Abstract
Lymphatic vessels are intimately involved in regulation of water and solute homeostasis by returning interstitial fluid back to the venous circulation and play an equally important role in immune responses by providing avenues for immune cell transport. Defects in the lymphatic vasculature result in a number of pathological conditions, including lymphedema and lymphangiectasia. Knowledge of molecular mechanisms underlying lymphatic development and maintenance is therefore critical for understanding, prevention and treatment of lymphatic circulation-related diseases. Research in the past two decades has uncovered several key transcriptional factors (Prox1, Sox18 and Coup-TFII) controlling lymphatic fate specification. Most recently, ERK signaling has emerged as a critical regulator of this transcriptional program. This review summarizes our current understanding of lymphatic fate determination and its transcriptional controls.
1. Development of the Lymphatic Vascular Network
The circulatory system in vertebrates is composed of two morphologically and functionally distinct vascular networks: blood and lymphatics. Whilst the blood vasculature transports oxygen and nutrients throughout the body, lymphatic system returns protein- rich interstitial fluid from extracellular space back into venous circulation. (Alitalo et al., 2005; Oliver, 2004; Yang and Oliver, 2014). Besides maintaining tissue fluid balance, lymphatic vessels are also involved in transport of fatty acids, white blood cells and antigen-presenting cells (Alitalo et al., 2005; Oliver, 2004; Yang and Oliver, 2014). In addition, recent studies have implicated skin lymphatics in control of blood pressure (Machnik et al., 2009). Congenital malformations or malfunction of the lymphatic vasculature due to disease or mechanical injury lead to lymphedema, lymphangiectasia, and various immune and metabolic abnormalities (Alitalo, 2011; Alitalo et al., 2005; Karpanen and Alitalo, 2008; Wang and Oliver, 2010).
Florence Sabin proposed close to a century ago that lymphatic vessels are derived from veins (Sabin, 1916). Modern genetic lineage-tracing studies confirmed this hypothesis by demonstrating that lymphatic endothelial cells (LECs) indeed originate from the venous vasculature in mice (Srinivasan et al., 2007). Time-lapse imaging and histological analysis in zebrafish and tadpoles also demonstrated development of lymphatic vessels from venous endothelial cells, thus establishing a highly conserved pattern of lymphatic fate specification in vertebrates (Kuchler et al., 2006; Ny et al., 2005; Yaniv et al., 2006).
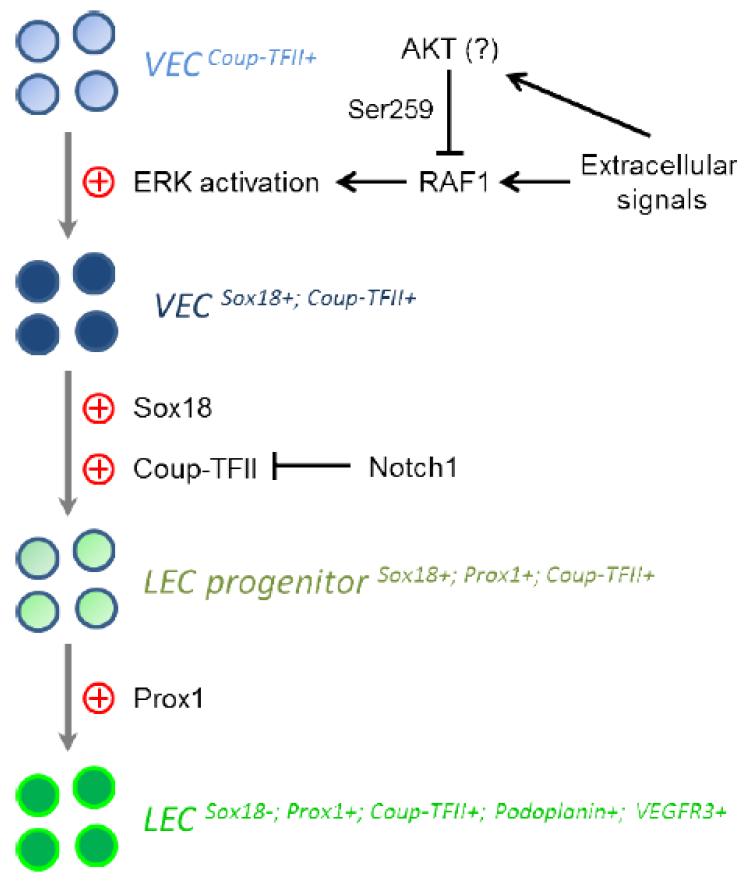
Assembly of the lymphatic vascular network is a stepwise process, during which endothelial cells gradually acquire LEC-specific gene signature (Francois et al., 2011; Oliver, 2004; Oliver and Harvey, 2002) (See Figure 1). At about E9.5, a subset of endothelial cells localized in the lateral parts of the anterior cardinal vein begin expressing prospero-related homeobox 1 (Prox1), considered the key transcription factor determining LEC fate (Srinivasan et al., 2007; Wigle and Oliver, 1999; Yang et al., 2012). Prox1 expression is required for subsequent lymphatic development and fate maintenance as the knockout of this gene results in complete failure of lymphatic network formation in mice (Johnson et al., 2008; Wigle and Oliver, 1999). The Prox1-positive LECs then bud from the cardinal vein at E9.75 and migrate dorsolaterally into the surrounding mesenchyme to form