of sucrose solution, however, after 10 days of food restriction they significantly over-consumed sucrose solution (Figure 9). Likewise, under normal feeding conditions (standard lab chow) these rats showed normal consumption of hypertonic saline solution, but, after 10 days maintenance on a diet essentially devoid of sodium, showed enhanced consumption of saline (Figure 10). Combined, these results show altered consumption of solutions by rats with PPTg lesions is not solely due to loss of cholinergic neurons and, furthermore, does not generalize to other highly consumed tastants but instead is dependent on specific properties of the tastant.
Previous interpretations of sucrose overconsumption by PPTg lesioned rats are in terms of disrupted behavioral control such as response-perseveration or loss of behavioral organization in conditions of high excitement (Olmstead et al., 1999; Alderson et al., 2001; Keating et al., 2002; Ainge et al., 2006; Winn, 2006). These theories have anatomical and behavioral support. The PPTg is highly integrated into cortico-thalamic and basal ganglia circuitry (to the extent that it has been argued it could be considered a part of basal ganglia (Mena-Segovia et al., 2004; Wilson et al., 2009b)). Of particular interest are reciprocal connections to the subthalamic nucleus (Kita & Kita, 2011) which plays an essential role in the stopping of ongoing behaviors (Schmidt et al., 2013). Behaviorally, in operant tasks requiring long sequences of lever presses to obtain a reward, ibotenic PPTg lesioned rats have higher levels of ‘late presses’ where they continue pressing despite the illumination of a light indicating pressing is no longer required (Wilson et al., 2009a). This behavior is not seen when only short sequences of lever presses are required and is suggestive of response-perseveration. We hypothesized that if sucrose overconsumption were the result of response-perseveration (continued execution of an ongoing behavior beyond a normal stopping point (Chambers & Self, 2002)) that we would see an increase in burst duration (the number of licks in each individual drinking episode). Alternatively, if overconsumption were due to loss of or disrupted behavioral control, we predicted a more general change in drinking patterns, for example, an increase in number of drinking episodes (bursts) and a decrease in length of drinking episodes (licks per burst). However, close analysis of the behavioral microstructure during overconsumption revealed no evidence of either change: the average burst duration (licks per burst – figure 3D) and the length of longest burst (figure 3F) were not increased in the lesion group. Likewise, the distribution of long versus short bursts (figure 3F) was also unaffected by PPTg lesion. While the total number of bursts was higher in the lesion group, this increase was not statistically significant (figure 3C). The overconsumption appeared to be a result of a significantly increased number of licks (figure 3B), distributed over an otherwise normal drinking pattern. If due to response-perseveration, we also predicted that the overconsumption effect should generalize to other highly consumed tastants. However, this was not the case. Sham rats consumed equal quantities of 20% sucrose as 0.3% saccharin, but contrary to predictions, PPTg lesioned rats did not overconsume this tastant (Figure 4A) and in addition no measure of drinking microstructure was altered (Figure 4 B–E). Combined, the results of the analysis of the consumption of sucrose versus saccharin and the apparently normal behavioral microstructure underlying sucrose consumption do not support the hypothesis that overconsumption is driven by response-perseveration or altered behavioral control in conditions of high excitement.
