Abstract
Chronic lymphocytic leukemia (CLL) is a B-cell malignancy in need of new, effective, and safe therapies. The recently identified IgM receptor FcμR is overexpressed on malignant B cells in CLL and mediates the rapid internalization and lysosomal shuttling of IgM via its Fc fragment (Fcμ). To exploit this internalization and trafficking pathway for targeted drug delivery, we engineered an IgM derived protein scaffold (Fcμ) and linked it with the cytotoxic agent monomethylauristatin F. This Fcμ-drug conjugate was selectively toxic for FcμR expressing cell lines in vitro and for CLL cells but not autologous normal T cells ex vivo. Notably, the cytotoxic activity of the Fcμ-drug conjugate was maintained in CLL cells carrying a 17p deletion, which predicts resistance to standard chemotherapy. Next, we tested the possible therapeutic application of the Fcμ-drug conjugate in immunodeficient NSG mice engrafted with peripheral blood cells from leukemia patients. Three intravenous injections of the Fcμ-drug conjugate over a 10 day period were well tolerated and selectively killed the human CLL cells but not the co-engrafted autologous human T cells. In summary, we developed a novel strategy for targeted cytotoxic therapy of CLL based on the unique properties of FcμR. FcμR-targeted drug delivery showed potent and specific therapeutic activity in CLL, thus providing proof-of-concept for FcμR as a valuable therapeutic target in CLL and for IgM-based antibody drug conjugates as a new targeting platform.
Keywords: Leukemia, Targeted Therapy, Antibody-drug conjugate, Fc-receptor, human mouse xenograft model
Introduction
Based on their ability to selectively deliver highly cytotoxic drugs into tumor cells, antibody-drug conjugates (ADCs) are among the most promising next-generation antibody therapeutics for cancer therapy (1, 2). The promise of ADCs is the targeted delivery of a potent cytotoxic drug selectively into tumor cells thereby minimizing toxicity toward normal cells. The Food and Drug Administration (FDA) approval of brentuximab vedotin for the therapy of Hodgkin lymphoma and anaplastic large cell lymphoma in 2011 and of trastuzumab emtansine for HER2+ metastatic breast cancer in 2013 were milestones that established the therapeutic utility of ADCs (3, 4). Brentuximab vedotin consists of a chimeric mouse/human anti-human CD30 monoclonal antibody (mAb) in IgG1 format as the carrier protein conjugated to monomethylauristatin E (MMAE) as the cytotoxic payload (5). MMAE is a synthetic anti-tubulin agent active at subnanomolar concentrations. Each MMAE drug is linked to the antibody molecule through a linker that harbors a valine-citrulline-para-aminobenzylcarbamate (Val-Cit-PABC) linker that is cleaved by lysosomal proteases such as cathepsin B. The linker also contains a maleimide group that reacts with thiol groups in the IgG1 hinge region. This random conjugation results in an ADC mixture with an average drug to antibody ratio (DAR) of 4:1 (range 0:1 to 8:1). Trastuzumab emtansine is based on the humanized anti-human HER2 mAb trastuzumab randomly conjugated to an average of 3.5 maytansinoid drugs through the ε-amino group of lysine (Lys) using a non-cleavable linker (6). This new generation of ADCs has demonstrated two important treatment advances; first, patients who relapse or are refractory to first-line therapy can be rescued using targeted cytotoxic drug delivery; and second, an ADC can replace systemic cytotoxic therapy demonstrating higher efficacy with lower toxicity (7, 8).
Chronic lymphocytic leukemia (CLL), the most common leukemia in Western countries, is characterized by the accumulation of mature monoclonal B cells in the blood, bone marrow, spleen, and lymph nodes. The median age at diagnosis is 75 years, precluding use of allogeneic hematopoietic stem cell transplantation, the only curative treatment option, in the majority of patients. Current first line treatment is chemoimmunotherapy with an anti-CD20 mAb and an alkylating agent with or without the addition of a purine analog (9-11). One of the most commonly used regimens for younger patients is the combination of fludarabine, cyclophosphamide, and rituximab (FCR) (11, 12). While initial response rates are high, most patients relapse. FCR is less active in high risk disease, less tolerable in elderly or frail patients, and increases the risk of infections. Fludarabine and cyclophosphamide are also toxic for normal T cells and myeloid cells leading to often long-lasting cytopenias. In contrast, the anti-CD20 mAbs rituximab, ofatumumab, and obinutuzumab selectively target B cells (13). These mAbs induce cell death mostly through immunologic effector mechanisms such as complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity (13, 14). As single agents the efficacy of anti-CD20 mAbs in CLL is limited and they are used primarily in combination regimens. Notably, CD20 is expressed at higher levels on normal B cells than on CLL cells (15), and anti-CD20 mAbs effectively kill normal B cells which can contribute to life-threatening viral, bacterial, and fungal infections (16). Recently kinase inhibitors that target B-cell receptor (BCR) signal transduction have shown impressive clinical activity in CLL (17-19). However, while well tolerated as single agents, these inhibitors achieve mostly partial responses and have to be taken continuously. In fact, even after years of treatment with a kinase inhibitor residual disease is easily detected in virtually all patients and resistant clones emerge that lead to relapse. Thus there is a great clinical need for targeted cytotoxic agents that could be combined with kinase inhibitors to eradicate the disease.