jugular lymph sacs. This process is stimulated by the lymphangiogenic vascular endothelial growth factor C (VEGF-C) (Wigle and Oliver, 1999; Yang et al., 2012). Once formed, jugular lymphatics sacs undergo sprouting to give rise to peripheral lymphatic vessels, which then remodel into a mature vascular lymphatic network (Adams and Alitalo, 2007; Oliver and Srinivasan, 2008). The critical role of VEGF-C in regulation of this step is demonstrated by the fact that while initial LEC fate specification occurs in VEGF-C knockout mice, no LEC outmigration and jugular lymphatic sac formation take place (Karkkainen et al., 2004). As LECs migrate out of the jugular sacs, they begin expressing Podoplanin and differentiate into mature LECs (Yang et al., 2012; Yang and Oliver, 2014).
Figure 1.
Differentiation of a lymphatic endothelial cell (LEC) from a venous endothelial cell (VEC) is driven by ERK activation, Sox18, Coup-TFII and Prox1. During this process, LECs gradually acquire specific gene signature.
A recent study provided a detailed 3D analysis of this stage of lymphatic development by combining selective plane illumination-based ultramicroscopy and 3 dimensional reconstructions of image stacks (Hagerling et al., 2013). In particular, LECs exiting from the cardinal vein were observed to aggregate close to the intersomitic vessels and then condensate to form a peripheral longitudinal lymphatic vessel. Meanwhile, LECs also accumulate close to the cardinal vein to generate the primitive thoracic duct with a large lumenized structure (Hagerling et al., 2013).
Lymphatic endothelial hyaluronan receptor (Lyve1) is the receptor for the glycosaminoglycan hyaluronan that is involved in wound healing, inflammation and some other physiological processes (Prevo et al., 2001). Lyve1 labels entire lymphatic endothelium during embryonic stage, while its expression is only limited to lymphatic capillaries after birth (Adams and Alitalo, 2007). However, Lyve1 is not a key determinant of lymphatic fate specification as mice with homozygous disruption of its expression exhibit normal lymphatic development (Gale et al., 2007).
2. Transcriptional Control of Lymphatic Fate Determination
2.1. Prox1
The transcriptional factor Prox1 is the key regulator of lymphatic fate specification (Table 1). It was initially cloned in Drosophila and shown to affect neuronal fate determination in the central nervous system (Doe et al., 1991). During mouse development, Prox1 is expressed in a number of different tissues including the heart, lens, pancreas and lymphatics (Oliver et al., 1993; Wigle and Oliver, 1999). Prox1 expression in mouse lymphatics is detectable as early as E9.5, when it is first seen in a highly polarized group of LEC progenitors in the lateral walls of the anterior cardinal vein (Wigle and Oliver, 1999). Ablation of Prox1 prevents venous endothelial cells from committing to a lymphatic fate thus resulting in a complete failure of lymphatic vessel development in mouse embryos (Wigle et al., 2002; Wigle and Oliver, 1999). Prox1 heterozygous mutants die soon after birth and exhibit a number of findings including milky chylous ascites in the peritoneal and thoracic cavities indicating severe lymphatic vascular dysfunction (Harvey et al., 2005). However, a small number of Prox1 heterozygous animals on the NMRI background can survive to adulthood. These mice show adult-onset obesity that has been attributed to lymph leakage caused by abnormal lymphatic development (Harvey et al., 2005).
Table 1.
