Abstract
Increases in step width have been reported for several clinical populations, including older adults and stroke survivors. These populations often also exhibit decreased hip abductor strength, suggesting that walking with wider steps may be an adaptive response in order to reduce the mechanical demands on the hip abductors. The purpose of this study was to quantify the relationship between step width and gluteus medius (GM) activity during walking. Fourteen young, uninjured adults walked on a treadmill at 1.25 m/s for four step width conditions (Normal, Narrow, Medium, and Wide) while step width and stance phase GM electromyographic (EMG) activity were quantified. We also measured hip abduction torque and GM activity during maximum voluntary isometric contractions (MVICs) at three hip angles (neutral, abducted 10°, and abducted 20°). During walking trials, GM activity was significantly (p<0.0001) influenced by step width; compared to Normal walking, GM activity was 47% higher with Wide steps and 24% lower with Narrow steps. We also observed a weak positive correlation (r=0.18±0.14) between step width and GM activity during Normal walking, as GM activity was higher with wider steps. These results cannot be attributed to changes in GM conformation under the recording electrode, as GM activity was not influenced by hip angle during MVICs. The increased GM activity with wider steps does not support the proposal that increasing step width would be a beneficial adaptation to weakened hip abductors. A likely alternative explanation is that increased step width is a response to decreased gait balance.
Keywords: Biomechanics, Gluteus medius, Hip abduction, Locomotion, Step width
1. Introduction
Kinematic characteristics of human walking are commonly used to identify atypical gait patterns, and may provide insight into the underlying deficits. For example, an increased step width has been reported with increased age [1, 2] and in patients post-stroke [3, 4]. Walking with wide steps increases the metabolic rate [5, 6], raising the question of why individuals would walk with this less economical gait pattern.
One possible explanation is that increases in step width are an adaptive response to reduced muscular strength. Specifically, decreases in hip abduction strength have been reported both with increased age [7] and following a stroke [8]. It is possible that walking with wider steps would reduce the mechanical demands on the hip abductors, allowing the task to be accomplished with less need for active force production by these weak muscles.
The purpose of this study was to determine whether step width influenced hip abductor activity among young, uninjured participants. We quantified step width and bilateral gluteus medius activity during trials in which step width was prescribed and trials in which participants walked with freely chosen step widths. In order to interpret changes in measured muscle activity, we accounted for possible effects of varying frontal plane hip angle on surface electromyographic (EMG) signals, which can potentially result from changing the conformation of the underlying muscle relative to the recording electrode [9]. Based on the potential link between hip abductor strength and step width among clinical populations, we hypothesized that walking with wider steps would decrease the required stance phase gluteus medius activity.
2. Methods
2.1. Participants
Fourteen adults (10 female, 4 male; age = 24±2 yrs; mass = 64.8±11.2 kg; leg length = 0.88±0.06 m) participated in this study. Potential participants with self-reported current lower extremity injuries, or a history of cardiac, respiratory, or neurological disease were excluded. Written informed consent was obtained from each participant using a form approved by the Medical University of South Carolina Institutional Review Board and consistent with the Declaration of Helsinki.
2.2. Equipment
All walking trials were performed on a treadmill (Bertec; Columbus, Ohio), while participants wore a harness attached to an overhead rail which did not support body weight, but would have prevented a fall in case of a loss of balance. Spatiotemporal walking data were collected at 120 Hz using active LED markers placed on the left and right heels (PhaseSpace; San Leandro, California). In separate trials, a dynamometer (Biodex Medical Systems; Shirley, New York) was used to quantify isometric hip abduction strength (sampled at 1000 Hz) in a standing posture.
During both walking and strength testing trials, electromyographic (EMG) activity of the gluteus medius (GM) muscles was sampled at 1000 Hz using bipolar surface electrodes with two 12mm sensor disks separated by 17mm (Motion Lab Systems; Baton Rouge, Louisiana). Electrodes were placed based on previously published SENIAM guidelines [10], after cleaning the skin over the GM with alcohol. Prior to testing, we ensured that the electrode placement allowed clear detection of GM activity with a minimal risk of cross-talk from other hip muscles. Participants performed isolated hip contractions (abduction, adduction, extension, and flexion) against manual resistance while we ensured that activity was clearly present during abduction contractions and minimal during contractions in other directions, a typical method of testing for cross-talk [11, 12]. In the case of visually detectable activity during a contraction in one of these other directions, the electrodes were moved and retested.
