Summary
Cancer cells are typically subject to profound metabolic alterations, including the Warburg effect wherein cancer cells oxidize a decreased fraction of the pyruvate generated from glycolysis. We show herein that the mitochondrial pyruvate carrier (MPC), composed of the products of the MPC1 and MPC2 genes, modulates fractional pyruvate oxidation. MPC1 is deleted or underexpressed in multiple cancers and correlates with poor prognosis. Cancer cells re-expressing MPC1 and MPC2 display increased mitochondrial pyruvate oxidation, with no changes in cell growth in adherent culture. MPC re-expression exerted profound effects in anchorage-independent growth conditions, however, including impaired colony formation in soft agar, spheroid formation, and xenograft growth. We also observed a decrease in markers of stemness and traced the growth effects of MPC expression to the stem cell compartment. We propose that reduced MPC activity is an important aspect of cancer metabolism, perhaps through altering the maintenance and fate of stem cells.
Introduction
The fate of pyruvate is one of the most important metabolic decisions made by eukaryotic cells. Most differentiated mammalian cells direct pyruvate into mitochondria where it is oxidized for efficient ATP production. Cancer cells, however, divert pyruvate and its precursors to fuel other anabolic processes or convert it to lactate for excretion from the cell (Vander Heiden et al., 2009). This metabolic adaptation was first described by the eminent biochemist Otto Warburg in the 1920s and is known as the Warburg effect (Warburg et al., 1927). Multiple mechanisms contribute to this metabolic derangement in cancer, but the synthesis and metabolism of pyruvate play a central role (Bayley and Devilee, 2012).
First, the synthesis of pyruvate in glycolysis is catalyzed by pyruvate kinase. Cancer cells tend to express a partially inhibited splice variant of pyruvate kinase (PK-M2), leading to decreased pyruvate production (Christofk et al., 2008a; Christofk et al., 2008b; Luo and Semenza, 2011; Yang et al., 2011; Yeh et al., 2008). Second, the two proteins that mediate pyruvate conversion to lactate and its export, lactate dehydrogenase A (LDHA) and the monocarboxylate transporter MCT-4, are commonly upregulated in cancer cells leading to decreased pyruvate oxidation (Azuma et al., 2007; Le Floch et al., 2011). Third, the enzymatic step following mitochondrial entry is the conversion of pyruvate to acetyl-coA by the pyruvate dehydrogenase (PDH) complex. Cancer cells frequently exhibit increased expression of the PDH kinase PDK1, which phosphorylates and inactivates PDH (Kim et al., 2006; McFate et al., 2008). This PDH regulatory mechanism is required for oncogene-induced transformation and reversed in oncogene-induced senescence (Kaplon et al., 2013). Further, the PDK inhibitor dichloroacetate has shown some clinical efficacy, which correlates with increased pyruvate oxidation (Michelakis et al., 2010). Altered pyruvate metabolism appears to be critical in enabling and promoting the transformed phenotype in many cancers.
One of the simplest mechanisms to explain decreased mitochondrial pyruvate oxidation in cancer cells, a loss of mitochondrial pyruvate import, has been observed repeatedly over the past 40 years (Eboli et al., 1977; Paradies et al., 1983). This process has been impossible to study at a molecular level until recently, however, as the identities of the protein(s) that mediate mitochondrial pyruvate uptake were unknown (Halestrap, 1975b; Papa and Paradies, 1974). We and others recently described the Mitochondrial Pyruvate Carrier (MPC) as a multimeric complex that is necessary for efficient mitochondrial pyruvate uptake (Bricker et al., 2012; Herzig et al., 2012). The MPC contains two distinct proteins, MPC1 and MPC2; the absence of either leads to a loss of mitochondrial pyruvate uptake and utilization in yeast, flies and mammalian cells (Bricker et al., 2012; Herzig et al., 2012). Several groups subsequently confirmed this discovery in multiple contexts (Colca et al., 2013; Divakaruni et al., 2013; Li et al., 2014; Patterson et al., 2014; Rohatgi et al., 2013; Timon-Gomez et al., 2013).
Identification of the MPC genes and proteins finally permits the use of molecular genetics to interrogate the contribution of mitochondrial pyruvate uptake to cancer metabolism. Given the decades-old observation that the MPC might be inactivated in cancer cell lines and tumors (Eboli et al., 1977; Paradies et al., 1983) and the decrease in pyruvate oxidation associated with the Warburg effect, we first asked whether MPC expression or activity is lost in cancer. Indeed both genes, but particularly MPC1, are underexpressed or deleted in most cancers and low expression correlates with poor survival. To determine whether MPC underexpression is an important feature of cancer, we re-expressed MPC1 and MPC2 in colon cancer cells and assessed their metabolic and proliferative phenotypes. MPC-expressing cells exhibited enhanced pyruvate oxidation and decreased glycolysis, consistent with reversal of the Warburg effect. While growth in standard adherent cell culture was unaffected, MPC re-expression impaired anchorage-independent growth, including in mouse xenograft assays. This was accompanied by decreased expression of stem cell markers. These data lead us to conclude that decreased MPC expression promotes the Warburg effect and the maintenance of stemness in colon cancer cells.
Results
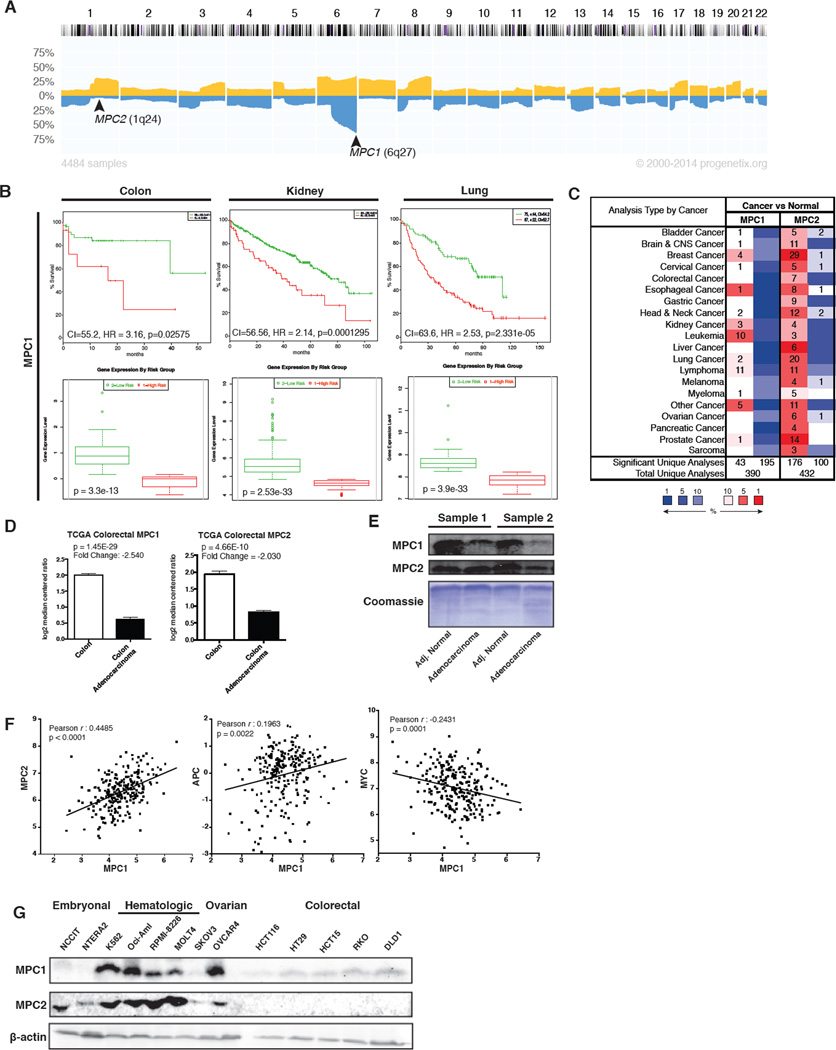
The discovery of the genes that encode the mitochondrial pyruvate carrier enabled the assessment of the genomic status, expression and impact of these genes in cancer. We first examined whether either MPC1 or MPC2 is deleted in cancer. While the genomic locus of MPC2 does not appear to be frequently lost, MPC1 is found within the most frequently deleted region across all cancer sample sets surveyed: 6q27 (Figure 1A) (Cai et al., 2014). The data are particularly striking for ovarian cancer, wherein 6q27 is deleted in 60–80% of epithelial ovarian carcinoma (Figure S1A) (Tibiletti et al., 1998). The minimal interval of this deletion has been determined to contain only three genes, one of which is MPC1 (Bignone et al., 2006; Liu et al., 2002). Colon and rectal cancer risk associates strongly with various haplotypes that include the MPC1 locus (Slattery et al., 2011). In addition, this region is frequently deleted in ulcerative colitis patients with neoplastic transformation (Chen et al., 2005). Low MPC1 expression correlates with poor survival in almost all cancers examined, including colon, kidney, lung, bladder, and brain (Figure 1B and S1B) (Aguirre-Gamboa et al., 2013). The correlation of survival with MPC2 expression was more variable, but was associated with poor prognosis in kidney and colon cancers (Figure S1C). The correlation of low MPC1 expression with poor survival is more significant (Hazard Ratio (HR)=3.16, p=0.02575) than a number of metabolic genes with validated roles in the cancer phenotype, including low PDHA1 (p = 0.1603), high PKM2 (p = 0.1232), high LDHA (p = 0.2442), high MCT1 (p = 0.09482), and high MCT4 (p = 0.1115).