Summary of the mouse lymphatic defects and human lymphatic diseases caused by the deficiency/activation of the genes associated with lymphatic fate specification. LEC: lymphatic endothelial cell.
| Gene | Lymphatic Phenotype in mouse models | Links to human disease |
|---|---|---|
| Prox1 |
|
No |
| Sox18 |
|
Hypotrichosis-lymphedema- telangiectasia |
| COUP-TFII |
|
No |
| ERK | Endothelial expression of RAF1S259A: greatly enhanced activation of ERK; dramatically increased lymphatic fate determination; expression of Sox18 in the entire cardinal vein; substantial induction of Prox1 in the venous endothelium and even dorsal arteries; enlarged lymph sacs. |
Noonan syndrome; LEOPARD syndrome |
| Notch |
|
No |
Prox1 activity is further required to maintain the lymphatic identity in mature lymphatic vessels. Conditional deletion of Prox1 during postnatal development or in adult mice reprograms LECs into blood endothelial cells (BECs) (Johnson et al., 2008). Downregulation of Prox1 in cultured LECs also leads to disappearance of lymphatic markers and acquisition of BEC gene signature (Johnson et al., 2008). Moreover, several studies suggest that Prox1 is also sufficient to drive BECs to adopt a lymphatic fate. Ectopic expression of Prox1 in cultured BECs suppresses blood vascular markers and promotes the expression of LEC-specific genes (Hong et al., 2002; Petrova et al., 2002). Conditional induction of Prox1 specifically in mouse BECs in vivo results in the sustained expression of lymphatic marker genes in blood vasculature, enlarged lymph sacs and edema (Kim et al., 2010).
Recent studies have identified several transcriptional targets of Prox1. Overexpression of Prox1 in cultured endothelial cells induces podoplanin (Hong et al., 2002), the calcitonin receptor-like receptor (Fritz-Six et al., 2008), Cyclin E and p57kip2 (Petrova et al., 2002). In endothelial cells derived from mouse embryonic stem cells, FoxC2, angiopoietin-2 and HoxD8 were found to be controlled by Prox1 (Harada et al., 2009). Prox1 also stimulates endothelial cell motility through regulating the expression of integrin α9 (Mishima et al., 2007). However, it remains unknown whether Prox1 activates the transcription of those genes directly or indirectly. Studies using chromatin immunoprecipitation (ChIP) and luciferase reporter-based promoter analysis demonstrated that Prox1 and p50 NF-κB directly bind and synergistically activate the promoter of vascular endothelial growth factor receptor 3 (VEGFR3), which is a key regulator of lymphangiogenesis (Adams and Alitalo, 2007; Flister et al., 2010).
Consistent with this finding, inflammatory stimuli-dependent activation of NF-κB signaling and Prox1 expression is followed by up-regulation of VEGFR3 during inflammation-induced lymphangiogenesis (Flister et al., 2010). A member of Ets family transcription factors, Ets2, together with Prox1, also occupies the promoter region of VEGFR3 to stimulate its transcription and therefore enhance inflammatory lymphangiogenesis (Yoshimatsu et al., 2011). These results collectively suggest that VEGFR3 is a direct transcriptional target of Prox1. Interestingly, the ability of Prox1 to activate VEGFR3 is modulated by sumoylation as Prox1 with a mutated sumoylation site loses its ability to induce VEGFR3 (Pan et al., 2009). In addition, Prox1 expression is regulated by microRNAs, including miR-181 and miR-31 (Kazenwadel et al., 2010; Pedrioli et al., 2010).
2.2. SRY-Related HMG-box 18 (Sox18)
Members of Sox gene family encode transcription factors with a DNA-binding HMG domain. They play important roles in a diverse range of developmental processes (Bowles et al., 2000). Among them, Group F subfamily, composed of Sox7, Sox17 and Sox18 plays a particularly important role in vascular development (Bowles et al., 2000). Mutations in Sox18 are found in patients with the hypotrichosis-lymphedema-telangiectasia syndrome (Irrthum et al., 2003). Recent studies have shown that Sox18 is a critical regulator of lymphatic fate specification which it accomplishes by the transcriptional control of Prox1 expression (Francois et al., 2008; Hosking et al., 2009) (see Table 1).
During mouse embryonic development, Sox18 expression in endothelial cells in the dorsolateral part of the cardinal vein at E9 precedes the appearance of Prox1. It persists in Prox1-positive LECs that have emerged from the cardinal vein as well as in LECs of jugular lymphatic sacs. However, this expression is transient and Sox18 levels decline sharply by E14.5 indicating a specific role of Sox18 during early stage of lymphatic development (Francois et al., 2008).