In order to understand why ibotenic PPTg lesioned rats overconsume sucrose yet have normal consumption of saccharin, an examination of the similarities and differences between sucrose and saccharin is required. Both sucrose and saccharin are sweet tasting. Sucrose is one of the most common naturally occurring sugars and is moderately rich in calories. Saccharin, in contrast, is an artificial sweetener devoid of any nutritional value. Despite rats being willing to work to obtain either as rewards, saccharin has considerably lower reinforcing properties and does not drive behavior as readily as sweetness-matched sucrose (Agmo & Marroquin, 1997; Beeler et al., 2012). For example, Scheggi and colleagues showed that sated (i.e. free-fed) rats lever pressed to obtain either sucrose or saccharin at comparable rates. However, after even relatively mild food deprivation (18 hours) rats pressed significantly more for the sucrose solution while the number of presses of saccharin was unchanged (Scheggi et al., 2013). Examination of the neural systems underlying these differences in sucrose and saccharin reveals a paralleled distinction in dopamine (DA) signaling. Initial experience of either a sucrose or saccharin solution induces phasic firing of midbrain DA neurons and increased levels of DA in the nucleus accumbens (NAcc) and other regions. However, when repeatedly presented to a hungry animal, the DA responses to saccharin rapidly diminishes, while those of sucrose stay elevated (Beeler et al., 2012; McCutcheon et al., 2012a; Scheggi et al., 2013). It is likely that many other neural differences exist between sucrose and saccharin, but the rapid reduction in DA signaling to saccharin and sustained response to sucrose may well be a considerable contributing factor to the higher reinforcing effects of sucrose. In this context, it is also worth noting that quinine solution (the only other tastant showing enhanced consumption by PPTg lesioned rats) also causes persistent changes in DA signaling that do not rapidly diminish with repeated exposures. However, this change is generally observed as a decrease rather than increase in DA activity (Roitman et al., 2008; McCutcheon et al., 2012b). Given that we observed altered consumption of sucrose and quinine (highly DA responsive) and not saccharin (where DA responses rapidly diminish) is it possible that the altered consumption is related to the DA activating properties of these tastants?
Support for the view that the altered consumption in PPTg lesioned rats is a result of altered DA responses comes from studies assessing the behavior of mice with a global knock out of the dopamine transporter (DAT-KO). These mice have enhanced DA signaling and an increased susceptibility to engage in some drug induced stereotypies (Smith & Cutts, 1990; Fox et al., 2013). However, of particular interest to our studies are the reports that DAT-KO mice also have increased consumption of both sucrose and quinine (Costa et al., 2007) while their consumption of water and saccharin is not increased (Savelieva et al., 2002) - a pattern that mirrors our rats with excitotoxic PPTg lesions.
Excitotoxic lesions of the PPTg are also known to alter the behavioral response to dopaminergic agonists. The lesions do not alter the locomotor activating properties of amphetamine or apomorphine, but do significantly change the profile and severity of the stereotypies induced by these drugs. In normal animals, both amphetamine and apomorphine dose dependently increase the frequency and magnitude of stereotyped sequences of behavior including sniffing, licking, rearing and, in the case of apomorphine, self-biting (Fray et al., 1980; Balsara et al., 1985; Chipkin et al., 1987; Arnt et al., 1988). In rats with ibotenic PPTg lesions, the severity of these stereotypies in response to apomorphine and damphetamine is enhanced (Inglis et al., 1994; Miller et al., 2002). Of particular interest is the finding that stereotyped biting develops in PPTg lesioned rats after administration of d-amphetamine. In normal rats, biting is induced by apomorphine but not amphetamine (Fray et al., 1980; Inglis et al., 1994; Miller et al., 2002) and is a result of activation of D2 receptors in the caudate-putamen (Chopra & Kulkarni, 1988; Allen & Winn, 1995; Rots et al., 1996). The enhanced development of stereotypies, particularly those attributable to D2 activation, suggests that excitotoxic PPTg lesion creates a general increased response to DA agonists and perhaps a bias towards D2 mediated behaviors (Arnt et al., 1988; Inglis et al., 1994). Consistent with this, excitotoxic lesion of the PPTg significantly augmented amphetamine induced elevation of DA levels (Miller et al., 2002).
There is also an association between sucrose consumption and D2 mediated systems. In normal rats, the individual differences in the preference for sucrose solution is significantly correlated with the density of striatal D2 receptors (Tonissaar et al., 2006). Sucrose seeking behavior is blocked by selective D2 receptor antagonists at doses which have no effect on locomotion (Rauhut et al., 2010). Furthermore, in models of obesity featuring increased sucrose consumption, selective D2 receptor antagonists are considerably more effective than D1 antagonists at reducing sucrose intake without reducing levels of overall behavior (Hajnal et al., 2007). These studies are consistent with the proposal that D1 systems process an array of possible behavioral actions which can be rapidly executed, whereas D2 systems integrate more complex information regarding current physiological needs and motivations (for recent discussion of the possible roles of D1/D2 systems, see: (Keeler et al., 2014)). The association between D2 systems and sucrose intake, and the known effect of ibotenic PPTg lesions to enhance D2 mediated behaviors, adds support to the possibility that sucrose overconsumption in PPTg lesioned rats might be reflective of an altered response to DA signaling, and in particular an increased propensity to engage in D2 driven behavior.