Figure 1. MPC expression is altered in cancer.
(A) Progenetix histoplot of copy number across all chromosomes for 4484 cancer samples. (B) Top: Kaplan-Meier survival curves of censored Cox analysis for Director’s Challenge NCI60 Lung, TCGA Colon Adenocarcinoma, and TCGA Kidney Clear Cell Carcinoma stratified by maximized MPC1 expression risk group. Bottom: MPC1 expression levels stratified by risk group (SurvExpress). (C) MPC1 and MPC2 expression profiles across multiple cancer types, compared to normal tissue (Oncomine). The number of studies in which over-expression or under-expression was observed is indicated in red or blue boxes, respectively. Color intensity corresponds to the magnitude of expression differences (threshold (p value) set to 0.05, threshold (fold change) to all, threshold (gene rank) set to all; MPC1 queried as BRP44L, MPC2 as BRP44). (D) MPC1 and MPC2 mRNA abundance in TCGA colorectal adenocarcinoma, compared to normal tissue (Oncomine). (E) Western blot of MPC1 and MPC2 in human colon adenocarcinoma samples versus adjacent normal/grossly uninvolved tissue. (F) Co-expression analysis for MPC1 in colorectal cancer versus MPC2, MYC, and APC. Plotted data are log2 mRNA expression from RNA Seq RPKM (Data from TCGA Research Network). (G) Western blot of MPC1 and MPC2 across a panel of cancer cell lines.
We next investigated whether MPC1 or MPC2 gene expression is altered in a variety of cancers. MPC2 was inconsistent, being underexpressed in some cancers and overexpressed in others (Figure 1C) (Rhodes et al., 2004). MPC1, however, was underexpressed in all cancers examined with the exception of the hematologic malignancies, leukemia and lymphoma (Figure 1C). The data were particularly striking for colon adenocarcinoma, wherein the MPC1 and MPC2 mRNAs are both at much lower abundance in tumor compared to normal tissue (Figure 1D). MPC1 protein was also decreased in colon adenocarcinoma (Figure 1E). When we examined the statistical relationships between MPC1 and MPC2 expression along with a variety of other tumor suppressors, oncogenes, and metabolic genes in colorectal cancer, we found that MPC1 and MPC2 expression was positively correlated as expected (Figure 1F). The expression of MPC1 was negatively correlated with the MYC oncogene and positively correlated with the colon tumor suppressor APC (Figure 1F) (Cerami et al., 2012; Gao et al., 2013; Network, 2012).
These data demonstrate that MPC1, and perhaps MPC2, associate with cancer risk, with deletion or under-expression being strongly correlated with cancer onset and poor prognosis (Figure S1D) (Hong et al., 2007). Reductions in MPC1 expression were not general to the locus as the adjacent gene, RPS6KA2, was increased in early onset colorectal cancer (Figure S1D). To begin to address the functional relevance of these findings, MPC1 and MPC2 expression was assessed in a variety of transformed and untransformed cell lines. We found consistently low expression among colon cancer cell lines, particularly as compared to leukemia and lymphoma. Embryonal carcinoma cells were the only cancer type that exhibited levels of MPC1 and MPC2 expression consistently lower than those found in colon cancer (Figure 1G).
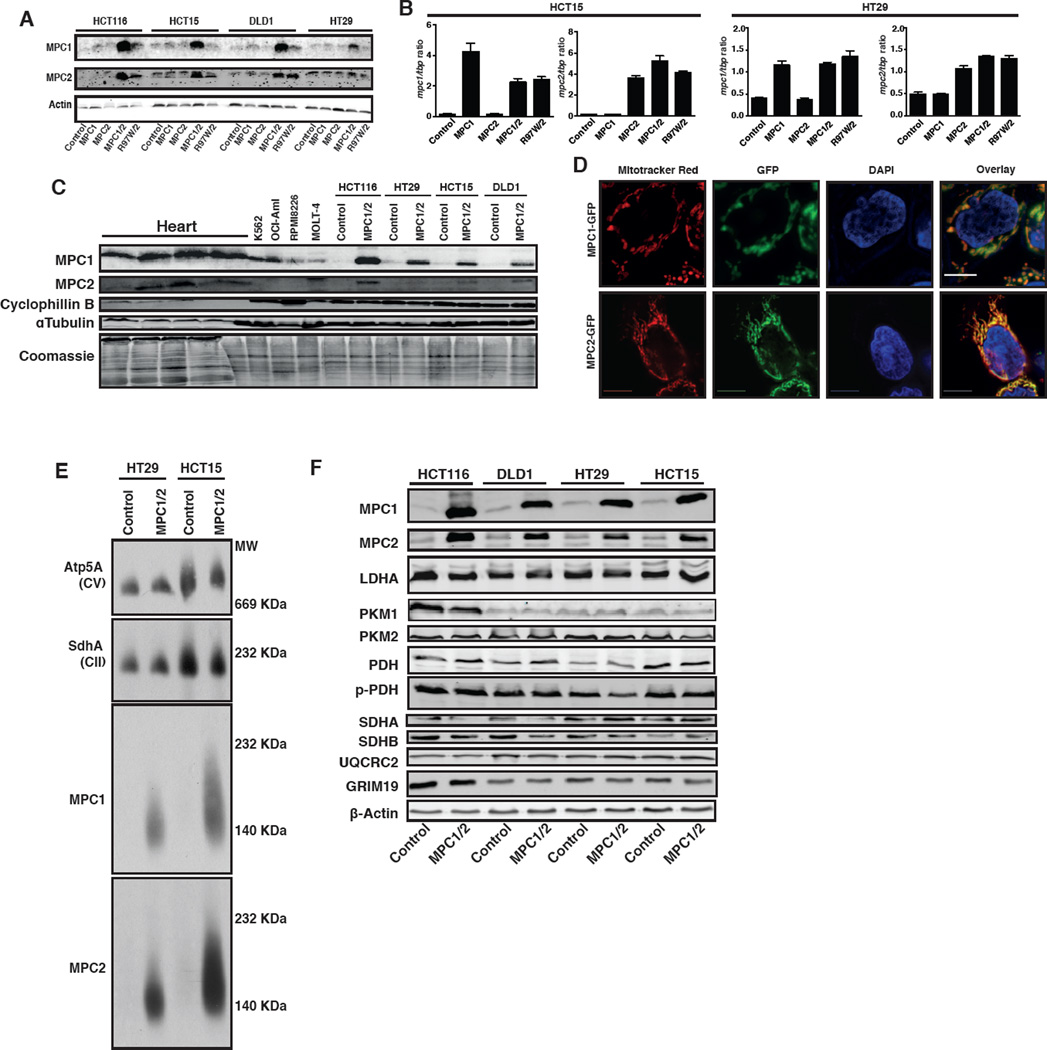
Based on the consistent observation of reduced MPC expression, we employed a re-expression system to test the necessity of low MPC expression for the metabolic and proliferative phenotypes in colon cancer. We expressed MPC1, MPC2, or both in four different colon cancer cell lines: HCT116, HCT15, DLD1 and HT29. When expressed alone, MPC1 and MPC2 did not accumulate (Figure 2A), despite robust mRNA expression (Figure 2B). When both genes were expressed, however, both MPC1 and MPC2 accumulated in all cell lines (Figure 2A). We also expressed a combination of MPC2 and the R97W mutant of MPC1, which we previously described in a human patient (Bricker et al., 2012). This mutant MPC1 protein appeared to be unstable, as it did not accumulate despite wild-type mRNA abundance (Figure 2A, 2B). We had hoped that MPC1-R97W would be an appropriate negative control, but the lack of stability limits its utility and, therefore, we did not use it for subsequent experiments. While MPC1 and MPC2 protein abundance is greatly increased over endogenous expression in colon cancer cells, it is similar to higher MPC-expressing cancers and less than normal heart tissue (Figure 2C).