2.3. Experimental Protocol
Participants walked at 1.25 m/s, a typical walking speed for young adults previously used to investigate the relationship between step width and metabolic rate [5]. Walking speed was constant across trials and individuals to avoid any complicating effects of walking speed on preferred step width [13]. To become comfortable with walking while looking straight ahead, participants first performed a 5-minute warm-up trial. Each participant’s preferred step frequency was measured during the final minute of this trial, and used to prescribe step frequency during the remaining trials.
Participants performed a series of four 3-minute walking trials in randomized order: Normal, Narrow, Medium, and Wide. For the Normal trial, participants were simply instructed to walk normally. In the remaining trials, step width was prescribed through verbal instructions. For the Narrow trial, participants were instructed to walk with narrow steps, such as by placing their feet directly in front of each other. For the Medium trial, participants were instructed to walk with a typical step width (i.e. not narrow or wide), but to keep this step width constant from step to step. For the Wide trial, participants were instructed to walk with the widest steps they could comfortably maintain throughout the walking period. Visual targets of the prescribed step width were intentionally not provided in order to prevent participants from “aiming” their steps. Instead, participants were instructed to look straight ahead while walking. The purpose of performing the Medium trial was to determine whether simply asking participants to pay attention to their step width had an influence on muscle activity (a psychological effect), beyond any potential effects of step width itself. To prevent participants from stepping off the treadmill belt, an experimenter provided verbal feedback if participants moved substantially away from the middle of the treadmill. For each trial, participants were instructed to match their step frequency to a metronome, which was set to the individual’s previously measured preferred step frequency. A 3 minute rest period separated walking trials.
Following the walking trials, participants performed a series of hip abduction maximum voluntary isometric contractions (MVICs) with their right leg. All trials were performed while standing on the left leg, following previously described methods [14]. The mediolateral location of the right hip joint center [15] was aligned with the axis of a dynamometer (Fig. 1A). Participants were permitted to stabilize their posture by holding onto the frame of the dynamometer, and an experimenter provided verbal and tactile feedback (i.e. lightly touching the participant’s trunk) to prevent participants from leaning laterally.
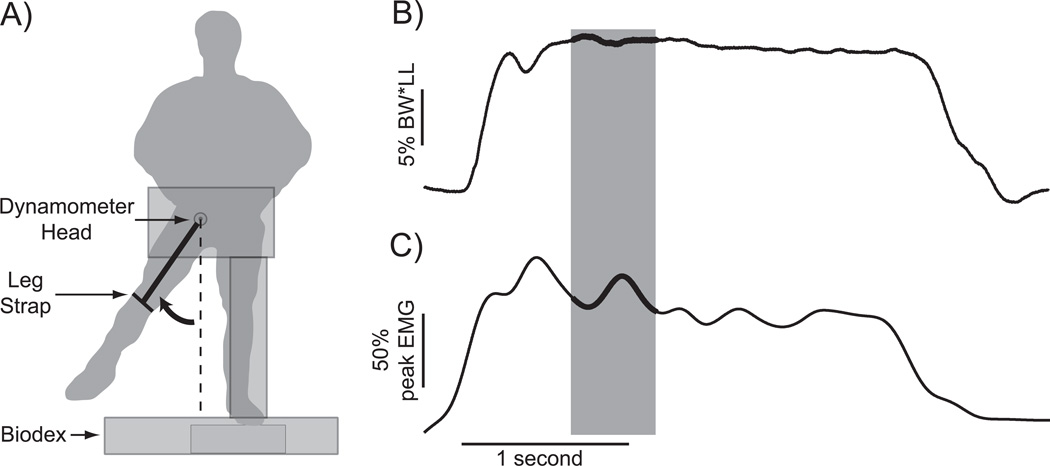
Figure 1.
Participants performed MVIC hip abduction contractions while torque and gluteus medius activity were recorded. A) Contractions were performed from a standing posture. B) Abduction torque was quantified during the 0.5 second period in which the average torque was highest (shaded box). C) Processed gluteus medius activity was quantified during the same time period.