DA systems have long been associated with the overlapping processes of reward, motivational control, value perceptions and salience signaling (Berridge & Robinson, 1998; Schultz, 1999; Berridge, 2007; Humphries & Prescott, 2010; Schultz, 2010). While the consumption of pleasant and aversive tastants could be linked to many if not all of these DA associations, our results are perhaps best considered with particular reference to salience. Salience is the property of a stimulus which makes it stand out relative to its neighbors and which, in turn, influences behavior (Bromberg-Martin et al., 2010). Many stimuli, for example painful or aversive stimuli, are generally always salient and can persistently affect behavior (Borsook et al., 2013). However, the salience of the majority of stimuli has plasticity in that it can be varied. For example, when hungry, food has increased salience compared to when sated; in an experimental setting, one of two neutral visual cues can have incentive salience attributed to it by being paired with a food or drug reward while the second cue remains neutral (Schultz, 2010). Based on the results of our first experiments conducted in food restricted rats, we hypothesized that the salience of the solution being consumed may be the determining factor leading to overconsumption following ibotenic PPTg lesion. Our rationale was that, to a food restricted rat (rats in experiment 1 were food restricted), sucrose has salience as it is both sweet and a source of nutritional calories, and quinine has persistent salience as it is aversive (both of these tastants also persistently lead to increased DA signaling, see above sections) (Roitman et al., 2008; McCutcheon et al., 2012b). However, saccharin which has no calorific value (something which is quickly learnt by rats) and saline solution (which is of no nutritional value to rats maintained on a balanced standard lab chow diet) are both palatable solutions which will be consumed, but have low salience. To further test the hypothesis that PPTg lesions cause an enhanced behavioral response to salient tastants, we systematically varied the salience of sucrose solution (by testing rats maintained on lifelong free-food and after a period of food deprivation) and saline solution (by testing rats maintained on standard lab chow and after dietary induced sodium deprivation). Our results were clear, for both solutions in the low salience condition (free food and no sodium deprivation) rats with ibotenic PPTg lesions consumed normal amounts of the solution (sucrose, figure 9; saline, figure 10). However, after food control ibotenic PPTg lesioned rats consumed significantly more sucrose than shams (figure 9) and after sodium deficiency they consumed significantly more saline than shams (figure 10). The overconsumption of sucrose when food deprived but not free-fed has been reported previously (Olmstead et al., 1999). However, to the best of our knowledge, the effect on sodium deprivation has never been observed. This finding establishes that the factor leading to generalizability of the overconsumption effect is not simply the amount consumed, but the relevance of the solution to the subject, in other words, how salient the solution is.
The interpretation has so far been based on the hypothesis of altered responses to salient tastants following PPTg lesion. Alternative or perhaps additional contributing factors may lie in altered signaling of reward magnitude during consumption or altered incentive motivation. PPTg neurons respond to sensory and somatosensory input at very short latency (8–100ms) (Dormont et al., 1998; Kobayashi et al., 2002; Winn, 2006). Moreover, distinct populations of PPTg neurons encode specific aspects of this input, such as the actual size of a reward being delivered and the expected size of a reward based on previous experience in similar situations (Okada et al., 2009; Norton et al., 2011; Hong & Hikosaka, 2014). Importantly, these responses also show plasticity – if reward magnitude pairing are changed, the firing pattern of PPTg neurons updates to the new pairings within a few experiences of the new pairing combinations (Okada et al., 2009). The PPTg is a major input to midbrain DA systems and cortico-striatal and cortico-thalamic circuitry. This has led some to hypothesize that the PPTg may be an ideal candidate for sending short-latency reward-related information into these systems (Wilson et al., 2009b; Maclaren et al., 2013) including salience related information (Hong & Hikosaka, 2014). This view is supported by the discovery that inactivation of the PPTg blocks the sensory elicited firing (but not baseline firing rates) of midbrain DA neurons (Pan & Hyland, 2005). Therefore, in rats with excitotoxic PPTg lesions, disruption or loss of PPTg signaling may result in the mis-calculation of reward magnitude in interconnected systems that normally depend on this input. This mis-representation of may in turn contribute to increased consumption. Changes in incentive motivation would explain the deprivation dependent effects of sucrose and saline (as deprivation will increase the incentive motivation to consume the corresponding solution). However, they cannot readily explain the increased consumption of quinine: quinine is an aversive taste to rats and is therefore salient, but presumably elicits little incentive motivation to consume it. While not directly assessing incentive motivation, changes in incentive motivation are also not immediately compatible with previous studies showing PPTg lesions have no effect on reward directed motivation (Taylor et al., 2004; Maclaren et al., 2013).