ADCs are also currently being tested as treatment options for CLL. A phase II clinical trial (NCT01461538) is investigating brentuximab vedotin in CD30+ malignancies, including CLL. In addition, a recently launched phase I clinical trial (NCT01290549) is based on an analogous ADC that targets CD79B of the BCR complex in non-Hodgkin lymphoma and CLL. Notably, none of the cell surface antigens targeted by ADCs in clinical trials are overexpressed in CLL. On the contrary, CD79B was not detected on the tumor cells of 43% of the CLL patients tested, while it is expressed on normal B cells (20), and CD30 is expressed at higher levels on activated normal B and T cells compared to CLL cells (21, 22). Thus, the current panel of clinically investigated ADCs for CLL does not bypass immunosuppression, underscoring the need for pursuing new ADCs that selectively target CLL.
The Fc receptor for IgM (FcμR), also known as TOSO or Fas apoptotic inhibitory molecule 3 (FAIM3), is highly expressed on CLL cells (23-26). FcμR is a ~60–kDa type I single-pass transmembrane protein whose expression in normal human cells and tissues is virtually restricted to the lymphoid lineage (23, 27). FcμR-deficient mice are viable and exhibit normal development (28-30). However, they have reduced numbers of marginal zone B cells (29), and FcμR-deficient B cells are less responsive to BCR stimulation and more readily undergo apoptosis. FcμR is overexpressed on the CLL B cells compared to B cells from normal donors and compared to the non-clonal T cells in the blood of CLL patients. FcμR may play a role in the pathogenesis of CLL, possibly by contributing to concomitant BCR and TLR activation that could enhance leukemic cell proliferation and survival (26, 31, 32). However, exactly what functional role, if any, FcμR has in the pathogenesis of CLL remains to be defined.
Fc receptors are highly effective in targeting specific molecules for binding to and internalization into select target cells. The existence of a variety of distinct Fc receptors with restricted cellular expression provides the basis for selective targeting approaches. For example, FcγRIII on dendritic cells has been used for internalization of vaccine components (33). However, delivering cytotoxic agents through Fc receptor-mediated internalization as anti-cancer therapy has not been established. In previous work we have shown that FcμR on CLL cells is functional, rapidly internalizes IgM, and delivers it to the lysosome where it is degraded (26). In fact, more than 50% of IgM bound to the CLL cell surface is internalized via FcμR within 1 min, and internalization is complete by 5 min. We hypothesized that the overexpression of FcμR on CLL cells and its ability to internalize bound IgM could be employed for targeted delivery of anti-leukemic therapy.
Here we describe the derivation of an FcμR targeting strategy that can deliver a cytotoxic payload selectively into CLL cells and show that it has anti-tumor activity against primary human tumor cells in vivo. Based on the work by Kubagawa and colleagues we knew that binding of IgM to FcμR critically depends on the integrity of sequences in the Fc region of IgM (Fcμ) in particular the Cμ2-4 domains (23). We therefore engineered a protein scaffold consisting of Fcμ and chose a site directed conjugation technology we have previously developed (34, 35). In this approach the introduction of a C-terminal selenocysteine (Sec) makes site-specific as opposed to random conjugation of the payload possible thereby preserving the structure of critical protein domains.
Materials and Methods
Primary cells and cell lines
With written informed consent peripheral blood was collected from treatment naïve CLL patients at the Clinical Center, National Institutes of Health (Bethesda, MD; www.clinicaltrials.gov identifier NCT00923507). PBMC were isolated by gradient centrifugation (Lymphocyte Separation Medium, MP Biomedicals) and cultured in serum-free AIM-V medium (Life Technologies). Mantle cell lymphoma cell lines Mino, JeKo-1, and HBL-2 were grown in RPMI 1640 medium (Life Technologies) and HeLa cells in DMEM (Lonza) supplemented with 10% (v/v) fetal bovine serum (HyClone), 2 mM L-glutamine, 1,000 units/mL penicillin G, and 100 mg/mL streptomycin (all from Life Technologies).
