Abstract
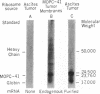
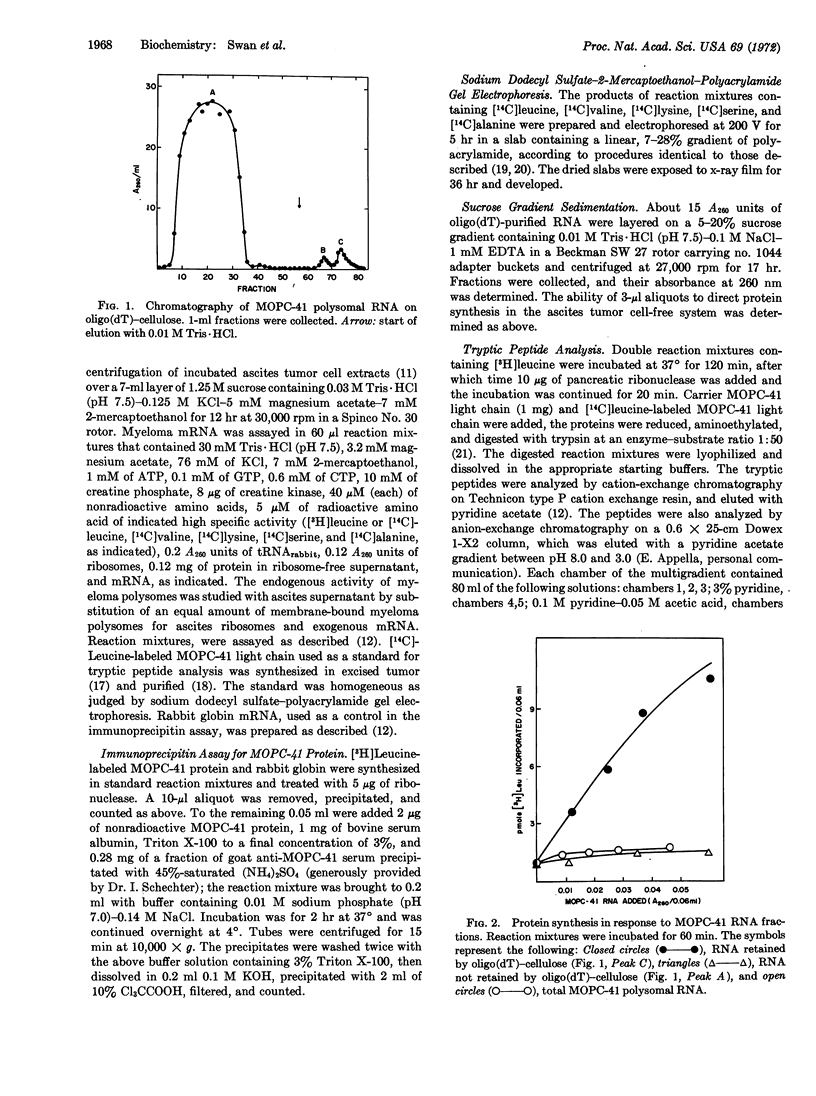
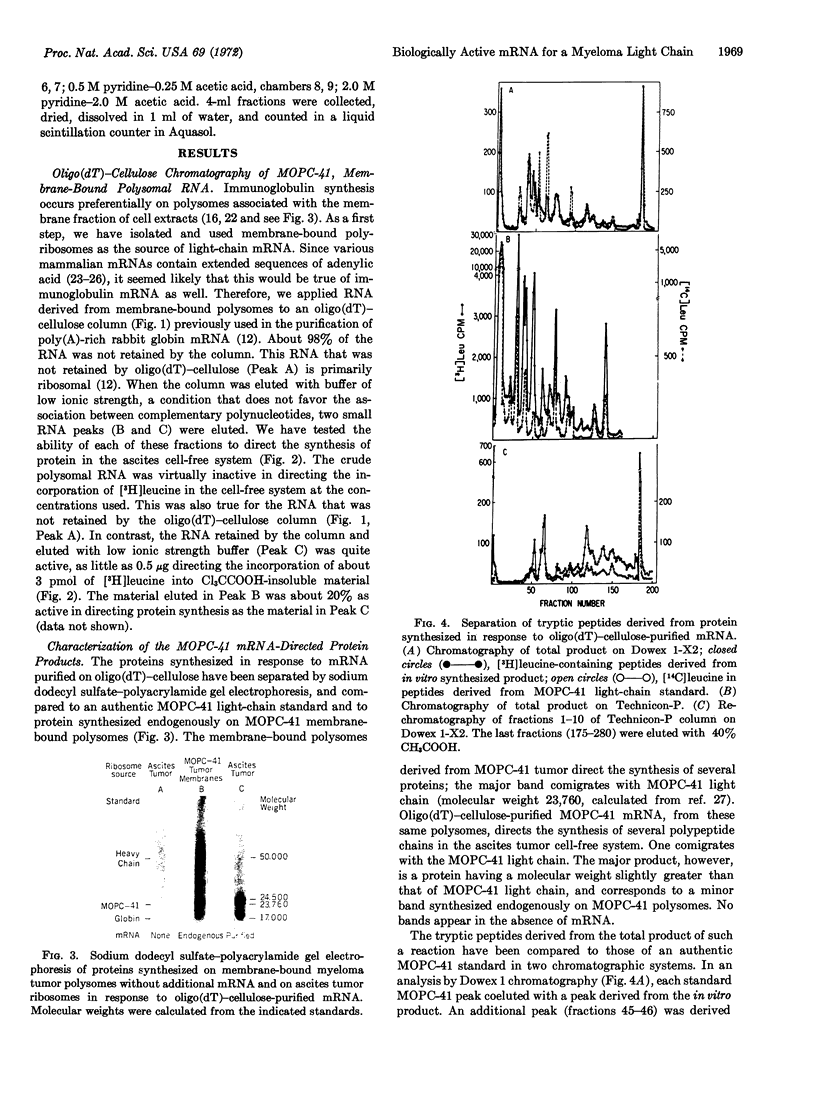
A cell-free system derived from Krebs II ascites tumor has been used to assay biologically active mRNA for myeloma (MOPC-41) light chain during its purification by oligothymidylate-cellulose chromatography and sucrose gradient centrifugation. The purified mRNA directs the synthesis of a product that yields tryptic peptides corresponding to those derived from authentic myeloma protein and that forms a specific immunoprecipitate with antibody directed against the MOPC-41 protein. The fact that the light-chain mRNA anneals to oligothymidylic acid-cellulose suggests that it, like several other eukaryotic mRNAs, contains a region rich in adenylic acid residues. The most active fractions of light-chain mRNA, representing about 0.1% of the RNA originally extracted from membrane-bound myeloma polysomes, sediment as a discrete peak with an s20,w of about 13, roughly corresponding to an RNA molecule containing 850 bases. The results suggest that the light-chain mRNA is monocistronic and that it contains about 200 more bases than would be necessary to encode the variable and constant regions of a single light-chain molecule.
Keywords: poly(A)-mRNA, oligo d(T)-cellulose, monocistronic 13S mRNA
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Boime I., Aviv H., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA. II. The in vitro synthesis of high molecular weight proteins and elements of the viral capsid. Biochem Biophys Res Commun. 1971 Nov 5;45(3):788–795. doi: 10.1016/0006-291x(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer W. J., Bennett J. C. The molecular basis of antibody formation: a paradox. Proc Natl Acad Sci U S A. 1965 Sep;54(3):864–869. doi: 10.1073/pnas.54.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. Somatic translocation of antibody genes. Nature. 1970 Jul 25;227(5256):341–348. doi: 10.1038/227341a0. [DOI] [PubMed] [Google Scholar]