Finally, it is worth considering which regions within the PPTg contribute to the observed effects. The PPTg is a heterogeneous collection of glutamatergic, GABAergic and cholinergic neurons (Wang & Morales, 2009). Previous lesion and inactivation studies utilize methods that are generally not selective for the neuronal sub-population they target (e.g. excitotoxic lesions or transient muscimol inactivation). The results from the current study show that loss of cholinergic neurons alone is not sufficient to produce behavioral changes in fluid consumption, implicating either that loss of non-cholinergic neurons or the combined loss of cholinergic and at least one other neuronal population is required for the observed effects. Additionally, different sub-regions within PPTg have differential connection patterns. For example, in terms of midbrain DA connections, the posterior region of the PPTg (pPPTg) projects to both VTA and substantia nigra (SN) whereas the anterior PPTg (aPPTg) projections only innervate the SN. There is also evidence to suggest inputs to PPTg are also segregated, inputs from cortical regions preferentially target pPPTg whereas basal ganglia targets the aPPTg (for review see (Martinez-Gonzalez et al., 2011). In line with these differential connection patterns, different PPTg sub-regions are implicated in different behaviors: for example excitotoxic lesions of the pPPTg (but not of the aPPTg) produce a persistent learning impairment (Wilson et al., 2009a). Therefore, in future studies it would be interesting to consider not only which PPTg non-cholinergic neuronal population is responsible for our behavioral effects, but also whether the effects are attributable to a specific anatomical sub-region and projection pathway.
Despite the uncertainty behind the mechanisms leading to overconsumption, the conditions under which rats with ibotenic PPTg lesions overconsume remains clear. In situations of low physiological relevance (drinking sucrose having never experienced the absence of any food and subsequent increases in hunger, and drinking saline when eating a sodium balanced diet) the consumption patterns and amount consumed by PPTg lesioned rats is entirely normal. However, when the relevance of the solution is increased (by restricting access to calories or removing sodium from the diet) rats with excitotoxic lesions of the PPTg then consume significantly more of the solution than sham operated controls. The critical factor here appears to be the salience of the tastant: experimentally increasing the salience of the solution being consumed leads to overconsumption in rats with a lesioned PPTg. No change in consumption of any solution was observed after selective depletion of the cholinergic sub-population of PPTg neurons, implicating that the effect either originates in the non-cholinergic (glutamatergic and GABAergic) neurons, or from the combined loss of cholinergic and at least one other population of neurons. Salience detection is a key mechanism for adaptation and survival as it enables the limited attentional and cognitive resources of an organism to be rapidly directed at the most relevant aspects of a crowded environment. While salience detection is often attributed to systems higher in the neuraxis, it is perhaps not surprising that such a fundamental process receives contributions from the brainstem.
Table 2.