Confocal immunofluorescence microscopy
We monitored Fcμ-Sec internalizationas previously described (26). HeLa cells stably transfected with FcμR were grown on coverslips, incubated for 15 min at 4°C with Cμ3-Cμ4-Sec, washed with ice-cold PBS, and incubated at 37°C with DMEM (Lonza) for 30 or 120 min. Cells were then fixed with 3% (w/v) paraformaldehyde (Electron Microscopy Sciences), washed with PBS, and incubated in Staining Buffer (0.05% (w/v) saponin, 10 mM glycine, and 5% (v/v) FBS in PBS) for 15 min. Cells were co-stained with rabbit anti-human LAMP-1 pAbs (Abcam) for 1 h, washed twice with PBS, and incubated with donkey anti-human Fcμ pAbs conjugated to DyLight 488 and donkey anti-rabbit IgG pAbs conjugated to Cy5 (all from Jackson ImmunoResearch Laboratories). Cells were then washed twice with PBS and labeled for 5 min at room temperature with 1 μg/mL Hoechst 33258 (Life Technologies) diluted in Staining Buffer. Subsequently, coverslips were washed twice with PBS, mounted with Fluoromount-G (SouthernBiotech), and visualized by confocal microscopy. Images were acquired using a Leica TCS SP5 laser scanning confocal microscope (LAS AF software) using the HCX PLAPO 63X objective (numerical aperture: 1.4). Images were processed with Adobe Photoshop and analyzed using the same settings.
Flow cytometry
Mino, JeKo-1, and HBL-2 cells were stained with Cμ3-Cμ4-Sec/DyLight 488 or Cμ2-Cμ3-Cμ4-Sec/DyLight 488. FcμR expression in cryopreserved PBMC samples from 30 CLL patients with a mouse anti-human FcμR mAb (Abnova) and a FITC-conjugated goat anti-mouse secondary antibody (Sigma-Aldrich) as previously described (26). B cells and normal T cells were distinguished with an APC-conjugated mouse anti-human CD19 mAb and a PE-conjugated mouse anti-human CD3 mAb (BD Biosciences), respectively. All samples were analyzed using a FACSCanto II flow cytometer (Becton-Dickinson) and FlowJo software (Tree Star).
In vitro cytotoxicity assays
Cytotoxicity toward MCL cell lines in vitro was measured by using the CellTiter 96 Aqueous Cell Proliferation Assay (Promega). Briefly, Mino or HBL2 cells were distributed in a 96-well flat bottom plate (Corning) at a density of 5×104 cells/well. Subsequently, compound 1a (0.001-1,000 nM) or Fcμ-Sec protein selectively conjugated to compound 1b (1-100 nM) were added to the cells. In parallel, unconjugated Fcμ-Sec protein (1-100 nM) was also tested. The cells were exposed to the compound 1a and 1b reagents for 1 h at 37°C, washed three times with RPMI 1640 medium, and incubated for an additional 71 h at 37°C. After adding 20 μL Assay Reagent to each well, the cells were further cultured for 3 h at 37°C and the absorbance at 490 nm was measured using a SpectraMax M5 microplate reader with SoftMax Pro software. Data were computed as mean ± SD of triplicates.
Ex vivo cytotoxicity assays
Cytotoxicity toward primary malignant B cells and primary normal T cells ex vivo was measured by flow cytometry using TO-PRO-3 and Annexin V staining. Briefly, cryopreserved PBMC samples from 30 different CLL patients (5 × 105 cells in 100 μL serum-free AIM-V medium) were incubated with 100 nM compound 1a, Cμ2-Cμ3-Cμ4-Sec/compound 1b conjugate, Cμ2-Cμ3-Cμ4-Sec alone, or were left untreated for 1 h at 37°C, washed and then cytotoxicity against malignant B cells and normal T cells was evaluated by flow cytometry using PE-conjugated Annexin V, a PE-Cy5-conjugated mouse anti-human CD19 mAb, a FITC-conjugated mouse anti-human CD3 mAb (all from BD Biosciences), and TO-PRO-3 (Invitrogen) according to the manufacturer's instructions.
Circulatory half-life
Two groups of two NSG mice (JAX strain 5557; The Jackson Laboratory) were injected i.v. (tail vein) or i.p. with 5 mg/kg of the Fcμ-carrier protein (Cμ2-Cμ3-Cμ4-Sec). One control mouse was injected with PBS. Blood was collected via the tail vein at 0.5, 24, 48, and 72 h after injection and serum was isolated. Sera were diluted 25-fold in 1% (w/v) BSA in PBS and analyzed by sandwich ELISA. To do this, 100 ng of rabbit anti-human Fcμ pAbs (Jackson ImmunoResearch Laboratories) was coated on a 96-well Costar 3690 plate (Corning). After blocking with 3% (w/v) BSA in PBS, the diluted sera or purified Cμ2-Cμ3-Cμ4-Sec as standard were added to duplicate wells, washed 10 times with PBS, and incubated with a mouse anti-His6 mAb conjugated to HRP (GenScript). All steps were carried out for 1 h at 37°C. The plate was washed 10 times with PBS and colorimetric detection was performed using 2,2’-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS; Roche) as substrate according to the manufacturer's instructions. Absorbance values were measured at 405 nm using a Victor3 plate reader (PerkinElmer).