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- Hood L. E. Two genes, one polypeptide chain--fact or fiction? Fed Proc. 1972 Jan-Feb;31(1):177–187. [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Genes and antibodies. Science. 1959 Jun 19;129(3364):1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- Labrie F. Isolation of an RNA with the properties of haemoglobin messenger. Nature. 1969 Mar 29;221(5187):1217–1222. doi: 10.1038/2211217a0. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Lamm M. E., Vassalli P. Synthesis of immunoglobulin heavy and light chains by the free ribosomes of a mouse plasma cell tumor. Proc Natl Acad Sci U S A. 1970 Jun;66(2):425–432. doi: 10.1073/pnas.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Cohn M. Immunoglobulin biosynthesis. V. Light chain assembly. J Mol Biol. 1970 Nov 14;53(3):305–320. doi: 10.1016/0022-2836(70)90067-7. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Uhr J. W. Immunoglobulin synthesis and secretion. V. Incorporation of leucine and glucosamine into immunoglobulin on free and bound polyribosomes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1183–1189. doi: 10.1073/pnas.66.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O. Antibody variability. Somatic recombination between the elements of "antibody gene pairs" may explain antibody variability. Science. 1967 Jul 21;157(3786):267–273. doi: 10.1126/science.157.3786.267. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Szilard L. THE MOLECULAR BASIS OF ANTIBODY FORMATION. Proc Natl Acad Sci U S A. 1960 Mar;46(3):293–302. doi: 10.1073/pnas.46.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P., Lisowska-Bernstein B., Lamm M. E., Benacerraf B. Studies on cell-free synthesis of rat immunoglobulins, II. Synthesis of immunoglobulin and of antibody to the dinitrophenyl hapten. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2422–2429. doi: 10.1073/pnas.58.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]