Licking microstructure of rats bearing bilateral excitotoxic (non-selective) lesions of the PPTg. Table shows group means. S.E.M. shows standard error of the mean. Sig indicates whether lesion group is significantly different to corresponding sham group.
| Total licks | Total bursts | Burst duration | Inter-lick interval | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Sham | Lesion | Sham | Lesion | Sham | Lesion | Sham | Lesion | |
|
| ||||||||
| Water alone | 3910 | 3107 | 39.5 | 39.4 | 108.44 | 89.1 | 0.20 | 0.20 |
| S.E.M. | ± 92.8 | ± 21.3 | ± 4.0 | ± 4.5 | ± 9.7 | ± 12.2 | ± 0.005 | ± 0.009 |
| Sig (vs sham) | p = 0.03 | n.s. | n.s. | n.s. | ||||
|
| ||||||||
| 20% sucrose | 17162 | 19759 | 292.0 | 306.9 | 70.4 | 67.75 | 0.21 | 0.20 |
| S.E.M. | ±722.5 | ±1575.3 | ± 37.5 | ±40.4 | ± 6.9 | ±9.7 | ± 0.006 | ±0.003 |
| Sig (vs sham) | p = 0.003 | n.s. | n.s. | n.s. | ||||
|
| ||||||||
| 0.3% saccharin | 20296 | 17983 | 672.50 | 597.6 | 40.9 | 38.79 | 0.21 | 0.21 |
| S.E.M. | ±1946.5 | ±2800.8 | 0±109.6 | ±105.6 | ±6.7 | ±6.4 | ±0.007 | ±0.005 |
| Sig (vs sham) | n.s. | n.s. | n.s. | n.s. | ||||
|
| ||||||||
| 0.01% quinine | 100 | 306 | 18.56 | 33.6 | 4.4 | 7.77 | 0.21 | 0.23 |
| S.E.M. | ±28.3 | ±102 | ±4.2 | ±4.0 | ±0.3 | ±1.7 | ±0.011 | ±0.012 |
| Sig (vs sham) | p = 0.049 | p = 0.015 | p = 0.045 | n.s. | ||||
|
| ||||||||
| 0.9% saline | 3247 | 3717 | 60.65 | 73.1 | 51.4 | 60.70 | 0.24 | 0.22 |
| S.E.M. | ±889.7 | ±445.9 | ±11.7 | ±10.2 | ±7.9 | ±8.3 | ±0.014 | ±0.009 |
| Sig (vs sham) | n.s. | n.s. | n.s. | n.s. | ||||
n.s. = non-significant difference.
Highlights.
Rats with lesions of the pedunculopontine tegmentum consume significantly more 20% sucrose than controls
Excitotoxic, but not selective cholinergic lesions, produced overconsumption
Using a contact lickometer system it was found that overconsumption is not due to perseveration
Overconsumption only occurs for salient solutions– both sucrose and saline are only overconsumed when salient
An intact PPTg is important for the normal behavioral response to salient stimuli
Acknowledgments
We would like to thank the staff of the Laboratory Animal Facility for daily care of our rats. Michael Marsh, Jasmine Nurse, Anandita Ananthakumar and Lauren Mueller assisted in histological processing. Dr Jessica Santollo provided valuable help in conducting and interpreting our experiments. We also thank Dr Hans-Peter Nothacker for his contributions in the development of the Dtx-UII toxin. SDC is supported by a grant from the National Institutes of Health, USA (R00DA024754).