Figure 2. Re-expressed MPC1 and MPC2 form a mitochondrial complex.
(A) Western blot and (B) qRT-PCR analysis of the indicated colon cancer cell lines with retroviral expression of MPC1 (or MPC1-R97W) and/or MPC2. (C) Western blots of human heart tissue, hematologic cancer cells, and colon cancer cell lines with and without MPC1 and MPC2 re-expression. (D) Fluorescence microscopy of MPC1-GFP & MPC2-GFP overlaid with Mitotracker Red in HCT15 cells. Scale bar: 10µm. (E) Blue-native PAGE analysis of mitochondria from control and MPC1/2-expressing cells. (F) Western blots of metabolic and mitochondrial proteins across four colon cancer cell lines with or without MPC1/2 expression.
Both proteins co-localize with mitochondria as expected (Figure 2D) and subsequent experiments focused on the effects of MPC1 and MPC2 co-expression. When expressed together, MPC1 and MPC2 assembled into an intact MPC complex, identical to what we previously observed for the native MPC (Figure 2E and S2A) (Bricker et al., 2012). The re-expression of MPC1 and MPC2 had no consistent effect on the abundance of a variety of mitochondrial proteins, nor on a variety of enzymes relevant to pyruvate metabolism, including LDHA and PDH (Figure 2F and S2B). There was also no effect on mitochondrial morphology (Figure S2C), suggesting that increased MPC abundance does not grossly alter metabolic gene expression, or mitochondrial biogenesis or removal.
We next sought to assess the metabolic effects of MPC re-expression, focusing on the HCT15 and HT29 cell lines. MPC re-expression caused increased pyruvate oxidation, as measured by oxygen consumption with pyruvate as the sole substrate (Figure 3A). The magnitude of the effect was enhanced when measuring maximally stimulated pyruvate oxidation in the presence of FCCP (Figure 3A). We also performed isotope tracing to determine the fate of glucose-derived carbon (Figure 3B). After culture with d-[U-13C]glucose, unlabeled citrate (m+0) was modestly reduced in cells re-expressing MPC, while higher-order citrate labeling (m+3, m+4 and m+5) was modestly increased (Figure 3C). Species that contain higher order labeling result from transfer of glucose-derived pyruvate into the mitochondria followed by activity of pyruvate dehydrogenase, pyruvate carboxylase, and TCA cycling (Figure 3C). Labeling differences were more pronounced at shorter labeling times, however, stable reductions in m+0 citrate suggest an increased rate of glucose contribution to the TCA cycle (Figure S3A). These data were further supported by in silico modeling experiments that varied pyruvate transport through the MPC (Figure S3B and S3C). In these experiments, m+0 citrate decreases significantly with increasing MPC flux due to the accumulation of 13C in citrate resulting in higher abundances of m+3,4, and 5, but with very small changes in the abundance of m+2 citrate. The modeled citrate abundances mirror closely the observed data upon MPC re-expression. As expected, we also observed a decrease in lactate production and extracellular acidification rate (Figure 3D and S3D). These data are all consistent with MPC expression increasing pyruvate flux into mitochondria for oxidation via the TCA cycle.
Figure 3. MPC re-expression alters mitochondrial pyruvate metabolism.
(A) Oxygen consumption rate (OCR) at baseline and maximal respiration in HCT15 (n=7) and HT29 (n=13) with pyruvate as the sole carbon source (mean ± SEM). (B and C) Schematic and citrate mass isotopomer quantification in cells cultured with d-[U-13C]glucose and unlabeled glutamine for 6 hours (mean ± SD, n=2). (D) Glucose uptake and lactate secretion normalized to protein concentration (mean ± SD, n=3). (E) Western blots of PDH, phospho-PDH, and PDK1; (F) PDH activity assay, and (G) citrate synthase activity assay with or without MPC1 and MPC2 expression (mean ± SD, n=4). (H-I) Effects of MPC1/2 re-expression on mitochondrial membrane potential and ROS production (mean ± SD, n=3). *,p<0.05; **,p<0.01; ***,p<0.001; ****,p<0.0001
After pyruvate enters the mitochondria it is converted to acetyl-coA by PDH, which is phosphorylated and inactivated by PDH kinases, including PDK1. Upon MPC expression, HT29 cells exhibited decreased PDK1 expression, decreased PDH phosphorylation, and increased PDH activity (Figure 3E and F). We did not observe the same effects in HCT15 cells, although we did observe increased 14CO2 production from [1-14C]-pyruvate (Figure 3F and S3E). In neither case did we observe a change in the activity of the TCA cycle enzyme citrate synthase (CS) (Figure 3G). Mitochondrial membrane potential was elevated upon MPC expression (Figure 3H), while reactive oxygen species (ROS) decreased in these cells (Figure 3I). As increased oxidative metabolism is often associated with increased generation of ROS, this result was surprising. Therefore, we also tested ROS levels in HCT116 and DLD1 cells with and without MPC re-expression and observed a similar effect (Figure S3F). While the mechanism remains to be determined, perhaps MPC re-expression leads to increased mitochondrial pyruvate, which is known to act as an antioxidant (Bassenge et al., 2000; Huang et al., 2013; Kang et al., 2001; Wang et al., 2007).
We next assessed the role of the MPC in cancer cell proliferation. MPC re-expression had no effect on proliferation rate in adherent cell culture conditions (Figure 4A), nor did it alter cell viability, apoptosis, or necrosis (Figure 4B). We examined cell cycle progression using an EdU pulse-chase and observed no difference in the timing or quantity of S phase entry or progression across a 24-hour time-course (Figure 4C and S4A). We also assessed the effect of MPC re-expression on proliferation under non-adherent conditions, which are often used to model tumor initiation and metastasis. Interestingly, we found that MPC1 and MPC2 expression significantly suppressed colony formation in soft agar in both HCT15 and HT29 cells under a variety of different media conditions, from multiple independently derived biological replicates (Figure 4D and Table S1). As a complementary measure, we plated HCT15 and HT29 cells in low attachment dishes and monitored spheroid formation (Carpentino et al., 2009; Takaishi et al., 2009). MPC re-expression was maintained in low attachment culture (Figure S4B), and decreased the number and size of spheroids in both cell lines (Figure 4E and S4C). To confirm the specific role of pyruvate transport by the MPC in these effects, we treated cells with the MPC inhibitor UK5099 (Halestrap, 1975a) and found that it rescued spheroid formation ability in both cell lines (Figure 4F).
Figure 4. MPC re-expression alters growth under low-attachment conditions.