A series of experiments employing ChIP, luciferase and electrophoretic mobility shift assays, have shown that Sox18 directly binds to the Prox1 promoter. A 4-kb Prox1 promoter containing the Sox18 binding sites is sufficient to drive a reporter gene expression in lymphatic vessels in transgenic mice, while the same promoter fragment with Sox18 binding regions mutated fails to do so in vivo. Ectopic expression of wild-type but not dominant-negative Sox18 stimulates Prox1, VEGFR3 and other lymphatic markers in differentiating embryonic stem cells and BECs (Francois et al., 2008).
Ragged Opposum (Raop) is a natural mouse mutant expressing a dominant-negative form of Sox18 (Pennisi et al., 2000). Homozygous Raop mice have markedly reduced numbers of LEC progenitors leading to reduced formation of the lymph sacs resulting in gross subcutaneous edema by E13.5. Some of the heterozygous Raop mice survive to adulthood but exhibit morphologically defective lymphatics (Francois et al., 2008). Homozygous disruption of Sox18 expression in mice on a pure C57BL/6 background shows a lymphatic phenotype similar to that of Raop mice. However, lymphatic vascular defects in Sox18-null mice on a mixed background are very mild. A further study identified Sox7 and Sox17 as strain-specific modifiers of the Sox18 mutant phenotype (Hosking et al., 2009). Sox7 and Sox17 do not express in LECs normally, but are activated to play a compensative role in the absence of Sox18 function and only in certain genetic backgrounds (Hosking et al., 2009). Sox18 can compensate for Sox17 function in a strain-specific manner as well. In a mixed genetic background, double knock-out of both Sox17 and Sox18, rather than single deletion of Sox17, induces vascular abnormalities in mouse embryos (Sakamoto et al., 2007). However, endothelial-specific inactivation of only Sox17 on a C57BL/6 background shows profound defects in arterial differentiation and vascular remodeling (Corada et al., 2013).
During lymphatic fate specification, Sox18 is expressed in both arteries and veins (Pennisi et al., 2000). However, Sox18 does not induce Prox1 in arterial endothelial cells, indicating that additional mechanisms are present that modulate Sox18-dependent regulation of Prox1 expression. One such modulator could be the co-factor Mef2c which partners with Sox18 to stimulate its transcriptional activity in endothelial cells (Hosking et al., 2001). Along similar lines, there likely exist venous endothelium-restricted partner proteins that operate concurrently with Sox18 to induce Prox1 expression.
2.3. Chicken Ovalbumin Upstream Transcription Factor II (COUP-TFII)
COUP-TFII is an orphan nuclear receptor expressed in multiple tissues and involved in a wide range of cellular and developmental processes (Pereira et al., 2000) (Table 1). In the vasculature, COUP-TFII is expressed in vein, lymphatic and capillary endothelial cells but not in the arterial endothelium (Pereira et al., 1999; Qin et al., 2010a; Qin et al., 2010b). Homozygous disruption of COUP-TFII expression in mice is embryonic lethal with the embryos exhibiting defective angiogenesis and heart development (Pereira et al., 1999). COUP-TFII also controls venous differentiation. Conditional disruption of COUP-TFII in the endothelium leads to ectopic expression of arterial markers (e.g. EphrinB2 and Neuropilin 1) in veins due to disinhibition of Notch signaling pathway (You et al., 2005). Venous expression of COUP-TFII is epigenetically promoted by Brg1, a chromatin-remodeling protein, by enhancing the accessibility of transcriptional machinery to the COUP-TFII promoter (Davis et al., 2013). Brg1 ablation therefore enables veins to acquire arterial identity, a phenotype similar to that seen in COUP-TFII knockout mice (Davis et al., 2013). Under pathological conditions, ablation of COUP-TFII suppresses tumor angiogenesis in pancreatic islets. Mechanistically, COUP-TFII transcriptionally represses soluble VEGFR1, which traps extracellular VEGF ligands, and thus promotes VEGFR2 signaling in endothelial cells (Qin et al., 2010b). COUP-TFII also functions in a non-cell-autonomous manner to regulate cancer cell growth and metastasis by modulating Angiopoietin-1 expression in pericytes (Qin et al., 2010a).