Abbreviations
- ANOVA
analysis of variance
- ChAT
choline acetyltransferase
- DAB
3,3′-diaminobenzidin
- DPX
distyrene plasticizer xylene
- Dtx-UII
diphtheria toxin-urotensin II
- IAL
interaural line
- ip
intraperitoneal
- NeuN
neuronal nuclei
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PBN
parabigeminal nucleus
- PPTg
pedunculopontine tegmental nucleus
- SEM
standard error of the mean
- SN
substantia nigra
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A, Marroquin E. Role of gustatory and postingestive actions of sweeteners in the generation of positive affect as evaluated by place preference conditioning. Appetite. 1997;29:269–289. doi: 10.1006/appe.1997.0101. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Keating GL, Latimer MP, Winn P. The pedunculopontine tegmental nucleus and responding for sucrose reward. Behav Neurosci. 2006;120:563–570. doi: 10.1037/0735-7044.120.3.563. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Faulconbridge LF, Gregory LP, Latimer MP, Winn P. Behavioural sensitisation to repeated d-amphetamine: effects of excitotoxic lesions of the pedunculopontine tegmental nucleus. Neuroscience. 2003;118:311–315. doi: 10.1016/s0306-4522(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Jenkins TA, Kozak R, Latimer MP, Winn P. The effects of excitotoxic lesions of the pedunculopontine tegmental nucleus on conditioned place preference to 4%, 12% and 20% sucrose solutions. Brain Res Bull. 2001;56:599–605. doi: 10.1016/s0361-9230(01)00733-x. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience. 2004;125:349–358. doi: 10.1016/j.neuroscience.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Latimer MP, Winn P. A functional dissociation of the anterior and posterior pedunculopontine tegmental nucleus: excitotoxic lesions have differential effects on locomotion and the response to nicotine. Brain Struct Funct. 2008;213:247–253. doi: 10.1007/s00429-008-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LF, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus disinhibit orofacial behaviours stimulated by microinjections of d-amphetamine into rat ventrolateral caudate-putamen. Exp Brain Res. 1995;104:262–274. doi: 10.1007/BF00242012. [DOI] [PubMed] [Google Scholar]
- Arnt J, Bogeso KP, Hyttel J, Meier E. Relative dopamine D1 and D2 receptor affinity and efficacy determine whether dopamine agonists induce hyperactivity or oral stereotypy in rats. Pharmacology & toxicology. 1988;62:121–130. doi: 10.1111/j.1600-0773.1988.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Balsara JJ, Bapat TR, Gada VP, Nandal NV, Chandorkar AG. Effect of ergometrine on methamphetamine and apomorphine stereotypy in the guinea-pig. J Pharm Pharmacol. 1985;37:514–517. doi: 10.1111/j.2042-7158.1985.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Baunez C, Amalric M, Robbins TW. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J Neurosci. 2002;22:562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. 2012;36:2533–2546. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: The value of salience circuits. Progress in Neurobiology. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipkin RE, McQuade RD, Iorio LC. D1 and D2 dopamine binding site up-regulation and apomorphine-induced stereotypy. Pharmacol Biochem Behav. 1987;28:477–482. doi: 10.1016/0091-3057(87)90509-0. [DOI] [PubMed] [Google Scholar]
- Chopra K, Kulkarni SK. Potentiation of Dopamine Receptor-Mediated Responses by B-Ht-920 in Mice. Arch Int Pharmacod T. 1988;294:46–55. [PubMed] [Google Scholar]
- Clark SD, Alderson HL, Winn P, Latimer MP, Nothacker HP, Civelli O. Fusion of diphtheria toxin and urotensin II produces a neurotoxin selective for cholinergic neurons in the rat mesopontine tegmentum. J Neurochem. 2007;102:112–120. doi: 10.1111/j.1471-4159.2007.04529.x. [DOI] [PubMed] [Google Scholar]
- Costa RM, Gutierrez R, de Araujo IE, Coelho MR, Kloth AD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Dopamine levels modulate the updating of tastant values. Genes Brain Behav. 2007;6:314–320. doi: 10.1111/j.1601-183X.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Cyr M, Parent MJ, Mechawar N, Rosa-Neto P, Soucy JP, Aliaga A, Kostikov A, Maclaren DA, Clark SD, Bedard MA. PET imaging with [(1)(8)F]fluoroethoxybenzovesamicol ([(1)(8)F]FEOBV) following selective lesion of cholinergic pedunculopontine tegmental neurons in rat. Nucl Med Biol. 2014;41:96–101. doi: 10.1016/j.nucmedbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Dormont JF, Conde H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res. 1998;121:401–410. doi: 10.1007/s002210050474. [DOI] [PubMed] [Google Scholar]