(A) Cell number of control and MPC1/2 re-expressing cell lines in adherent culture (mean ± SD, n=7). (B) Cell viability determined by Trypan blue exclusion and Annexin V/PI staining (mean ± SD, n=3). (C) EdU incorporation of MPC re-expressing cell lines at 3 hours post EdU pulse. Growth in 3D culture evaluated by (D) soft agar colony formation (mean ± SD, n=12, see also Table S1) and by (E, F) spheroid formation ± MPC inhibitor UK5099 (mean ± SEM, n=12). *,p<0.05; **,p<0.01; ***,p<0.001; ****,p<0.0001
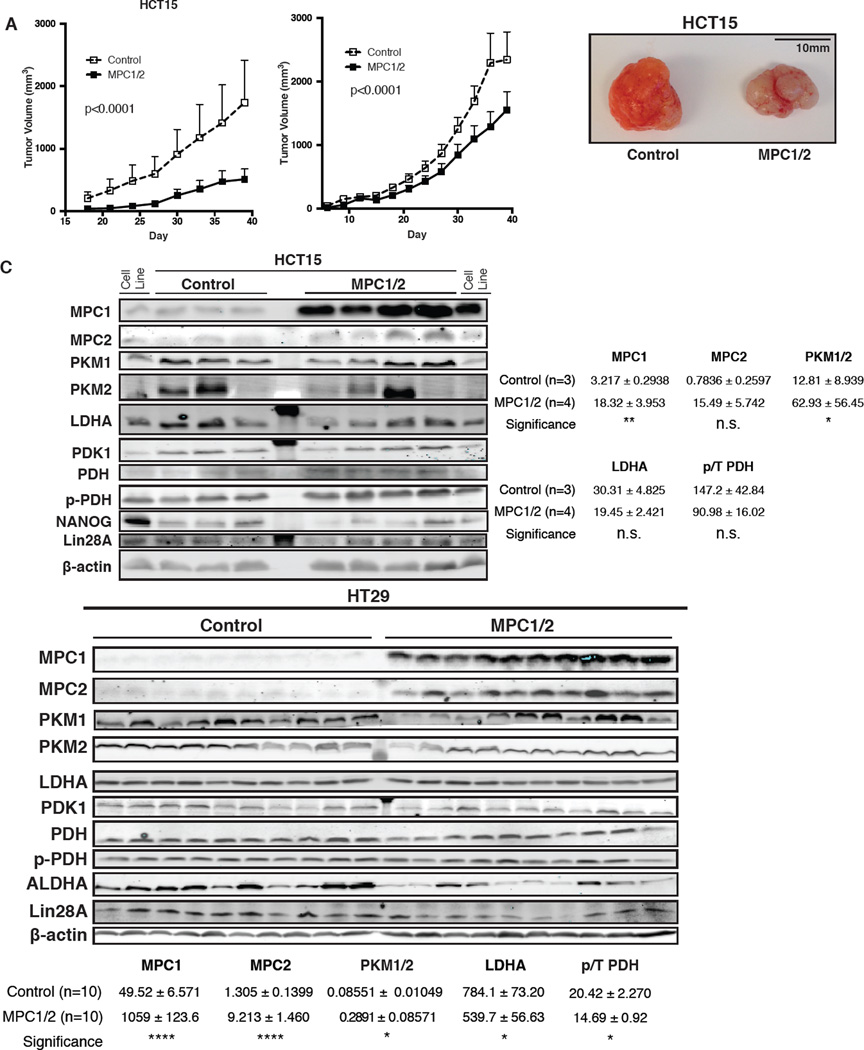
We extended these studies to xenograft assays in nude mice. In both HCT15 and HT29 cell lines, MPC re-expression slowed tumor growth across the 40-day course of the experiment (Figure 5A and B). Upon completion of the experiment, we examined MPC1 and MPC2 protein content and found it to be similar to the parental cells (Figure 5C). We did, however, observe changes that reflect adaptation to the enhanced metabolic demand in the xenograft system. The content of PK-M2 and LDHA, both of which tend to exhibit increased expression in cancer cells and promote aerobic glycolysis, were decreased in MPC-expressing HT29 xenografts (Figure 5C). In all experiments, we observed that MPC expression reduced the phosphorylation of PDH, consistent with pyruvate inhibition of PDH kinase (Figure 5C) (Pratt and Roche, 1979; Whitehouse et al., 1974).
Figure 5. MPC re-expression reduces tumor growth in vivo.
(A-B) Tumor growth of HCT15 (n=4) and HT29 (n=10) xenografts as determined by caliper measurement (mean ± SEM), and representative HCT15 xenograft tumor images. (C) Western blots of excised tumors and band quantification after normalization to β-actin.
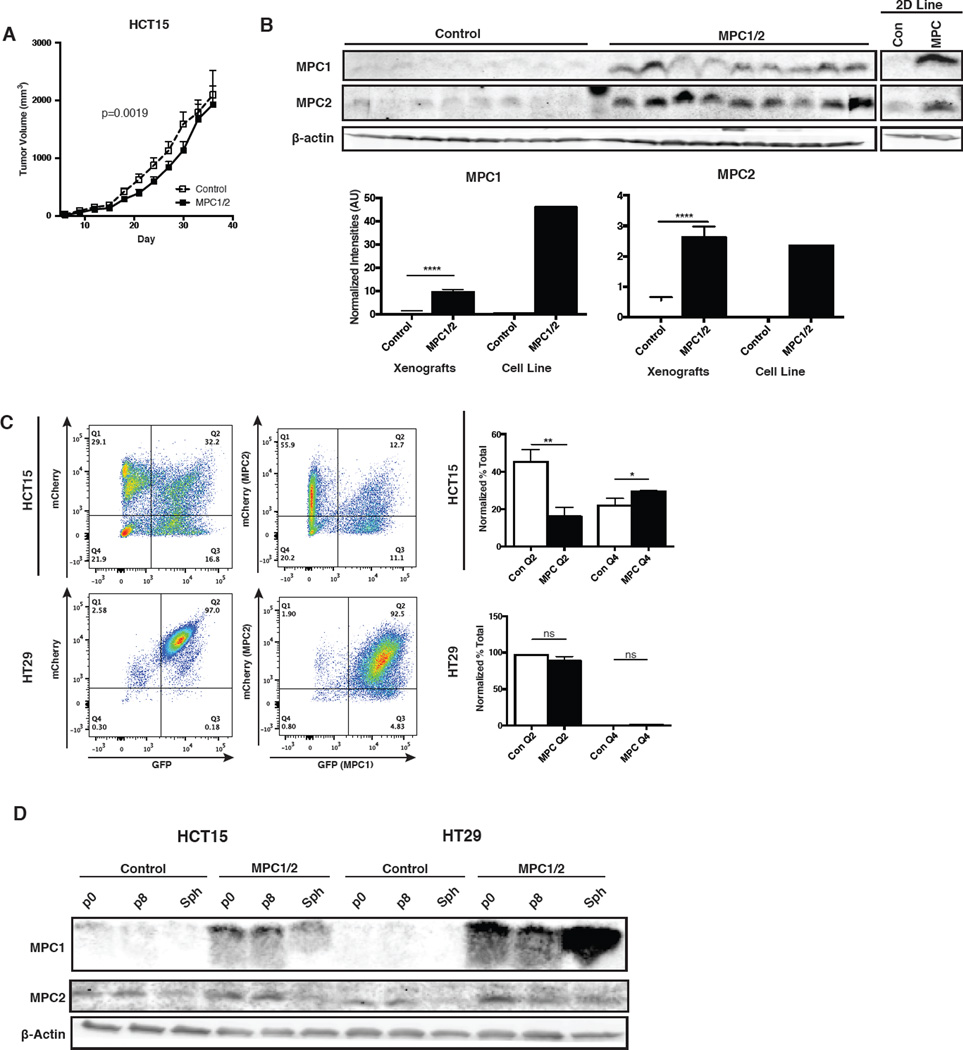
Because our first experiment with control and MPC-expressing HCT15 cells showed profound effects using a low number of mice per group (n=4), we repeated the experiment after additional passaging of the cells. In the second xenograft experiment (n=10), we again observed that MPC-expressing tumors grew slower than control tumors (Figure 6A). In this experiment, however, the effects were more modest and by the end, the tumor volume of MPC-expressing tumors had “caught up” to the controls. Puzzled by this discrepancy, we examined the protein levels in the harvested tumor samples. Unlike HT29 tumors, we found that MPC1 protein levels were ~5-fold lower in the tumor sample relative to the parental MPC-expressing cell line. There was no difference in the MPC2 protein level between the tumor and cells cultured in vitro (Figure 6B). Compared to the control xenografts, MPC-expressing xenografts also had lower c-Myc, Grim19 and PCNA (Figure S5A). These data are consistent with a selection against MPC1 protein expression in the context of xenograft tumors.