Several recent studies have shown that COUP-TFII is also a key player in lymphatic fate specification. COUP-TFII directly binds to the Prox1 promoter, as shown by ChIP, luciferase and electrophoretic mobility shift assays (Srinivasan et al., 2010). Selective ablation of COUP-TFII in endothelial cells driven by Tie2-Cre greatly reduces Prox1 expression and the emergence of lymphatic progenitors. This suggests that COUP-TFII is required for Prox1 expression in cardinal veins (Srinivasan et al., 2007). However, deletion of Rbpj, a critical mediator of Notch-induced gene transcription in the endothelium, fails to rescue lymphatic defects caused by COUP-TFII deficiency. This implies that, unlike its role in vein identity determination, COUP-TFII regulation of Prox1 expression and lymphatic fate specification is not mediated via Notch signaling (Srinivasan et al., 2010).
One unresolved question is why COUP-TFII, whose expression is not polarized, only induces Prox1 at dorsolateral parts of cardinal veins. This suggests that additional factors are present that modulate COUP-TFII-dependent regulation of Prox1 expression. COUP-TFII activity is also required for maintaining Prox1 level during early stage of lymphatic fate determination once Prox1 expression has been initiated. Conditional disruption of COUP-TFII expression in Prox1-positive lymphatic progenitors results in the loss of Prox1 expression in those cells (Srinivasan et al., 2010). However, once progenitor cells differentiate into mature LECs, Prox1 expression is no longer dependent on COUP-TFII (Srinivasan et al., 2010). Besides regulating Prox1 initiation and maintenance, COUP-TFII also physically interacts with and functionally cooperates with Prox1 to control lymphatic specific gene expression including VEGFR3 and Lyve1 (Lee et al., 2009). Disruption of the interaction between COUP-TFII and Prox1 suppresses lymphatic fate specification in mice (Srinivasan et al., 2010).
COUP-TFII regulates other aspects of lymphatic vessel development as well. Embryonic inactivation of COUP-TFII results in irregular and dilated lymphatic capillaries and blood-filled lymphatics. Mutant LECs also exhibit defective proliferation and filopodia formation (Lin et al., 2010). Mechanistically, COUP-TFII directly controls the transcription of Neuropilin 2, which is a receptor for VEGF-C (Lin et al., 2010; Tammela and Alitalo, 2010).
2.4. ERK Signaling and Lymphatic Fate Determination
A recent study has shown that endothelial ERK signaling controls the transcriptional programs involved in lymphatic fate specification (Deng et al., 2013; Deng and Simons, 2013). Receptor Tyrosine Kinases (RTKs), such as VEGFR3, stimulate RAS-RAF1-MEK-ERK cascade (Olsson et al., 2006). The activity of RAF1, a critical regulatory kinase of this pathway, is modulated by phosphorylation. While phosphorylation of certain sites (e.g. Ser338 and Ser494) enhances RAF1 kinase activity, phosphorylation of Ser259 substantially suppresses it by recruiting 14-3-3 protein to promote RAF1 autoinhibition (Pandit et al., 2007; Wellbrock et al., 2004). AKT as well as some other kinases including PKA and PKCα, can phosphorylate Ser259 (Dumaz and Marais, 2003; Kolch et al., 1993; Zimmermann and Moelling, 1999), whereas protein phosphatase-2A dephosphorylates this site (Pandit et al., 2007).
Expression of the RAF1 S259A mutant (RAF1S259A), which cannot be inactivated by phosphorylation of Ser259, results in a constitutive activation of ERK (Deng et al., 2013; Pandit et al., 2007). Interestingly, expression of RAF1S259A in BECs leads to a profound induction of Sox18 and Prox1, as well as a number of other LEC-specific genes, including VEGFR3, Lyve1 and Podoplanin (Deng et al., 2013). Endothelial cell-specific expression of RAF1S259A in mice greatly enhances lymphatic fate specification. Sox18-positive endothelial cells are detected throughout the entire cardinal vein in the mutant mouse embryos rather than appearing only in dorsolateral parts. Similarly, Prox1 expression is also substantially induced in venous endothelium and even in dorsal arteries (Deng et al., 2013). This prolonged and spatially unrestricted Sox18 and Prox1 expression results in excessive generation of LECs from cardinal vein endothelial cells. This in turn leads to formation of massively enlarged and malformed jugular lymphatic sacs as well as of the subcutaneous lymphatic vasculature. RAF1S259A embryos exhibit obvious edema, suggesting the lymphatics formed do not function properly.