- Duclos M, Ouerdani A, Mormede P, Konsman JP. Food restriction-induced hyperactivity: addiction or adaptation to famine? Psychoneuroendocrinology. 2013;38:884–897. doi: 10.1016/j.psyneuen.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Dunbar JS, Hitchcock K, Latimer M, Rugg EL, Ward N, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus of the rat. II. Examination of eating and drinking, rotation, and reaching and grasping following unilateral ibotenate or quinolinate lesions. Brain Res. 1992;589:194–206. doi: 10.1016/0006-8993(92)91278-m. [DOI] [PubMed] [Google Scholar]
- Fox MA, Panessiti MG, Hall FS, Uhl GR, Murphy DL. An evaluation of the serotonin system and perseverative, compulsive, stereotypical, and hyperactive behaviors in dopamine transporter (DAT) knockout mice. Psychopharmacology (Berl) 2013;227:685–695. doi: 10.1007/s00213-013-2988-x. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD. An observational method for quantifying the behavioural effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychopharmacology (Berl) 1980;69:253–259. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- Hajnal A, De Jonghe BC, Covasa M. Dopamine D2 receptors contribute to increased avidity for sucrose in obese rats lacking CCK-1 receptors. Neuroscience. 2007;148:584–592. doi: 10.1016/j.neuroscience.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Progress in Neurobiology. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Allen LF, Whitelaw RB, Latimer MP, Brace HM, Winn P. An investigation into the role of the pedunculopontine tegmental nucleus in the mediation of locomotion and orofacial stereotypy induced by d-amphetamine and apomorphine in the rat. Neuroscience. 1994;58:817–833. doi: 10.1016/0306-4522(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW. Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav Brain Res. 2001;123:117–131. doi: 10.1016/s0166-4328(01)00181-4. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- Keating GL, Walker SC, Winn P. An examination of the effects of bilateral excitotoxic lesions of the pedunculopontine tegmental nucleus on responding to sucrose reward. Behav Brain Res. 2002;134:217–228. doi: 10.1016/s0166-4328(02)00032-3. [DOI] [PubMed] [Google Scholar]
- Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience. 2002;112:687–696. doi: 10.1016/s0306-4522(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Keeler JF, Pretsell DO, Robbins TW. Functional implications of dopamine D1 vs D2 receptors: A ‘Prepare and Select’ model of the striatal direct vs. indirect pathways. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Kita T, Kita H. Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. The European journal of neuroscience. 2011;33:433–443. doi: 10.1111/j.1460-9568.2010.07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H. Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol. 2002;88:715–731. doi: 10.1152/jn.2002.88.2.715. [DOI] [PubMed] [Google Scholar]
- Lydall ES, Gilmour G, Dwyer DM. Analysis of licking microstructure provides no evidence for a reduction in reward value following acute or sub-chronic phencyclidine administration. Psychopharmacology (Berl) 2010;209:153–162. doi: 10.1007/s00213-010-1779-x. [DOI] [PubMed] [Google Scholar]
- MacLaren DA, Santini JA, Russell AL, Markovic T, Clark SD. Deficits in motor performance after pedunculopontine lesions in rats - impairment depends on demands of task. Eur J Neurosci. 2014 doi: 10.1111/ejn.12666. [DOI] [PubMed] [Google Scholar]
- Maclaren DA, Wilson DI, Winn P. Updating of action-outcome associations is prevented by inactivation of the posterior pedunculopontine tegmental nucleus. Neurobiol Learn Mem. 2013;102:28–33. doi: 10.1016/j.nlm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Frontiers in neuroanatomy. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2012a;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012b;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev. 2008;58:265–271. doi: 10.1016/j.brainresrev.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Menani JV, De Luca LA, Jr, Johnson AK. Role of the lateral parabrachial nucleus in the control of sodium appetite. Am J Physiol Regul Integr Comp Physiol. 2014;306:R201–210. doi: 10.1152/ajpregu.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki EG, Daniels D. Ghrelin reduces hypertonic saline intake in a variety of natriorexigenic conditions. Exp Physiol. 2011;96:1072–1083. doi: 10.1113/expphysiol.2011.