NSG/CLL xenograft model
The NSG/CLL xenograft model was first introduced by Bagnara et al. (40). We used a modified protocol established in our laboratory that replicates important aspects of CLL biology and that we have successfully used in drug studies (41). Mice were housed and handled in accordance with the guidelines set by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute, Bethesda, MD. All animal experiments were carried out on an approved animal protocol. Thirty NSG mice were preconditioned with an i.p. injection of 25 mg/kg busulfan (Otsuka America Pharmaceutical) and on the following day (day 1) i.v. injected with 5 × 107 PBMC from one out of four CLL patients. All mice were bled on day 4 and PBMC were isolated by gradient centrifugation using Lymphocyte Separation Medium. On day 4, 6, and 8 different cohorts of mice were i.v. injected with 10 mg/kg Cμ2-Cμ3-Cμ4-Sec carrier protein or Cμ2-Cμ3-Cμ4-Sec/compound 1b conjugate or an equal volume of PBS. On day 11, retro-orbital puncture bleeds and spleens were harvested from all mice. PBMC were isolated as above. Spleens were homogenized using the gentleMACS Dissociator (MiltenyiBiotec). The homogenate was filtered through a 70-μm nylon cell strainer (BD Biosciences) and washed with ACK Lysing Buffer (Quality Biological) to remove erythrocytes and platelets. PBMC and splenocytes were stained with a mouse anti-human CD45 mAb conjugated to V450, a mouse anti-human CD19 mAb conjugated to PE-Cy5, a mouse anti-human CD3 mAb conjugated to FITC, PE-conjugated Annexin V (all from BD Biosciences), and TO-PRO-3 (Life Technologies), and then analyzed by flow cytometry as described above. Normalized titers of human lymphocyte populations in PBMC isolated on day 4 and day 11 were determined with 5.0-5.9-μm AccuCountBlank Particles (Spherotech) according to the manufacturer's instructions.
RESULTS
Generation and characterization of Fcμ-Sec
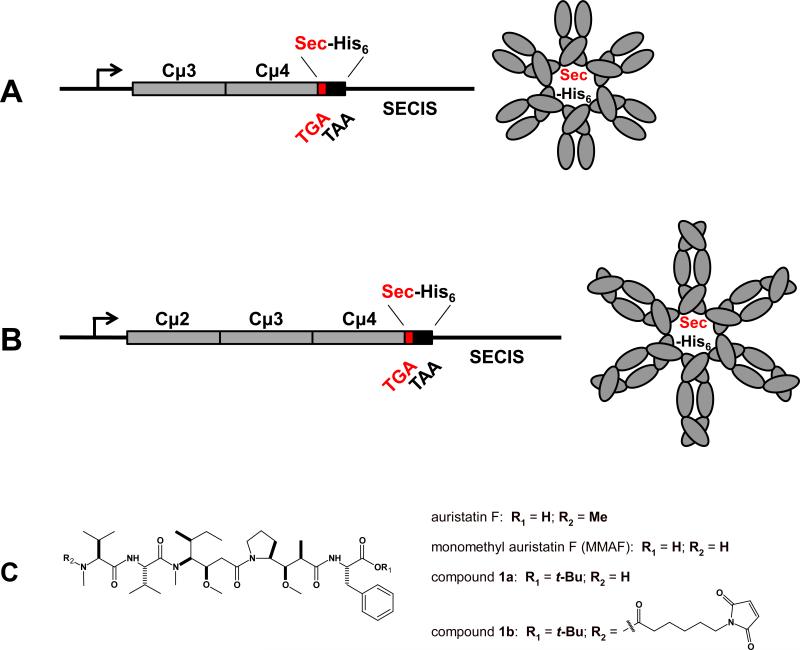
To be able to target a payload to FcμR, we first generated IgM derived protein scaffolds consisting of two (Cμ3-Cμ4) or three (Cμ2-Cμ3-Cμ4) of the C-terminal constant domains of human IgM. In the expression plasmids the Fcμ encoding sequence was followed by a TGA stop codon, a sequence encoding a hexa-histidine (His6) tag, a TAA stop codon, and finally a downstream Selenocysteine (Sec) incorporation sequence (SECIS). In the presence of SECIS the TGA stop codon instructs the translational insertion of Sec (Fig. 1A and B). Because termination at the TGA codon competes with and dominates over Sec insertion, the expression cassette yields a mixture of ~10% Fcμ-Sec and ~90% Fcμ-stop. As we have previously demonstrated for Fcγ-Sec (35), Fcμ-Sec and Fcμ-stop are anticipated to form mixed multimers. The respective Fcμ-Sec proteins were generated in human embryonic kidney (HEK) 293E cells and purified utilizing the His6 tag. Due to pentamerization and hexamerization of IgM in the absence of the J chain (36, 37), Fcμ comprises ten and twelve polypeptide chains, respectively. Thus, based on a 10:1 ratio of terminated polypeptide chains to polypeptide chains having a C-terminal Sec residue, the dominant product of our expression cassette contains a single Sec (Fig. 1A and B).