Figure 6. Adaptation to MPC re-expression in xenograft and spheroids.
(A) Tumor growth of HCT15 xenografts as determined by caliper measurement (n=10, mean ± SEM). (B) Western blots of excised tumors versus parental cell line and band quantification after normalization to β-actin (mean ± SEM). (C) Dissociated spheroid FACS plot of GFP and mCherry fluorescence after 8 passages in low-attachment culture. Fractions plotted of high-high (Q2) vs. low-low (Q4) expressers (mean ± SD, n=6.). (D) Western blot of 8-passage cells in 2D and 3D culture. *,p<0.05; **,p<0.01
This adaptation was recapitulated in vitro after serial passaging of spheroids. Bicistronic vectors were used to express MPC1 and GFP from the same transcript and MPC2 and mCherry from a distinct transcript. Maintenance of GFP and mCherry expression were used as surrogate markers of MPC1 and MPC2 expression, respectively, after 8 spheroid passages. As in xenografts, we observed little evidence of decreased MPC1 or MPC2 expression after spheroid passaging in HT29 cells (Figure 6C). On the other hand, we observed a ~60% decrease in GFP/mCherry double positive HCT15 cells. As in the xenografts this was due almost completely to a loss of GFP/MPC1 expression; we observed a doubling of the mCherry-positive, GFP-negative population (Figure 6C). These surrogate observations were confirmed by western blots, showing a loss of MPC1 protein in passaged HCT15 spheroids (Figure 6D). In contrast, MPC1 expression remained stable after eight passages in adherent culture (Figure S5B and C). These data suggest a selective pressure against high MPC1 expression in cancer cells grown in anchorage-free conditions in vitro or in vivo.
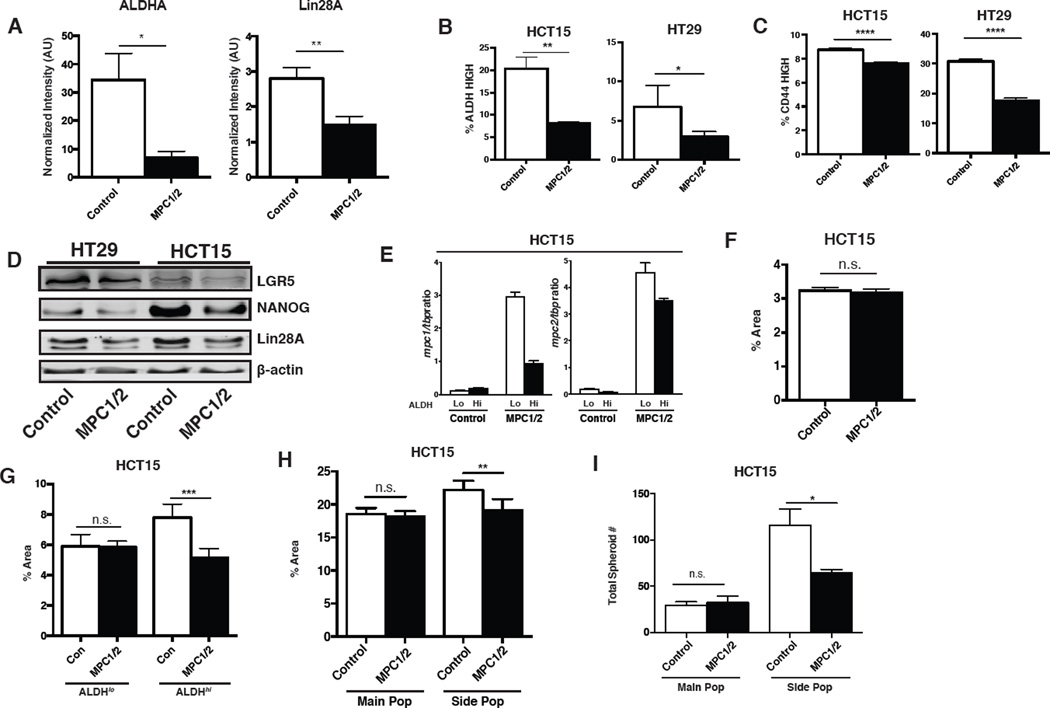
When MPC is re-expressed at levels comparable to differentiated cells and tissues, these cancer cells lose oncogenic potential, without signs of impaired cell health or viability. The first clue as to a potential mechanism came from examination of western blots from the xenograft experiments displayed in Figure 5. We found that aldehyde dehydrogenase, ALDHA, was significantly decreased in the MPC-expressing tumors relative to controls (Figure 5C and 7A). ALDHA is a stem cell marker and is a regulator of differentiation through its control of retinoic acid synthesis (Chute et al., 2006). The expression of this marker has also been shown to associate with more aggressive cancers and is commonly used to isolate cancer stem cells (Deng et al., 2014; Ginestier et al., 2007; Wang et al., 2012). Similarly, the LIN28A stem cell marker was also decreased in the MPC-expressing tumors relative to controls (Figure 7A and 5C). This led us to hypothesize that the defects in soft agar, spheroid, and xenograft growth upon MPC1 and MPC2 re-expression were a consequence of impaired stem cell properties. We went back to HCT15 and HT29 cells grown in adherent culture and examined whether the decrease in ALDH activity was present prior to injection into mice. Indeed, we found that the ALDHhi population was depleted in both HCT15 and HT29 cells upon MPC1 and MPC2 expression (Figure 7B and S6A). Similarly, FACS-based analysis of CD44hi (stem cell like) versus CD44lo (non-stem cell like) cells showed MPC expression decreased the CD44hi population in both cell lines also (Figure 7C and S6B). We also performed western blots of additional stem cell markers and found that LGR5 and LIN28A protein levels were decreased in adherent cells upon MPC expression in both cell lines, and NANOG was decreased in HCT15 cells (Figure 7D and S6C).
Figure 7. MPC re-expression alters the cancer initiating cell population.
(A) Western blot quantification of ALDHA and Lin28A from control or MPC re-expressing HT29 xenografts (mean ± SEM, n=10). (B-C) Percentage of ALDHhi (n=3) and CD44hi (n=5) cells as determined by flow cytometry (mean ± SEM). (D) Western blot analysis of stem cell markers in control and MPC re-expressing cell lines. (E) Relative MPC1 and MPC2 mRNA levels in ALDH sorted HCT15 cells (n=4, mean ± SEM). 2D growth of (F) whole-population HCT15 cells and (G) ALDH sorted cells. Area determined by ImageJ after crystal violet staining (mean ± SD, n=6). (H) Adherent and (I) spheroid growth of main population (MP) versus side population (SP) HCT15 cells. (mean ± SD, n=6). *,p<0.05; **,p<0.01; ***,p<0.001; ****,p<0.0001
We next isolated the top and bottom 5% of cells based on ALDH activity (ALDHhi and ALDHlo) from control and MPC-expressing HCT15 cells. Exogenous MPC1 expression was markedly suppressed in the stem cell population relative to the non-stem cell population (Figure 7E). Expression of MPC2 was also lower, albeit to a lesser extent (Figure 7E). We examined the proliferation rate of these cell populations; in all experiments, the number of cells entering the assay was normalized across all groups. As observed previously, MPC expression had no effect on proliferation when assessed in the entire cell population (Figure 7F) or in the ALDHlo population (Figure 7G). We observed impaired proliferation in MPC-expressing ALDHhi cells, however (Figure 7G). We next isolated stem cells using a complementary parameter, the ability to efflux Hoechst 33342 (Haraguchi et al., 2006; Patrawala et al., 2005; Wu et al., 2007). As with ALDH-selected stem cells, we observed no difference in HCT15 proliferation upon MPC expression in the main (non-stem cell) population (Figure 7H and S6D). In the side (stem cell) population, we observed decreased proliferation in the MPC-expressing cells (Figure 7H). When these same cells were assessed for ability to form spheroids, the results were more profound. Side population control cells exhibited a ~5-fold increased frequency of spheroid formation relative to the main population, and this difference was markedly blunted in the MPC-expressing cells (Figure 7I). MPC re-expression had no effect on the proficiency of spheroid formation in main population cells (Figure 7I).