The lymphatic abnormalities caused by endothelial RAF1S259A expression, including lymphedema and lymphangiectasia, are very similar to the pathology seen in patients with Noonan and LEOPARD syndromes (Tidyman and Rauen, 2009). Noonan syndrome is an autosomal dominant disorder characterized by dysmorphic facial features, auditory defects, congenital heart disease, coagulation difficulties and deficits in several other systems (Roberts et al., 2013; Tartaglia et al., 2010). A large number of these patients have peripheral lymphedema, which can develop during infancy, adolescence or in adulthood (Roberts et al., 2013). Lymphangiectasia in the intestines or lung, abnormal lymphatic vessels in the leg or thoracic cage, lack of the thoracic duct, and some other forms of lymphatic vasculature abnormalities are also reported in Noonan syndrome patients (Roberts et al., 2013; White, 1984).
LEOPARD syndrome is a genetic multisystem disorder which is named based on its clinical characteristics, including Lentigines, Electrocardiographic conduction abnormalities, Ocular hypertelorism, Pulmonic stenosis, Abnormal genitalia, Retardation of growth, and sensorineural Deafness (Gorlin et al., 1971). LEOPARD syndrome patients, similar to those with Noonan syndrome, exhibit severe lymphatic defects (Sevick-Muraca and King, 2014; Tartaglia et al., 2010). Recent studies have shown that missense mutations in RAS-RAF-ERK signaling components can cause both Noonan and LEOPARD syndromes. For example, PTPN11 which encodes protein tyrosine phosphatase SHP-2 is affected in 50% of the Noonan syndrome patients (Sevick-Muraca and King, 2014). Mutations in the SH2 (N terminal) and PTP domains of SHP-2 impair its autoinhibition, which requires the interaction between the two domains, and thereby lead to enhanced activity of SHP-2 and its downstream signaling (Tartaglia and Gelb, 2005). Missense mutations in RAF1 Ser259 (e.g. S259A) and its flanking regions are also frequently detected in patients with Noonan and LEOPARD syndromes (Pandit et al., 2007). These and other mutations leading to excessive activation of the RAS signaling cascade have been termed RASopathies (Tidyman and Rauen, 2009). Patients with these mutations frequently demonstrate lymphatic abnormalities including lymphangiectasia (pathological dilation/expansion of lymphatic vessels) (Sevick-Muraca and King, 2014).
Of note, inhibition of ERK signaling rescues the lymphatic defects in RAF1S259A mice (Deng et al., 2013), implying that ERK may be a potential therapeutic target to alleviate lymphatic symptoms in patients with various RASopathies.
2.5. Notch signaling and lymphatic fate determination
The mammalian Notch signaling pathway is composed of four receptors (Notch1-4) and five ligands (Jagged1 and 2, and Delta-like (Dll) 1, 3, and 4). Receptor-ligand interaction mediated by direct cell-cell contact induces proteolytic cleavage of the Notch receptor, releasing the receptor intracellular domain into the cytosol. The Notch intracellular domain (NICD) subsequently translocates to the nucleus, where it binds to transcriptional regulators and controls gene expression of downstream targets (D’Souza et al., 2010; Kopan and Ilagan, 2009).
Recent studies have shed new light on the role of Notch signaling in lymphatic vascular development. Using cultured LECs in vitro, one study found that ectopic expression of the activated NICD represses Prox1, COUP-TFII, and Podoplanin levels (Kang et al., 2010). Consistent with these results, the addition of soluble, recombinant forms of the Notch ligands Dll4 and Jagged1 down-regulates Prox1, COUP-TFII, and Podoplanin expression. This repression was found to proceed through Notch target transcription factors Hey1 and Hey2. Treatment with siRNAs targeting these two downstream effectors can rescue the repression of Prox1 by ectopic NICD, suggesting that in vitro Notch signaling suppresses lymphatic phenotypes. Additional studies have further implicated the importance of feedback loops among Prox1, COUP-TFII and Notch in critically maintaining the balance between early arterial, venous and lymphatic cell fates (Aranguren et al., 2013).