Participants performed hip abduction contractions at three hip angles in randomized order: neutral position, 10° abduction, and 20° abduction. The purpose of these trials was to determine whether the measured GM activity was influenced by hip angle, as could be caused by changes in muscle conformation under the recording electrodes. The chosen 20° range exceeded published values of hip abduction displacement during walking [16], indicating that this range would be sufficient to detect relevant position-dependent changes in measured GM activity. Similarly, the 20° range exceeded the changes in hip abduction angle (quantified using a modified Helen Hayes marker set) during pilot walking trials in which participants (n=2) walked with narrow and wide steps. For each hip angle, the contribution of the weight of the leg to the measured torque was first quantified by recording the torque while participants remained relaxed, as confirmed by monitoring gluteus medius muscle activity. Participants then performed a practice contraction in which they were instructed to push their right leg laterally against the padded radial arm of the dynamometer with approximately 50% of their maximum force, while in the same posture as used for the maximum contractions. Finally, participants performed a series of three maximum contractions, in which they were instructed to gradually ramp up the force. These contractions lasted approximately 3 seconds, while verbal encouragement was provided by an experimenter. Contractions were separated by at least one minute of rest, during which the right leg was returned to neutral position and participants were able to bear weight on either leg. To avoid any learning effects, only the final two maximum contractions were included in subsequent analysis.
2.4. Data Collection and Processing
Heel-strike events were identified using anteroposterior velocity of the heel markers [17]. Stride period was calculated as the time between subsequent right heel-strikes, and step width was calculated as the mediolateral distance between the heels upon each heel-strike. For group analysis, each individual’s step width was normalized by their leg length (LL), defined as the vertical distance from the greater trochanter of the right leg to the ground while wearing their own athletic shoes. For all trials, only the final 100 strides (200 steps) were analyzed.
EMG data were high-pass filtered at 20 Hz, rectified, and smoothed using a 50 Hz low-pass filter. EMG data were then divided into strides based on heel-strike timing. For each participant, the average EMG trace during a stride was calculated for the Normal walking trial, and all EMG data were normalized by the peak value of this trace, a standard normalization method for reducing intersubject variability during walking [18]. Based on typical patterns of activity [19, 20], average GM activity during stance was calculated from 1–40% of the gait cycle (see Fig. 3A for sample traces). This study focused on the stance phase (in which GM activity is consistently highest), not the brief period at the beginning of swing during which small bursts of GM activity have occasionally been reported [19, 21].
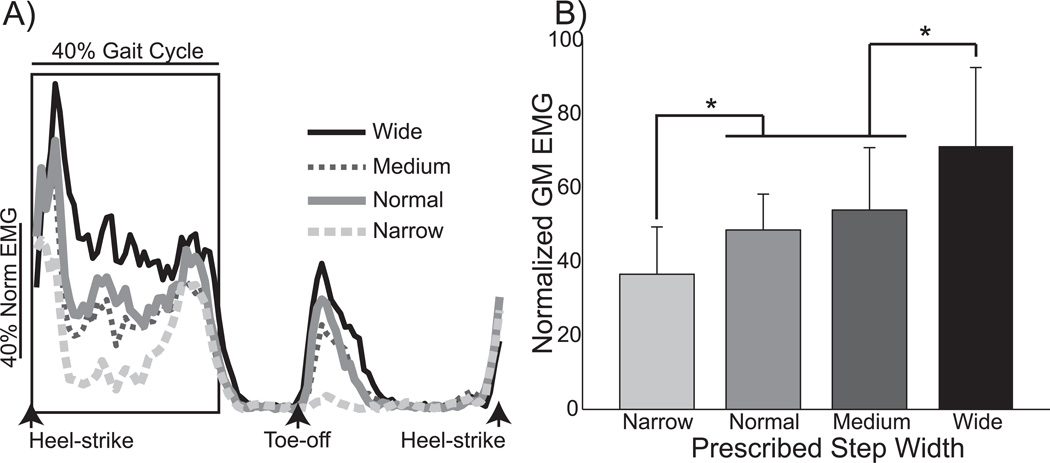
Figure 3.