Discussion
The Warburg effect, wherein cells oxidize carbohydrates at a reduced rate even in the presence of oxygen, is now recognized as a common feature of cancer. Otto Warburg’s observations in the early 20th century led him to conclude that cancer cells contain defective mitochondria. While mitochondria have subsequently been shown to be vital for cancer growth (Cavalli et al., 1997; Morais et al., 1994), we show herein that impaired import of pyruvate via loss of the mitochondrial pyruvate carrier (MPC) enforces the Warburg effect and MPC re-expression impacts the metabolic and growth properties of cancer cells. The oxidative machinery of cancer cells, at least those that were studied herein, is capable of effective oxidative metabolism (Zu and Guppy, 2004); pyruvate simply must be provided through increased expression and activity of the MPC. It is quite surprising that solely expressing MPC1 and MPC2 to generate more intact MPC complexes is sufficient to alter the metabolic program of cancer cells given the numerous, redundant and efficacious mechanisms in place to limit pyruvate oxidation in cancer cells. In the face of these counteracting mechanisms, the metabolic effects of MPC expression are impressive.
Our demonstration that the MPC is lost or underexpressed in many cancers might provide clarifying context for earlier attempts to exploit metabolic regulation for cancer therapeutics. The PDH kinase inhibitor dichloroacetate, which impairs PDH phosphorylation and increases pyruvate oxidation, has been explored extensively as a cancer therapy (Bonnet et al., 2007; Hamilton, 2010). It has met with mixed results, however, and has typically failed to dramatically decrease tumor burden as a monotherapy (Garon et al., 2014; Sánchez-Aragó et al., 2010; Shahrzad et al., 2010). Is one possible reason for these failures that the MPC has been lost or inactivated, thereby limiting the metabolic effects of PDH activity? The inclusion of the MPC adds additional complexity to targeting cancer metabolism for therapy, but has the potential to explain why treatments may be more effective in some studies than in others (Fulda et al., 2010; Hamanaka and Chandel, 2012; Tennant et al., 2010; Vander Heiden, 2011).
The redundant measures to limit pyruvate oxidation make it easy to understand why expression of the MPC leads to relatively modest metabolic changes in cells grown in adherent culture conditions. While subtle, we observed a number of changes in metabolic parameters, all of which are consistent with enhanced mitochondrial pyruvate entry and oxidation. There are at least two possible explanations for the discrepancy that we observed between the impact on adherent and non-adherent cell proliferation. One hypothesis is that the stress of nutrient deprivation and detachment combines with these subtle metabolic effects to impair survival and proliferation. It is also possible, however, that the metabolic effects themselves become much more profound during anchorage-independent growth. Regardless of the magnitude of the metabolic effect of MPC expression, it seems clear that the effects of MPC expression on stemness are indeed metabolic as they are reversed by an inhibitor of pyruvate transport. There is precedent for mitochondrial metabolism affecting stemness as HT29 cells differentiate following replacement of glucose with galactose, which promotes mitochondrial oxidative metabolism (Pinto et al., 1982). The mechanism(s) underlying the loss of stem cell markers and behavior that we observed, however, remain unknown. Filling this gap will be very important, as these mechanisms likely constitute a key vulnerability in cancer cells and a critical defining feature of stem cells.
Not surprisingly, cancer cells appear to select against MPC expression, principally through deleting or suppressing MPC1 gene expression. This is most convincingly demonstrated by the strong correlation of low MPC1 expression with poor prognosis across a wide variety of cancers. We also observed the same phenomenon in our ectopic MPC1 and MPC2 expression system. We found that both serial spheroid passage and xenograft growth appeared to suppress MPC1 gene expression in HCT15 cells. The disproportionate and consistent suppression of MPC1 is surprising, given that we have previously shown that both MPC1 and MPC2 are absolutely required for formation of a functional MPC complex. Moreover, in the present study, we have shown stable accumulation of either protein to be completely dependent on expression of the other. These data raise the interesting possibility that a decrease in the MPC1:MPC2 ratio has a functional consequence beyond just decreased intact MPC complexes and pyruvate transport.
Our attempts to define the molecular mechanism for the context-dependent impairment of cell proliferation upon MPC re-expression culminated in the observation of decreases in multiple measures of stemness. Interestingly, this occurs in cells growing in adherent culture, where we see no effect on proliferation, viability, apoptosis or necrosis. We measured six different markers of the stem cell compartment and each showed a decrease in both HCT15 and HT29 cells except the side population assay, which is a measure of the activity of the ATP-dependent drug transporters ABCG1, ABCC1-5, and ABCG2 to efflux Hoechst 33342 (Goodell et al., 1996). It is possible that the perturbation of metabolism by the MPC, perhaps through ATP production, somehow disconnects the side population assay from other measures of stem cell content (Liu et al., 2014). We see it as significant that, in spite of this, the side population is exclusively affected by MPC expression. Similarly, when selecting stem cells based on high ALDH activity, we also only observed effects of MPC expression on proliferation in the stem cell population. The data strongly suggest that the effects of MPC expression on proliferation in multiple contexts are related to its effects on the stem cell compartment.
This seemingly intimate connection between the MPC and stemness is consistent with recent observations that most stem cell populations exhibit a glycolytic metabolic phenotype similar to cancer cells (Folmes et al., 2012; Ito and Suda, 2014; Wanet et al., 2012). Our preliminary analysis of publically available datasets suggests that the MPC might be involved. Both MPC1 and MPC2 are expressed at a very low level in embryonic stem cells, and their expression increases upon differentiation (for example, MPC1, p=4.8E-6; MPC2, p=6.3E-5 for cardiomyocyte differentiation (Cao et al., 2008), and MPC1, p<0.0001; MPC2, p=0.0061 for neurons (Fathi et al., 2011)). This leads us to speculate that modulation of pyruvate metabolism via the MPC is vital for the maintenance of stem cell populations (Mandal et al., 2011; Schieke et al., 2008). In addition, glycolysis supports numerous biosynthetic pathways that may be altered by enhanced pyruvate oxidation, including the pentose phosphate pathway and one-carbon pools, which may in turn alter stemness (Shyh-Chang et al., 2013). Therefore, the studies of cancer cell metabolism described herein might also be informative as to the metabolic requirements for stem cell maintenance.
In conclusion, we propose that suppression of MPC1 expression and the concomitant decrease in mitochondrial pyruvate uptake and oxidation is an important mediator of the Warburg effect in colon cancer cells, and most likely in many cancer settings. It is well documented that, in many cancers, the extent of this glycolytic metabolism correlates with a poorer prognosis—consistent with the prognostic impact of MPC1 expression shown herein. We suggest that the data presented herein begin to establish a more complete framework for understanding and therapeutically exploiting cancer metabolism. We have shown that loss of MPC activity, and the concomitant loss of pyruvate supply to the TCA cycle, is required for the normal oncogenic function of cancer cells. It is possible that this metabolic inflexibility will confer enhanced sensitivity to agents that prevent anapleurotic contribution of other substrates, such as glutamine, to the TCA cycle. Therefore, placing the MPC properly in the metabolic network of cancer cells might enable a more personalized and sophisticated approach to target this axis for therapeutic benefit.
Experimental Procedures
Additional details can be found in Supplemental Experimental Procedures.
Mammalian cell culture
Unless otherwise stated, all cell lines were maintained in DMEM +2mM Glutamax (Invitrogen) with 10% FBS (Sigma Aldrich) and 1% primocin at 37°C in 5% CO2. Cells were obtained from the National Cancer Institute (Bethesda, MD) and ATCC (Manassas, VA). All retroviral transductions were performed with pseudotyped retroviral supernatants generated by co-transfection of 293T cells with Vsv-G, Gag-Pol, and a retroviral targeting vector (pQCXIP/B/Z) harboring cDNA for the gene of interest. All lentiviral transductions were performed with pseudotyped 3rd-generation lentiviral supernatants generated by cotransfection of 293T cells with vectors pMD2.G (Addgene #12259), pMDLG/pRRE (Addgene #12251), pRSV-Rev (Addgene #12253), and a lentiviral targeting vector (LeGO iG2/iC2) harboring cDNA for the gene of interest. Stable cell lines were generated by selection with appropriate antibiotic Blasticidin (6µg/mL), Puromycin (6µg/mL), Zeocin (400µg/mL), or by fluorescence sorting.