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Forster GL, Metcalf KM, Blaha CD. Excitotoxic lesions of the pedunculopontine differentially mediate morphine- and d-amphetamine-evoked striatal dopamine efflux and behaviors (vol 111, pg 351, 2002) Neuroscience. 2002;113:1015–1015. doi: 10.1016/s0306-4522(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci. 2011;33:1885–1896. doi: 10.1111/j.1460-9568.2011.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci. 2009;29:4858–4870. doi: 10.1523/JNEUROSCI.4415-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. Lesions of the pedunculopontine tegmental nucleus block drug-induced reinforcement but not amphetamine-induced locomotion. Brain Res. 1994;638:29–35. doi: 10.1016/0006-8993(94)90629-7. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Inglis WL, Bordeaux CP, Clarke EJ, Wallum NP, Everitt BJ, Robbins TW. Lesions of the pedunculopontine tegmental nucleus increase sucrose consumption but do not affect discrimination or contrast effects. Behav Neurosci. 1999;113:732–743. [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Fenton L, Bardo MT. Renewal of sucrose-seeking behavior in rats: Role of D(2) dopamine receptors. Pharmacol Biochem Behav. 2010;96:354–362. doi: 10.1016/j.pbb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostron CL, Farquhar MJ, Latimer MP, Winn P. The pedunculopontine tegmental nucleus and the nucleus basalis magnocellularis: do both have a role in sustained attention? BMC Neurosci. 2008;9:16. doi: 10.1186/1471-2202-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rots NY, Cools AR, Berod A, Voorn P, Rostene W, de Kloet ER. Rats bred for enhanced apomorphine susceptibility have elevated tyrosine hydroxylase mRNA and dopamine D2-receptor binding sites in nigrostriatal and tuberoinfundibular dopamine systems. Brain Res. 1996;710:189–196. doi: 10.1016/0006-8993(95)01379-2. [DOI] [PubMed] [Google Scholar]
- Savelieva KV, Caudle WM, Findlay GS, Caron MG, Miller GW. Decreased ethanol preference and consumption in dopamine transporter female knock-out mice. Alcoholism, clinical and experimental research. 2002;26:758–764. [PubMed] [Google Scholar]
- Scheggi S, Secci ME, Marchese G, De Montis MG, Gambarana C. Influence of palatability on motivation to operate for caloric and non-caloric food in non food-deprived and food-deprived rats. Neuroscience. 2013;236:320–331. doi: 10.1016/j.neuroscience.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. The Reward Signal of Midbrain Dopamine Neurons. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 1999;14:249–255. doi: 10.1152/physiologyonline.1999.14.6.249. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behavioral and brain functions : BBF. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. The Journal of comparative neurology. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Smith CF, Cutts S. Dopamine agonist activity of 8-OH-DPAT. Arch Int Pharmacodyn Ther. 1990;306:106–113. [PubMed] [Google Scholar]
- Tandon S, Simon SA, Nicolelis MA. Appetitive changes during salt deprivation are paralleled by widespread neuronal adaptations in nucleus accumbens, lateral hypothalamus, and central amygdala. J Neurophysiol. 2012;108:1089–1105. doi: 10.1152/jn.00236.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CL, Kozak R, Latimer MP, Winn P. Effects of changing reward on performance of the delayed spatial win-shift radial maze task in pedunculopontine tegmental nucleus lesioned rats. Behav Brain Res. 2004;153:431–438. doi: 10.1016/j.bbr.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Tonissaar M, Herm L, Rinken A, Harro J. Individual differences in sucrose intake and preference in the rat: circadian variation and association with dopamine D2 receptor function in striatum and nucleus accumbens. Neurosci Lett. 2006;403:119–124. doi: 10.1016/j.neulet.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Walker SC, Winn P. An assessment of the contributions of the pedunculopontine tegmental and cuneiform nuclei to anxiety and neophobia. Neuroscience. 2007;150:273–290. doi: 10.1016/j.neuroscience.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DI, MacLaren DA, Winn P. Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine. Eur J Neurosci. 2009a;30:504–513. doi: 10.1111/j.1460-9568.2009.06836.x. [DOI] [PubMed] [Google Scholar]
- Wilson DIG, MacLaren DAA, Winn P. The Basal Ganglia IX. Springer; New York: 2009b. On the Relationships Between the Pedunculopontine Tegmental Nucleus, Corticostriatal Architecture, and the Medial Reticular Formation; pp. 143–157. [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci. 2006;248:234–250. doi: 10.1016/j.jns.2006.05.036. [DOI] [PubMed] [Google Scholar]