Step width influenced gluteus medius activity. A) The average pattern of gluteus medius activity is plotted from heel-strike to heel-strike for a single participant. Differences between the prescribed step widths were evident during both stance and swing. B) Stance phase gluteus medius activity varied across walking conditions, scaling with the prescribed step width. Error bars represent standard deviation, and asterisks (*) indicate significant (p<0.05) results of post-hoc tests.
During the MVIC trials, we identified the 0.5 s period during the contraction in which average abduction torque was highest (Fig. 1B). The isometric torque produced by the voluntary muscle contraction was then calculated by subtracting the torque produced by the passive weight of the leg from this measured torque value. GM activity was processed as during walking, and average GM activity was calculated during this same 0.5 second period (Fig. 1C), as recommended for the simultaneous analysis of joint torque and EMG [22]. Abduction torque was normalized by the product of leg length (LL) and body weight (BW). GM activity was normalized by the maximum measured value across all analyzed trials in order to reduce variation across individuals, a standard normalization method for voluntary contractions [10].
2.5. Statistics
For the walking trials, we performed a series of one-way repeated measures ANOVA to determine whether walking condition (Normal, Narrow, Medium, or Wide) significantly influenced the average stride period, step width, or stance phase GM activity. To determine whether step width influenced GM activity from step to step during Normal walking, we performed a series of 28 linear regressions (14 participants * 2 legs) to calculate the Pearson correlation coefficients between step width and stance phase GM activity during the subsequent stride. For the MVICs, we performed a series of two-way repeated measures ANOVA with hip abduction angle and trial number as the independent variables, and abduction torque and GM EMG as the dependent variables. For all analyses, p values less than 0.05 were interpreted as significant. For all ANOVA, post-hoc Tukey tests were performed when appropriate.
3. Results
Gluteus medius activity varied with step width, increasing with wider steps during both prescribed and normal walking. During maximal isometric contractions, increasing the hip abduction angle decreased torque but did not change gluteus medius activity.
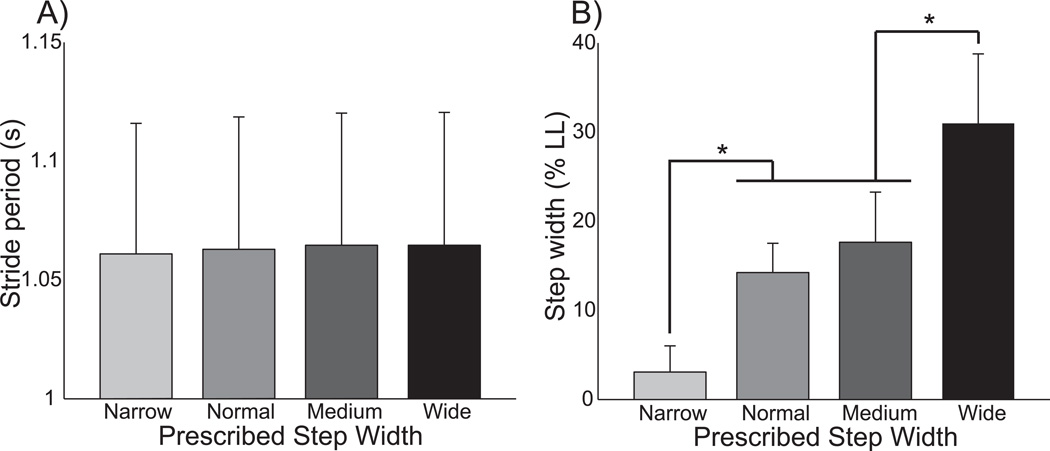
Participants varied their step width in response to instructions, influencing gluteus medius activity. Participants successfully followed the prescribed behavior, keeping stride period constant (p=0.25) but significantly (p<0.0001) changing step width across trials (Fig 2). GM activity also varied across trials, as illustrated for a single participant in Figure 3A. Stance phase activity was significantly (p<0.0001) influenced by walking condition (Fig. 3B). In comparison to normal walking, stance phase GM activity increased by 47% for wide steps and decreased by 24% for narrow steps. Stance phase GM activity did not differ significantly between normal and medium steps, indicating that simply paying attention to step width did not have a strong effect on this measure. While not the focus of this study, participants often exhibited a burst of GM activity early in the swing phase, with a magnitude that appeared to scale with step width (see Fig. 3A).