Xenografts
One million cells were injected for HCT15, and two million cells for HT29. Control and MPC overexpression cells were injected to each side of the same nude mouse. Tumor sizes were measured at indicated time by caliper.
Blue-Native PAGE
BN-PAGE was performed as described previously (Bricker et al., 2012). Mitochondria were resuspended in lysis buffer (50 mM NaCl, 5 mM 6-aminocaproic acid, 50 mM imidazole, 1 mM AEBSF, and protease inhibitor cocktail). Mitochondria were solubilized with ~1.5% digitonin. Lysates were resolved on a 3%–13% gradient native gel using a PROTEAN®II xi Cell gel running system (Bio-Rad). Western blot performed as described elsewhere using a Trans-blot transfer cell (Bio-Rad) and PVDF membranes.
Fluorescence microscopy
Stable cell lines with empty vector, MPC1 & MPC2, MPC1-GFP or MPC2-GFP were plated at 1000/cells per well and grown for at least 24 hours prior to imaging. The cells were then treated with 25nM Mitotracker Red CMXRos (Life Technologies) and 16.2µM Hoechst 33342, and incubated for 5 minutes prior to imaging. Cells were imaged on the Axio Observer Z1 imaging system (Carl Zeiss) equipped with 10×, 40× and 100× objectives (oil - immersion). Digital fluorescence and differential interference contrast (DIC) images were acquired using a monochrome digital camera (AxioCam MRm, Carl Zeiss). Z - stacks were obtained and deconvolved using the AxioVision software (Version 4.8, Carl Zeiss). 2D projection of the z - stacks was performed using the ImageJ software (summation – average intensity). The final images were adjusted and assembled using Adobe Photoshop CS5. Brightness and contrast were adjusted only using linear operation on the entire image.
Oxygen Consumption Studies; Seahorse
Metabolic measurements were carried out in standard 24-well Seahorse microplates on a Seahorse XF24 analyzer. Glycolysis was measured per the glycolysis stress test kit and displayed as extracellular acidification rate (ECAR). Pyruvate oxidation was measured using oxygen consumption rate (OCR) when cells were incubated in unbuffered Seahorse media containing 10mM sodium pyruvate as the only respiratory substrate. For all experiments 80,000 cells per well were plated 16–18 hours prior to analysis.
Statistical Methods
Statistical analyses were performed using Graphpad Prism 6. Unless otherwise noted, data were analyzed by Student’s t-test and considered significant at p < 0.05.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the following individuals for their contribution to this work: James Marvin for his consultation and flow cytometry training and Chris Leukel for lively discussion regarding flow cytometry. We thank Janet Bassett and our fellow students in the MD/PhD Program including Dr. Jess Maddox, Joe Cho, Jon Downie, and Alex Keefe. We thank Dr. Dave Jones for reviewing this manuscript. We thank Drs. Dean Tantin, Sue Hammoud, Don Ayer, Janet Shaw, Sihem Boudina, James Cox, Jerry Kaplan, Janet Lindsley, Amnon Schlegel, Adam Frost, Rich Dorsky, Jody Rosenblatt, Ed Levine, Joe Yost, Charlie Murtaugh, Carl Thummel, and members of the Rutter Lab for discussion and insight. We thank Drs. Nikolaos Diakos, Stavros Drakos, and Dean Li for human heart tissue samples and the Tissue Research Acquisition Core (TRAC) for the human colon tissue samples. We thank the Metabolic Phenotyping Core for their expertise and services. This project was supported by the Nora Eccles Treadwell Foundation (to JR); NIH R01 GM094232 (to JR); AJH was supported by an American Cancer Society - Daiichi Sankyo, Inc. Postdoctoral Fellowship, grant # PF-13-363-01-TBE; NIH Developmental Biology Training Grant 5T32HD07491 (to JCS); NIH Hematology Training Grant 5T32DK007115-40 (to KAO), NIH R01 CA157996 (to RJD); CPRIT: RP130272 (to RJD); NCI P30CA042014 (to TRAC); NCRR 1S10RR026802-01 (to Utah Flow Cytometry Core).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Wrote the manuscript: JCS, KAO, JR; Edited the manuscript: JCS, KAO, LJ, AJH, RJD. Designed experiments: JCS, KAO, LJ, AJH, JGV, RAE, RJD, JR. Performed experiments: JCS, KAO, LJ, AJH, JGV, RAE, EGE. JX developed proprietary antibodies essential to this work.
Literature Cited
- Aguirre-Gamboa R, Gomez-Rueda H, Martínez-Ledesma E, Martínez-Torteya A, Chacolla-Huaringa R, Rodriguez-Barrientos A, Tamez-Peña JG, Treviño V. SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PloS one. 2013;8:e74250. doi: 10.1371/journal.pone.0074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M, Shi M, Danenberg KD, Gardner H, Barrett C, Jacques CJ, Sherod A, Iqbal S, El-Khoueiry A, Yang D, et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics. 2007;8:1705–1713. doi: 10.2217/14622416.8.12.1705. [DOI] [PubMed] [Google Scholar]
- Bassenge E, Sommer O, Schwemmer M, Bunger R. Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. American journal of physiology Heart and circulatory physiology. 2000;279:H2431–H2438. doi: 10.1152/ajpheart.2000.279.5.H2431. [DOI] [PubMed] [Google Scholar]
- Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012;24:62–67. doi: 10.1097/CCO.0b013e32834deb9e. [DOI] [PubMed] [Google Scholar]
- Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AER, Charnock FML, Beck S, Dunham I, Mungall AJ, et al. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene. 2006;26:683–700. doi: 10.1038/sj.onc.1209827. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. Epub 1212012 May 1218024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Kumar N, Ai N, Gupta S, Rath P, Baudis M. Progenetix: 12 years of oncogenomic data curation. Nucleic Acids Research. 2014;42:D1055–D1062. doi: 10.1093/nar/gkt1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde Dehydrogenase-Expressing Colon Stem Cells Contribute to Tumorigenesis in the Transition from Colitis to Cancer. Cancer Research. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli LR, Varella-Garcia M, Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1997;8:1189–1198. [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Bronner MP, Crispin DA, Rabinovitch PS, Brentnall TA. Characterization of genomic instability in ulcerative colitis neoplasia leads to discovery of putative tumor suppressor regions. Cancer Genetics and Cytogenetics. 2005;162:99–106. doi: 10.1016/j.cancergencyto.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008a;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008b;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell-Conrad AS, Wolfe CL, Wheeler JS, Coulter KR, Kilkuskie PM, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One. 2013;8:e61551. doi: 10.1371/journal.pone.0061551. Print 0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Zhou J, Fang L, Cai Y, Ke J, Xie X, Huang Y, Huang M, Wang J. ALDH1 is an independent prognostic factor for patients with stages II-III rectal cancer after receiving radiochemotherapy. British Journal of Cancer. 2014;110:430–434. doi: 10.1038/bjc.2013.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. Epub 1303362013 Mar 1303360119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eboli ML, Paradies G, Galeotti T, Papa S. Pyruvate transport in tumour-cell mitochondria. Biochim Biophys Acta. 1977;460:183–187. doi: 10.1016/0005-2728(77)90166-9. [DOI] [PubMed] [Google Scholar]
- Fathi A, Hatami M, Hajihosseini V, Fattahi F, Kiani S, Baharvand H, Salekdeh GH. Comprehensive gene expression analysis of human embryonic stem cells during differentiation into neural cells. PloS one. 2011;6:e22856. doi: 10.1371/journal.pone.0022856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CD, Nelson TJ, Dzeja PP, Terzic A. Energy metabolism plasticity enables stemness programs. Ann N Y Acad Sci. 2012;1254:82–89. doi: 10.1111/j.1749-6632.2012.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nature Reviews Drug Discovery. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Gao, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Science Signaling. 2013;6:pl1–pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar F, et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. Journal of cancer research and clinical oncology. 2014;140:443–452. doi: 10.1007/s00432-014-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. The Journal of experimental medicine. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotanda Y, Akagi Y, Kawahara A, Kinugasa T, Yoshida T, Ryu Y, Shiratsuchi I, Kage M, Shirouzu K. Expression of monocarboxylate transporter (MCT)-4 in colorectal cancer and its role: MCT4 contributes to the growth of colorectal cancer with vascular endothelial growth factor. Anticancer research. 2013;33:2941–2947. [PubMed] [Google Scholar]