Figure 1.
Fcμ-Sec engineering and structural formulas of auristatin F derivatives. Schematic of the Fcμ-Sec expression cassettes, which encode A, two or B, three C-terminal constant domains of the heavy chain of human IgM (gray). A C-terminal Sec (red) followed by a His6 tag (black) was introduced by combining a TGA codon (red) with a 3’UTR that contains a SECIS element. The dominant product of these expression cassettes are pentameric and hexameric (shown) proteins with a single Sec-His6-displaying C-terminus. (C) We synthesized a tertiary butyl ester of the tubulin polymerization inhibitor monomethyl auristatin F (compound 1a) and its maleimidocaproyl derivative (compound 1b) for site-specific conjugation to the unique selenol group in Fcμ-Sec.
Both Coomassie staining and Western blotting after reducing SDS-PAGE revealed a ~50-kDa Cμ2-Cμ3-Cμ4-Sec monomer. In contrast, after non-reducing SDS-PAGE high molecular weight multimers similar to whole human IgM were visualized (Supplementary Fig. S1). Using gel filtration chromatography (data not shown), Cμ2-Cμ3-Cμ4-Sec multimers revealed a broad peak corresponding to 500-600 kDa, likely reflecting a mixture of pentamers (~500 kDa) and hexamers (~600 kDa). The Cμ3-Cμ4-Sec monomer revealed the expected lower molecular weight (~40 kDa; Supplementary Fig. S1A) with a diminished propensity to form stable and defined multimers (Supplementary Fig. S1B, and data not shown). Indicating successful incorporation of Sec (35), the His6 tag was detected in both purified Fcμ-Sec proteins (Supplementary Fig. S1C).
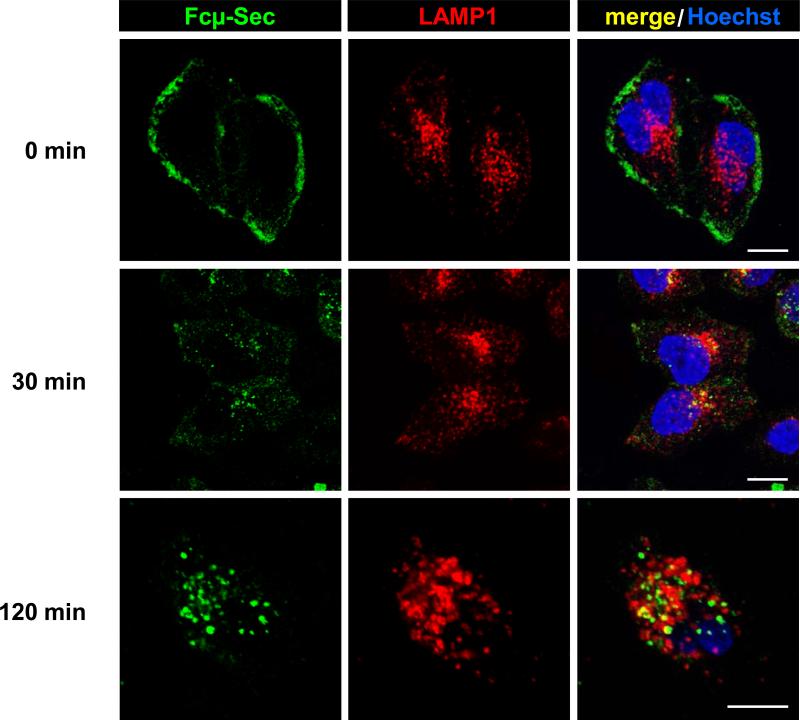
Binding, internalization, and trafficking of human IgM by HeLa cells transiently transfected with human FcμR cDNA was previously shown by flow cytometry and confocal immunofluorescence microscopy (26). We confirmed that purified Cμ3-Cμ4-Sec selectively bound to FcμR-expressing, but not wild-type HeLa cells, followed by rapid internalization and trafficking to lysosomes (Fig. 2). Thus, as described (23), the Fc fragment of IgM is sufficient for FcμR binding, internalization, and trafficking. We also confirmed the binding of both purified Fcμ-Sec proteins to the FcμR expressing mantle cell lymphoma (MCL) cell line Mino using Fcμ-Sec proteins that we labeled in a Sec dependent reaction with a maleimide derivative of DyLight 488. Two other MCL cell lines, JeKo-1 and HBL-2, which do not express FcμR, did not bind Fcμ-Sec/DyLight 488 (Fig. 3A and data not shown).