Figure 2.
The changes in spatiotemporal gait measures across walking conditions were restricted to the frontal plane. A) Stride period remained constant across trials. B) Step width varied across trials as prescribed. Error bars represent standard deviation, and asterisks (*) indicate significant (p<0.05) results of post-hoc tests.
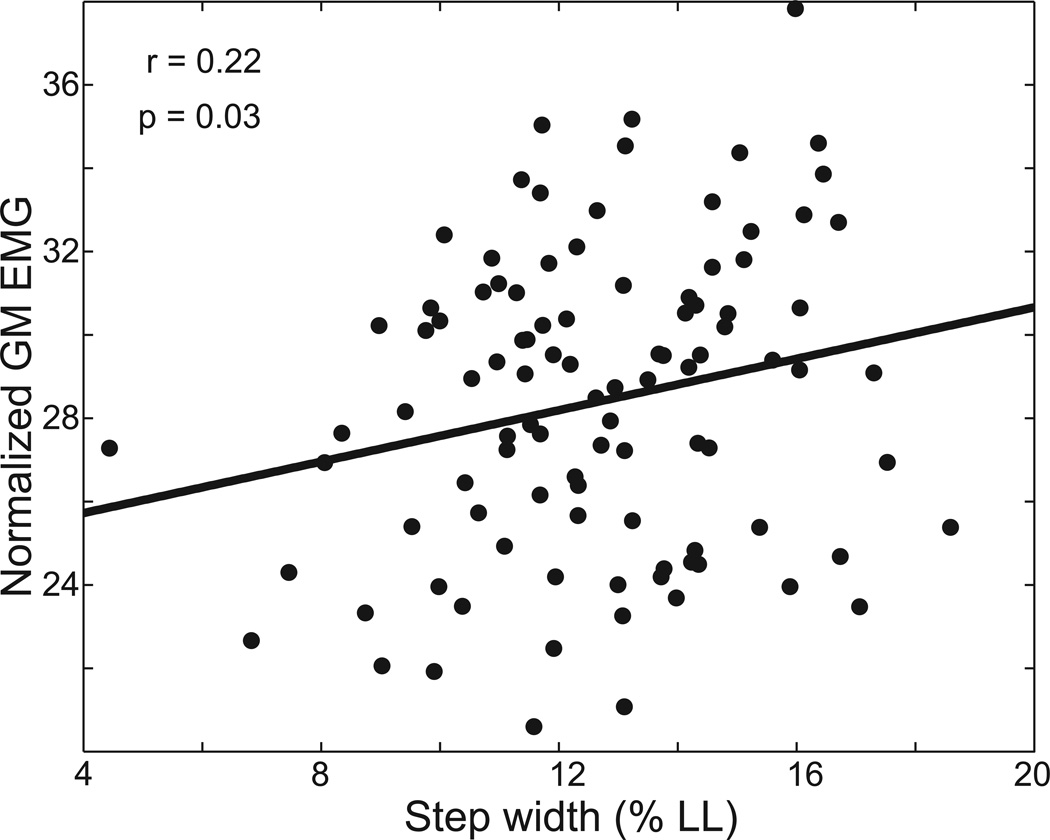
During normal walking, variation in step width and gluteus medius activity was present from step to step. The relationship between step width and stance phase GM activity during the subsequent stride is illustrated for a single participant in Figure 4. The correlation between step width and GM activity was calculated for all 28 legs; in 17 legs we found a significant (p<0.05) positive correlation, and in the other 11 legs we found no significant correlation. Across all 28 legs, the average Pearson correlation coefficient was 0.18±0.14 (mean±s.d.), a weak correlation but significantly (p<0.0001) greater than zero. Therefore, wider steps were generally associated with increased stance phase GM activity.
Figure 4.
From step to step, both step width and stance phase gluteus medius activity changed, as illustrated in a single participant. A weak (r=0.22) but significant (p=0.03) positive correlation was present between step width and stance phase gluteus medius activity during the subsequent stride.