- Halestrap AP. The Mitochondrial Pyruvate Carrier: Kinetics and Specificity for Substrates and Inhibitors. Biochem J. 1975a;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. The Biochemical journal. 1975b;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. The Journal of experimental medicine. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton In vitro cytotoxicity of combinations of dichloroacetate with anticancer platinum compounds. Clinical Pharmacology: Advances and Applications. 2010:177. doi: 10.2147/CPAA.S11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem cells (Dayton, Ohio) 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Hong Y, Ho KS, Eu KW, Cheah PY. A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: implication for tumorigenesis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1107–1114. doi: 10.1158/1078-0432.CCR-06-1633. [DOI] [PubMed] [Google Scholar]
- Huang CY, Kuo WT, Huang YC, Lee TC, Yu LC. Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell death & disease. 2013;4:e622. doi: 10.1038/cddis.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nature Reviews Molecular Cell Biology. 2014 doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Chung SJ, Kang IJ, Park JH, Bunger R. Intramitochondrial pyruvate attenuates hydrogen peroxide-induced apoptosis in bovine pulmonary artery endothelium. Molecular and cellular biochemistry. 2001;216:37–46. doi: 10.1023/a:1011040026620. [DOI] [PubMed] [Google Scholar]
- Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M, Shlomi T, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- Kim J-w, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clinical & Experimental Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL, Group TaAR. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. British Journal of Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floch R, Chiche J, Marchiq I, Naiken T, Naiken T, Ilc K, Ilk K, Murray CM, Critchlow SE, Roux D, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CL, Wang M, Ma XY, Zhang W. NRGA1, a Putative Mitochondrial Pyruvate Carrier, Mediates ABA Regulation of Guard Cell Ion Channels and Drought Stress Responses in Arabidopsis. Molecular plant. 2014 doi: 10.1093/mp/ssu061. [DOI] [PubMed] [Google Scholar]
- Liu PP, Liao J, Tang Z-J, Wu W-J, Yang J, Zeng Z-L, Hu Y, Wang P, Ju H-Q, Xu R-H, et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell death and differentiation. 2014;21:124–135. doi: 10.1038/cdd.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Emilion G, Mungall AJ, Dunham I, Beck S, Le Meuth-Metzinger VG, Shelling AN, Charnock FML, Ganesan TS. Physical and transcript map of the region between D6S264 and D6S149 on chromosome 6q27, the minimal region of allele loss in sporadic epithelial ovarian cancer. Oncogene. 2002;21:387–399. doi: 10.1038/sj.onc.1205067. [DOI] [PubMed] [Google Scholar]
- Luo W, Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2011;2 doi: 10.18632/oncotarget.299. 551-556-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem cells (Dayton, Ohio) 2011;29:486–495. doi: 10.1002/stem.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. The Journal of biological chemistry. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, et al. Metabolic modulation of glioblastoma with dichloroacetate. Science translational medicine. 2010;2 doi: 10.1126/scitranslmed.3000677. 31ra34. [DOI] [PubMed] [Google Scholar]
- Morais R, Zinkewich-Péotti K, Parent M, Wang H, Babai F, Zollinger M. Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer Research. 1994;54:3889–3896. [PubMed] [Google Scholar]
- Network TCGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Paradies G. On the Mechanism of Translocation of Pyruvate and Other Monocarboxylic Acids in Rat-Liver Mitochondria. European Journal of Biochemistry. 1974;49:265–274. doi: 10.1111/j.1432-1033.1974.tb03831.x. [DOI] [PubMed] [Google Scholar]
- Paradies G, Capuano F, Palombini G, Galeotti T, Papa S. Transport of pyruvate in mitochondria from different tumor cells. Cancer Res. 1983;43:5068–5071. [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Research. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- Patterson JN, Cousteils K, Lou JW, Manning Fox JE, Macdonald PE, Joseph JW. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. J Biol Chem. 2014 doi: 10.1074/jbc.M113.521666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VAF, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1,2, and 4 in colorectal carcinomas. Virchows Archiv : an international journal of pathology. 2008;452:139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- Pinto M, Appay MD, Simon-Assmann P, Chevalier G, Dracopoli N, Fogh J, Zweibaum A. Enterocytic Differentiation of cultured human colon cancer cells by replacement of glucose by galactose in the medium. Biol Cell. 1982;44:193–196. [Google Scholar]
- Pratt ML, Roche TE. Mechanism of pyruvate inhibition of kidney pyruvate dehydrogenasea kinase and synergistic inhibition by pyruvate and ADP. The Journal of biological chemistry. 1979;254:7191–7196. [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia (New York, NY) 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohatgi N, Aly H, Marshall CA, McDonald WG, Kletzien RF, Colca JR, McDaniel ML. Novel Insulin Sensitizer Modulates Nutrient Sensing Pathways and Maintains beta-Cell Phenotype in Human Islets. PLoS One. 2013;8:e62012. doi: 10.1371/journal.pone.0062012. Print 0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Aragó M, Chamorro M, Cuezva JM. Selection of cancer cells with repressed mitochondria triggers colon cancer progression. Carcinogenesis. 2010;31:567–576. doi: 10.1093/carcin/bgq012. [DOI] [PubMed] [Google Scholar]
- Schieke SM, Ma M, Cao L, McCoy JP, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283:28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrzad S, Lacombe K, Adamcic U, Minhas K, Coomber BL. Sodium dichloroacetate (DCA) reduces apoptosis in colorectal tumor hypoxia. Cancer letters. 2010;297:75–83. doi: 10.1016/j.canlet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery ML, Lundgreen A, Herrick JS, Wolff RK. Genetic variation in RPS6KA1, RPS6KA2, RPS6KB1, RPS6KB2, and PDK1 and risk of colon or rectal cancer. Mutation research. 2011;706:13–20. doi: 10.1016/j.mrfmmm.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi S, Okumura T, Tu S, Wang SSW, Shibata W, Vigneshwaran R, Gordon SAK, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem cells (Dayton, Ohio) 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nature reviews Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- Tibiletti MG, Trubia M, Ponti E, Sessa L, Acquati F, Furlan D, Bernasconi B, Fichera M, Mihalich A, Ziegler A, et al. Physical map of the D6S149-D6S193 region on chromosome 6Q27 and its involvement in benign surface epithelial ovarian tumours. Oncogene. 1998;16:1639–1642. doi: 10.1038/sj.onc.1201654. [DOI] [PubMed] [Google Scholar]
- Timon-Gomez A, Proft M, Pascual-Ahuir A. Differential regulation of mitochondrial pyruvate carrier genes modulates respiratory capacity and stress tolerance in yeast. PLoS One. 2013;8:e79405. doi: 10.1371/journal.pone.0079405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nature Reviews Drug Discovery. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A, Arnould T, Renard P, Lou PH, Peterson N. Mitochondrial Involvement in Stemness and Stem Cell Differentiation. Cellular Bioenergetics in Health and Disease: New Perspective in Mitochondrial Biology. 2012:195–215. [Google Scholar]
- Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132:1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-C, Yo Y-T, Lee H-Y, Liao Y-P, Chao T-K, Su P-H, Lai H-C. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. The American Journal of Pathology. 2012;180:1159–1169. doi: 10.1016/j.ajpath.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. The Journal of general physiology. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse S, Cooper RH, Randle PJ. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochemical Journal. 1974;141:761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Wei Q, Utomo V, Nadesan P, Whetstone H, Kandel R, Wunder JS, Alman BA. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Research. 2007;67:8216–8222. doi: 10.1158/0008-5472.CAN-07-0999. [DOI] [PubMed] [Google Scholar]
- Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C-S, Wang J-Y, Chung F-Y, Lee S-C, Huang M-Y, Kuo C-W, Yang M-J, Lin S-R. Significance of the glycolytic pathway and glycolysis related-genes in tumorigenesis of human colorectal cancers. Oncology reports. 2008;19:81–91. [PubMed] [Google Scholar]
- Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochemical and Biophysical Research Communications. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.