Figure 2.
Binding, internalization, and trafficking of Fcμ-Sec. HeLa cells stably transfected with human FcμR cDNA were incubated with 25 μg/mL Cμ3-Cμ4-Sec (Fcμ-Sec) for 30 min at 4°C. After washing, the cells were kept at 4°C or were incubated at 37°C for 30 and 120 min. The cells were then fixed, permeabilized, and co-stained with rabbit anti-human LAMP1 pAbs followed by donkey anti-rabbit IgG pAbs conjugated to Cy5 (red) and donkey anti-human Fcμ pAbs conjugated to DyLight 488 (green). Nuclei were stained with Hoechst 33258 (blue). Stained cells were visualized by confocal immunofluorescence microscopy. Note the co-localization (yellow) of Fcμ-Sec and lysosomal marker LAMP1 following internalization. Scale bars, 10 μm.
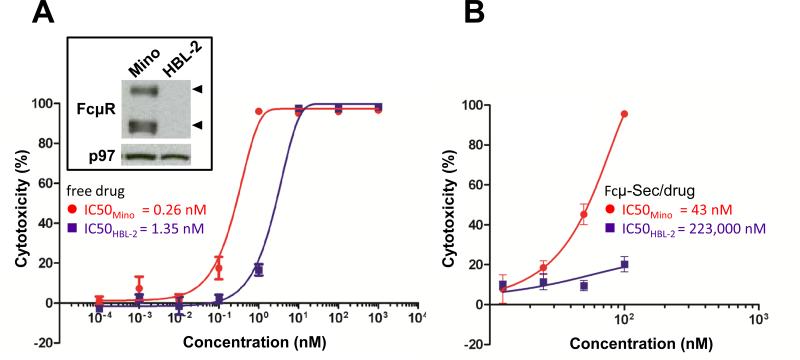
Figure 3.
In vitro cytotoxicity of Fcμ-drug conjugate. A, FcμR is expressed in mantle cell lymphoma cell line Mino, but not HBL-2, as previously shown (15). Western blot of cell lysates probed with anti FcμR and p97 for loading control (inset box). Viability of Mino (red circles) and HBL-2 cells (purple squares) exposed to free MMAF compound 1a was determined using a colorimetric assay. B, The same experiment was carried out with MMAF compound 1b conjugated to Cμ2-Cμ3-Cμ4-Sec (Fcμ-Sec/drug).
Generation and in vitro investigations of Fcμ-drug conjugate
As the cytotoxic payload we chose the tubulin polymerization inhibitor monomethylauristatin F (MMAF). MMAF differs from MMAE (the drug component of brentuximab vedotin) by having a C-terminal phenylalanine (38). As free drug control we synthesized a tertiary butyl ester of MMAF (MMAF-tBu; compound 1a), which freely diffuses into cells through the plasma membrane (38), and for Fcμ-Sec conjugation we synthesized a maleimide derivative with a stable alkyl linker (maleimidocaproyl-MMAF-tBu; compound 1b) (Fig. 1C).
To test the potency and specificity of free vs. conjugated drug, we used MCL cell lines Mino (FcμR-positive) and HBL-2 (FcμR-negative) (Fig. 3A). After exposure for 1 h and chase incubation for 71 h, the free drug potently killed both Mino and HBL-2 cells with IC50 values of 0.26 nM and 1.35 nM, respectively (Fig. 3A). As expected, the Fcμ-drug conjugate was less potent than the freely diffusing drug. Under the same conditions, the Fcμ-Sec-MMAF conjugate killed Mino and HBL-2 cells with IC50 values of 43 nM and 223 μM, respectively (Fig. 3B). Thus, FcμR-positive Mino cells were approximately 5,200-times more sensitive to targeted MMAF than FcμR-negative HBL-2 cells. Taking the different sensitivities to free drug into account, we observed a 1,000-fold increase in potency when FcμR, the target of the carrier protein, was present.