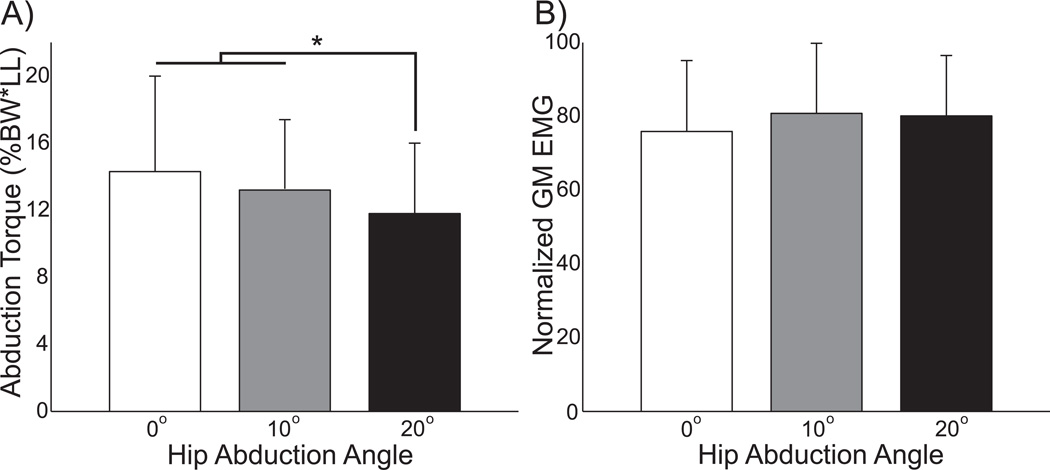
The position of the hip influenced hip abduction torque but not gluteus medius activity during MVICs. Hip abduction angle had a significant (p<0.0001) effect on hip abduction strength, as isometric torque decreased at a more abducted angle (Fig. 5A). Across participants, average abduction torque was 18% lower when the hip was abducted 20° than when the hip was in neutral position. Conversely, hip angle did not have a significant (p=0.38) effect on GM activity during maximum contractions (Fig. 5B). Trial number did not have a significant effect (p>0.34) on either abduction torque or GM activity.
Figure 5.
Abduction torque and muscle activity were quantified during maximal isometric contractions. A) Hip abduction torque decreased at more abducted angles. B) Gluteus medius activity did not vary significantly across joint angles. Error bars represent standard deviation, and asterisks (*) indicate significant (p<0.05) results of post-hoc tests.
4. Discussion
Contrary to our hypothesis, increases in step width caused increases in stance phase gluteus medius activity. A clear relationship between wider steps and increased gluteus medius activity was seen during prescribed walking trials. This relationship was also present, although not as obvious, during normal walking. Therefore, the present results do not support the proposal that walking with wider steps would allow individuals to reduce hip abductor activity by reducing the mechanical demands on these muscles.
The combination of our walking and MVIC muscle activity results indicate that the observed relationship between step width and gluteus medius activity is not simply due to changes in muscle conformation. The typical explanation for an increased surface EMG signal is an increase in muscle activation, whether from an increase in the number of motor units recruited or in the motor unit discharge rate. Alternatively, it is theoretically possible that the increased gluteus medius EMG with wider steps is due to changes in the muscle’s conformation. Specifically, the stance leg hip would likely be more abducted following a wide step, shortening the gluteus medius. With a shorter gluteus medius, more active motor units may be geometrically closer to the recording electrode, increasing the magnitude of the measured EMG signal for the same level of muscle activity [9]. This does not appear to be a likely explanation for our results, as the lack of a significant effect of joint angle on gluteus medius activity during isometric contractions indicates that any effect of muscle conformation on measured EMG is relatively small.
Our finding that wider steps increase gluteus medius activity appears to contradict previous model simulations which suggested that walking with wider steps could decrease the abduction torque that must be generated by active contractions of the gluteus medius [23]. However, the increased hip abduction angle required to produce these wider steps likely shortens the gluteus medius, moving the muscle further from its optimal length at a slightly adducted hip angle [24, 25]. In this more abducted angle, the ability to produce active abduction torque would be reduced (present results; [24]) so greater activation would be required to produce the same amount of torque [25]. Therefore, while the hip abduction torque demands may be reduced by walking with wider steps, such a gait pattern may still require increased gluteus medius activation, as observed in the present study.