However, the exact role played by Notch in lymphatic development remains poorly understood. A recent study has demonstrated that suppression of Notch signaling with a soluble form of Dll4 promotes lymphangiogenesis in adult mouse ears when performed in concert with VEGF stimulation (Zheng et al., 2011). LEC-specific deletion of Notch1 in mice leads to excessive Prox1-positive lymphatic endothelial progenitors, lymphatic overgrowth and failed separation of lymphatic vessels from the cardinal vein (Murtomaki et al., 2013) (Table 1). Conversely, activation of Notch signaling in LECs suppresses COUP-TFII expression, thereby resulting in loss of Prox1 and lymphatic development defects (Murtomaki et al., 2013) (Figure 1). A different study using LEC-specific Notch1 knockout mice showed that Notch1 deficiency promotes filopodia formation, proliferation, and survival of LECs, further supporting that the Notch signaling pathway serves as a critical negative regulator of developmental lymphangiogenesis (Fatima et al., 2014).
Contrary to these results implicating Notch as a negative regulator of lymphatic growth, another set of recent studies have suggested that Notch may also be a positive regulator of lymphatic specification and development. During zebrafish embryogenesis, the systematic silencing of Notch family receptors and their ligands impaired thoracic duct formation and reduced the number of lymphatic vessels sprouting from the posterior cardinal vein (Geudens et al., 2010). At a later stage of lymphatic development, Notch silencing prevented the proper navigation of lymphatic intersomitic vessels, resulting in stalled migration or misrouting of lymphangioblasts along their arterial templates (Geudens et al., 2010). Consistent with this view of Notch as a positive regulator of lymphangiogenesis, another study demonstrated that inhibiting Notch1 and Dll4 with specific blocking antibodies impairs lymphatic sprouting and growth in neonatal mice; additionally, Notch1-Dll4 blockade was shown to reduce the expression of lymphatic markers, cause disorganized mural cell coverage in lymphatic vessels, and impair lymphangiogenesis during wound healing (Niessen et al., 2011).
While these discrepancies regarding the role of Notch signaling in lymphatic development may be attributed to differences in developmental stage and model organisms, another explanation to consider is that Notch signaling can occur through different mechanisms. The canonical pathway proceeds through the nuclear translocation of NICD and its binding to Rbpj, but non-canonical activation of Notch signaling that proceeds independently of Rbpj has also been observed in vertebrates (Sanalkumar et al., 2010). The relative importance of these two Notch pathways in regulating early lymphatic fate and later developmental lymphangiogenesis remains a point of strong contention (Srinivasan et al., 2010).
3. Concluding Remarks
Despite significant progress in our understanding of lymphatic fate specification, our knowledge of this important process is still rather limited. Several critical questions remain to be answered. Among these is the induction of polarized distribution of Sox18 and Prox1 expression, identity of factors that activate ERK signaling that is required for induction of Sox18 expression and the precise role played by Notch signaling. A better understanding of these questions will not only further our knowledge of basic lymphatic biology, but will also aid the development of novel therapeutic strategies to treat human diseases associated with the lymphatic system.
Acknowledgement
Supported in part by NIH grants R01 HL053793 and HL084619 (both to MS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–80. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Alitalo K, et al. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–53. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Aranguren XL, et al. COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or hetero-dimerisation with PROX1. J Cell Sci. 2013;126:1164–75. doi: 10.1242/jcs.116293. [DOI] [PubMed] [Google Scholar]
- Bowles J, et al. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–55. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Corada M, et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun. 2013;4:2609. doi: 10.1038/ncomms3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza B, et al. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RB, et al. BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Development. 2013;140:1272–81. doi: 10.1242/dev.087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, et al. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest. 2013;123:1202–15. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Simons M. Lymphatic fate determination: playing RAF with ERK. Cell Cycle. 2013;12:1157–8. doi: 10.4161/cc.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ, et al. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–64. doi: 10.1016/0092-8674(91)90463-9. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem. 2003;278:29819–23. doi: 10.1074/jbc.C300182200. [DOI] [PubMed] [Google Scholar]
- Fatima A, et al. Murine Notch1 is required for lymphatic vascular morphogenesis during development. Dev Dyn. 2014 doi: 10.1002/dvdy.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flister MJ, et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115:418–29. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–7. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Francois M, et al. The transcriptional control of lymphatic vascular development. Physiology (Bethesda) 2011;26:146–55. doi: 10.1152/physiol.00053.2010. [DOI] [PubMed] [Google Scholar]
- Fritz-Six KL, et al. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geudens I, et al. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol. 2010;30:1695–702. doi: 10.1161/ATVBAHA.110.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, et al. The Leopard (multiple lentigines) syndrome revisited. Birth Defects Orig Artic Ser. 1971;07:110–5. [PubMed] [Google Scholar]
- Hagerling R, et al. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32:629–44. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, et al. Identification of targets of Prox1 during in vitro vascular differentiation from embryonic stem cells: functional roles of HoxD8 in lymphangiogenesis. J Cell Sci. 2009;122:3923–30. doi: 10.1242/jcs.052324. [DOI] [PubMed] [Google Scholar]