Ex vivo and in vivo investigations of Fcμ-drug conjugate
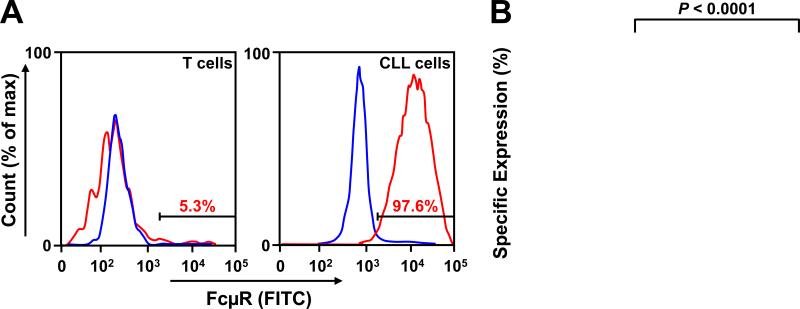
Next we tested whether the Fcμ-Sec-MMAF conjugate can selectively kill primary CLL cells ex vivo. FcμR expression on cryopreserved peripheral blood mononuclear cells (PBMC) from 30 CLL patients (Supplementary Table S1) was measured by flow cytometry. In patients with CLL there are very few or no normal B cells in circulation; therefore >99% of CD19+ cells are CLL cells (31). As expected virtually all CLL cells (CD19+) and much fewer T cells (CD3+) expressed FcμR (Fig. 4). These findings are consistent with previously published observations that FcμR is overexpressed in CLL and that some T cells, especially when activated, can also express FcμR (23), albeit at low cell surface levels (24). FcμR expression was equally high in treatment naïve and previously treated patients (Fig. S4), which indicates for wide clinical applicability of the FcμR targeting strategy.
Figure 4.
Cell surface expression of FcμR on PBMC samples from CLL patients. PBMC samples from 30 untreated CLL patients were analyzed for cell surface expression of FcμR by flow cytometry. A, histograms comparing FcμR expression (red) by CD19+ malignant B cells (left) and autologous CD3+ T cells (right) of a representative sample. The percentages of FcμR+ cells are indicated in red. The isotype control is shown in blue. B, the specific expression of FcμR by CD3+ T cells and CD19+ malignant B cells is plotted for all 30 PBMC samples (for color-code and patient characteristics, please see Supplementary Table S1). A paired two-tailed t-test was used to calculate P.
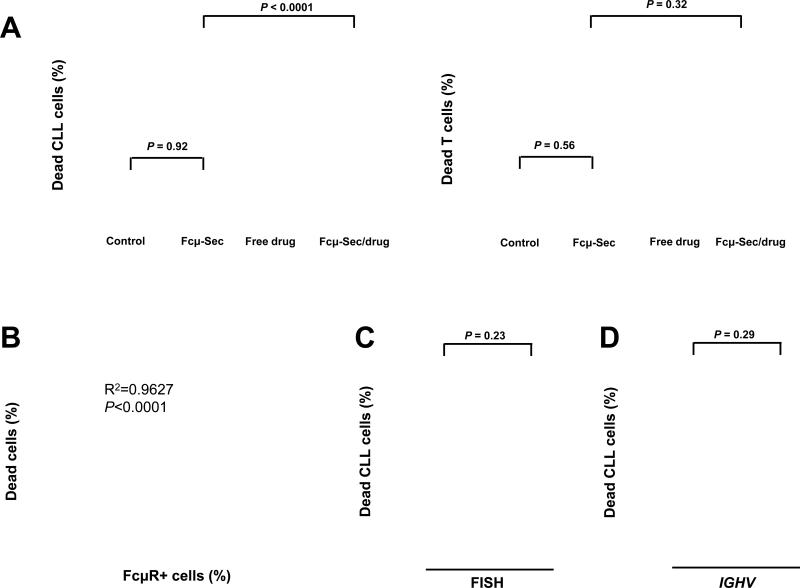
The same 30 CLL PBMC samples were then treated in parallel with 100 nM free MMAF or the Fcμ-Sec-MMAF conjugate for 1h, washed, and then analyzed for cell death 47 h later by flow cytometry using TO-PRO-3 and Annexin V staining. Clearly, malignant B cells were effectively killed by both free and targeted MMAF, whereas normal T cells were only killed by free MMAF (Fig. 5A). In fact, the average viability of CLL B cells was reduced from >90% in controls to <20% in Fcμ-drug treated cultures, while T-cell viability was unchanged. The Fcμ-Sec carrier protein alone did not affect cell viability. This exquisitely selective cytotoxicity and the stark differential expression of FcμR between malignant B cells and T cells provide substantial support to the idea that Fcμ-drug conjugates can be used to selectively deliver cytotoxic payloads to target cells without harming bystander cells (Fig. 5B). The Fcμ-drug conjugate was equally effective against tumor cells with high risk molecular features (expression of an unmutated IGVH gene and/or a deletion of chromosome 17p) and those without these characteristics (Fig. 5C and D).
Figure 5.