Despite the increased requirement for hip abductor activation, clinical populations may walk with wider steps in order to increase their perceived balance. A relationship between increased step width and improved balance has long been suggested [26], although this potential link appears to be more complex than a simple causal relationship [27]. While an increased base of support may improve lateral balance during standing posture, the potential benefits are not as clear during walking. In gait, the majority of time is spent is spent in single leg support, and walking with wider steps would likely require larger and faster mediolateral displacements of the center of mass, as reported in older adults [2]. Nevertheless, the stability benefits of walking with wide steps are suggested by model simulations which found that wide steps decrease the control precision required to maintain balance [28]. As indirect evidence for such a relationship, humans tend to walk with narrower steps when the need to actively stabilize their lateral motion is removed [1]. Additionally, humans walk with more laterally placed steps [21] and more metabolically costly gait patterns [29] when they perceive a challenge to their balance. Future experiments could more directly test whether wider steps improve balance by applying mechanical perturbations during walking with various step widths.
The present experiments have several limitations which prevent us from definitively stating that wide steps cannot be a beneficial adaptation to reduced hip abductor strength. Most notably, these experiments involved young, uninjured participants. It is possible that participants from other populations (e.g. older adults, stroke survivors) may respond differently to prescribed changes in step width. Additionally, the present experiments focused on changes in hip abductor activity, which will not necessarily scale in parallel with frontal plane hip torques [25]. The experimental details may have influenced step width, which can vary between treadmill and overground walking [30] and with gait speed [13]. Finally, we did not strictly prescribe step widths using visually presented targets. While this was an intentional choice to prevent participants from increasing muscular co-contraction in order to “aim” their feet toward specific locations on the treadmill, this decision did allow substantial variability across participants. While the present work does not support the proposal that wider steps reduce hip abduction demands, a future study should further investigate the effects of step width on abductor activity and hip torques at different overground walking speeds among clinical populations of interest.
In conclusion, increasing step width increases the need for strong stance phase hip abductor contractions among uninjured controls. Therefore, the altered mechanics of walking with wide steps appear to increase task difficulty in terms of both muscular and metabolic [5, 6] demand. While clinical populations may increase step width to improve balance, these resultant negative consequences could be a secondary contributor to reduced mobility.
Research highlights.
Step width during walking is often increased in clinical populations
Increased step width may be a response to hip abductor weakness
We found that gluteus medius activity increases when walking with wider steps
The increased gluteus medius activity is not due to muscle conformation changes
Wide steps do not appear to reduce the mechanical demands on the hip abductors
Acknowledgements
This work was partially supported by grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service (IK2 RX000750) and the National Institutes of Health (R21 HD064964). The funding sources had no involvement in the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare they have no conflicts of interest.