- Harvey NL, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–81. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Hong YK, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–7. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Hosking B, et al. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development. 2009;136:2385–91. doi: 10.1242/dev.034827. [DOI] [PubMed] [Google Scholar]
- Hosking BM, et al. SOX18 directly interacts with MEF2C in endothelial cells. Biochem Biophys Res Commun. 2001;287:493–500. doi: 10.1006/bbrc.2001.5589. [DOI] [PubMed] [Google Scholar]
- Irrthum A, et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470–8. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–91. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–50. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–97. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- Kazenwadel J, et al. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116:2395–401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- Kim H, et al. Embryonic vascular endothelial cells are malleable to reprogramming via Prox1 to a lymphatic gene signature. BMC Dev Biol. 2010;10:72. doi: 10.1186/1471-213X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W, et al. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–52. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchler AM, et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16:1244–8. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Lee S, et al. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–9. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, et al. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120:1694–707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnik A, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–52. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- Mishima K, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–9. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtomaki A, et al. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development. 2013;140:2365–76. doi: 10.1242/dev.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen K, et al. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood. 2011;118:1989–97. doi: 10.1182/blood-2010-11-319129. [DOI] [PubMed] [Google Scholar]
- Ny A, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4:35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- Oliver G, Harvey N. A stepwise model of the development of lymphatic vasculature. Ann N Y Acad Sci. 2002;979:159–65. doi: 10.1111/j.1749-6632.2002.tb04876.x. discussion 188-96. [DOI] [PubMed] [Google Scholar]
- Oliver G, et al. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Oliver G, Srinivasan RS. Lymphatic vasculature development: current concepts. Ann N Y Acad Sci. 2008;1131:75–81. doi: 10.1196/annals.1413.006. [DOI] [PubMed] [Google Scholar]
- Olsson AK, et al. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Pan MR, et al. Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J Cell Sci. 2009;122:3358–64. doi: 10.1242/jcs.050005. [DOI] [PubMed] [Google Scholar]
- Pandit B, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Pedrioli DM, et al. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30:3620–34. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet. 2000;24:434–7. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- Pereira FA, et al. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–49. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FA, et al. COUP-TF orphan nuclear receptors in development and differentiation. Cell Mol Life Sci. 2000;57:1388–98. doi: 10.1007/PL00000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–9. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevo R, et al. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–30. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- Qin J, et al. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci U S A. 2010a;107:3687–92. doi: 10.1073/pnas.0914619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, et al. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res. 2010b;70:8812–21. doi: 10.1158/0008-5472.CAN-10-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AE, et al. Noonan syndrome. Lancet. 2013;381:333–42. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin FR. The Method of Growth of the Lymphatic System. Science. 1916;44:145–58. doi: 10.1126/science.44.1127.145. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, et al. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–44. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Sanalkumar R, et al. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Mol Life Sci. 2010;67:2957–68. doi: 10.1007/s00018-010-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevick-Muraca EM, King PD. Lymphatic vessel abnormalities arising from disorders of Ras signal transduction. Trends Cardiovasc Med. 2014;24:121–7. doi: 10.1016/j.tcm.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–32. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD. Noonan syndrome and related disorders: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2005;6:45–68. doi: 10.1146/annurev.genom.6.080604.162305. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, et al. Noonan syndrome: clinical aspects and molecular pathogenesis. Mol Syndromol. 2010;1:2–26. doi: 10.1159/000276766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–6. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115–26. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellbrock C, et al. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- White SW. Lymphedema in Noonan’s syndrome. Int J Dermatol. 1984;23:656–7. doi: 10.1111/j.1365-4362.1984.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Wigle JT, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–8. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest. 2014;124:888–97. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K, et al. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–6. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu Y, et al. Ets family members induce lymphangiogenesis through physical and functional interaction with Prox1. J Cell Sci. 2011;124:2753–62. doi: 10.1242/jcs.083998. [DOI] [PubMed] [Google Scholar]
- You LR, et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Zheng W, et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood. 2011;118:1154–62. doi: 10.1182/blood-2010-11-317800. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]