References
- 1.Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Trans Biomed Eng. 2007;54:1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- 2.Schrager MA, Kelly VE, Price R, Ferrucci L, Shumway-Cook A. The effects of age on mediolateral stability during normal and narrow base walking. Gait Posture. 2008;28:466–471. doi: 10.1016/j.gaitpost.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roerdink M, Lamoth CJC, Kwakkel G, van Wieringen PCW, Beek PJ. Gait coordination after stroke: benefits of acoustically paced treadmill walking. Phys Ther. 2007;87:1009–1022. doi: 10.2522/ptj.20050394. [DOI] [PubMed] [Google Scholar]
- 4.Fung J, Perez CF. Sensorimotor enhancement with a mixed reality system for balance and mobility rehabilitation. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6753–6757. doi: 10.1109/IEMBS.2011.6091666. [DOI] [PubMed] [Google Scholar]
- 5.Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc R Soc Lond B. 2001;268:1985–1992. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wert DM, Brach J, Perera S, Van Swearingen JM. Gait biomechanics, spatial and temporal characteristics, and the energy cost of walking in older adults with impaired mobility. Phys Ther. 2010;90:977–985. doi: 10.2522/ptj.20090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 8.Neckel N, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. J NeuroEng Rehab. 2006;3:17. doi: 10.1186/1743-0003-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frigon A, Carroll TJ, Jones KE, Zehr EP, Collins DF. Ankle position and voluntary contraction alter maximal M waves in soleus and tibialis anterior. Muscle Nerve. 2007;35:756–766. doi: 10.1002/mus.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G. European Recommendations for Surface Electromyography. Enschede, Netherlands: Roessingh Research and Development; 1999. [Google Scholar]
- 11.Winter DA, Fuglevand AJ, Archer SE. Crosstalk in surface electromyography: theoretical and practical estimates. J Electromyogr Kinesiol. 1994;1:15–26. doi: 10.1016/1050-6411(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 12.Zazulak BT, Ponce PL, Straub SJ, Medvecky MJ, Avedisian L, Hewett TE. Gender comparison of hip muscle activity during single-leg landing. J Orthop Sports Phys Ther. 2005;5:292–299. doi: 10.2519/jospt.2005.35.5.292. [DOI] [PubMed] [Google Scholar]
- 13.Helbostad JL, Moe-Nilssen R. The effect of gait speed on lateral balance control during walking in healthy elderly. Gait Posture. 2003;18:27–36. doi: 10.1016/s0966-6362(02)00197-2. [DOI] [PubMed] [Google Scholar]
- 14.O’Dwyer C, Sainsbury D, O’Sullivan K. Gluteus medius muscle activation during isometric muscle contractions. J Sport Rehab. 2011;20:174–186. doi: 10.1123/jsr.20.2.174. [DOI] [PubMed] [Google Scholar]
- 15.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26:633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 16.Ko SU, Ling SM, Winters J, Ferrucci L. Age-related mechanical work expenditure during normal walking: the Baltimore Longitudinal Study of Aging. J Biomech. 2009;42:1834–1839. doi: 10.1016/j.jbiomech.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeni JA, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27:710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65:517–521. [PubMed] [Google Scholar]
- 19.Hof AL, Elzinga H, Grimmius W, Halbertsma JPK. Speed dependence of averaged EMG profiles in walking. Gait Posture. 2002;16:78–86. doi: 10.1016/s0966-6362(01)00206-5. [DOI] [PubMed] [Google Scholar]
- 20.Winter DA. The Biomechanics and Motor Control of Human Gait. Waterloo, Canada: Waterloo Biomechanics; 1987. [Google Scholar]
- 21.Rankin BL, Buffo SK, Dean JC. A neuromechanical strategy for mediolateral foot placement in walking humans. J Neurophysiol. doi: 10.1152/jn.00138.2014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soylu AR, Arpinar-Avsar P. Detection of surface electromyography recording time interval without muscle fatigue effect for biceps brachii muscle during maximum voluntary contraction. J Electromyogr Kinesiol. 2010;20:773–776. doi: 10.1016/j.jelekin.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Pandy MG, Lin YC, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech. 2010;43:2055–2064. doi: 10.1016/j.jbiomech.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Neumann DA, Soderberg GL, Cook TM. Comparison of maximal isometric hip abductor muscle torques between hip sides. Phys Ther. 1988;68:496–502. doi: 10.1093/ptj/68.4.496. [DOI] [PubMed] [Google Scholar]
- 25.Neumann DA, Soderberg GL, Cook TM. Electromyographic analysis of hip abductor musculature in healthy right-handed persons. Phys Ther. 1989;69:431–440. doi: 10.1093/ptj/69.6.431. [DOI] [PubMed] [Google Scholar]
- 26.Finley FR, Cody KA, Finizie RV. Locomotion patterns in elderly women. Arch Phys Med Rehabil. 1969;50:140–146. [PubMed] [Google Scholar]
- 27.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Rob Res. 1999;18:917–930. [Google Scholar]
- 29.Monsch ED, Franz CO, Dean JC. The effects of gait strategy on metabolic rate and indicators of stability during downhill walking. J Biomech. 2012;45:1928–1933. doi: 10.1016/j.jbiomech.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait Posture. 2010;31:380–384. doi: 10.1016/j.gaitpost.2010.01.002. [DOI] [PubMed] [Google Scholar]