Ex vivo cytotoxicity of Fcμ-drug conjugate. PBMC from 30 CLL patients were left untreated (control) or treated with (i) 100 nM Cμ2-Cμ3-Cμ4-Sec (Fcμ-Sec), (ii) 100 nM free MMAF compound 1a (free drug), or (iii) 100 nM MMAF compound 1b conjugated to Cμ2-Cμ3-Cμ4-Sec (Fcμ-Sec/drug). Cell viability was determined by flow cytometry using TO-PRO-3 staining. A, Fcμ-Sec/drug conjugate-mediated cytotoxicity of CD19+ malignant B cells (left panel) and autologous CD3+ T cells (right panel) in PBMC samples from CLL patients (Supplementary Table S1). Paired two-tailed t-tests were used to calculate P. B, Cytotoxicity of the Fcμ-Sec/drug conjugate and expression of FcμR are highly correlated (Spearman correlation: ρ = 0.8431, P < 0.0001). C, and D, the Fcμ-Sec/drug conjugate is equally effective against tumor cells with adverse prognostic features. C, comparison of cytotoxicity against cells with or without a deletion of chromosome 17p (del17p and no del17p, respectively). D, comparison of cytotoxicity against cells of the IGHV mutated (M-CLL) and of the IGHV unmutated CLL subtype (U-CLL).
In preparation for the investigation of the Fcμ-drug conjugate in vivo, we assessed the circulatory half-life of the Fcμ-Sec carrier protein in NOD/SCID/IL-2Rγnull (NSG) mice. After i.v. or i.p. injection of 100 μg (5 mg/kg) Fcμ-Sec in 100 μL isotonic saline, the serum concentration was measured by a sandwich ELISA. Regardless of the route of injection the peak serum concentration ranged between 8-10 μg/mL and the circulatory half-life was ~18 h (Supplementary Fig. S2). In comparison, the reported circulatory half-life of mouse IgM in mice ranges between 24 and 30 hours (39). Thus, despite their differences in molecular weight and composition, Fcμ-Sec and IgM share similar circulatory half-lives.
We chose the recently described NSG/CLL xenograft model to evaluate the activity of the Fcμ-drug conjugate in vivo. In these immunodeficient mice xenografted human CLL cells and the co-injected autologous T cells engraft in the murine blood and spleen (40, 41). Thirty NSG mice were preconditioned with busulfan and on the following day (day 1) i.v. injected with 5 × 107 PBMC (typically composed of ~90% tumor cells, and up to 10% T cells). Each set of mice was injected with cells from one out of four different CLL patients (Supplementary Table S1). On days 4, 6, and 8 half of the mice were i.v. injected with 10 mg/kg Fcμ-Sec-MMAF conjugate and the other half were left untreated. On day 11, mice were terminally bled by retro-orbital puncture and spleens were harvested. PBMC prepared from mouse blood before (day 4) and 72 hours after the last injection of the Fcμ-drug conjugate (day 11) were stained with anti-human CD45 (to identify human cells), CD19, and CD3 mAbs to distinguish malignant B cells (CD45+ CD19+) and normal T cells (CD45+ CD3+). The viability of the xenografted human cells was assessed by flow cytometry using Annexin V and TO-PRO-3 staining.
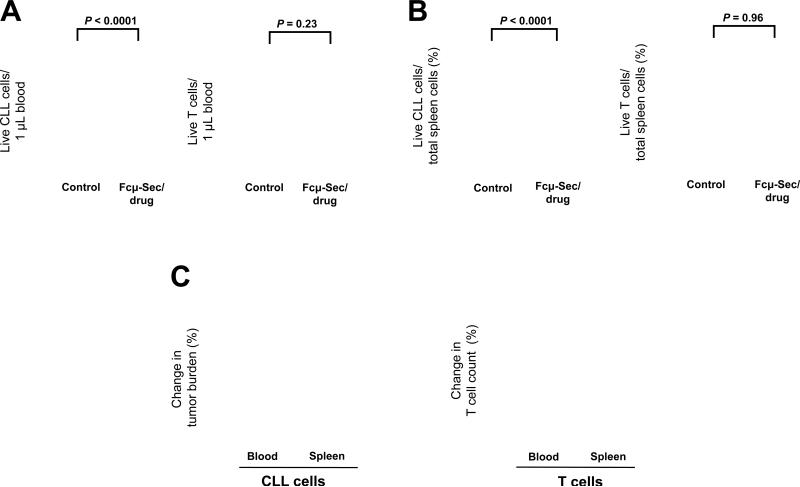
To quantify the reduction in tumor burden on treatment with the Fcμ-Sec-MMAF conjugate we measured a number of different endpoints. First, we determined the leukemic cell count in the blood using flow cytometry and counting beads. There was a substantial decrease in circulating malignant B cells in treated mice compared to controls (Fig. 6A). The average leukemic cell count over the treatment period of one week decreased by 74.4 ± 6.6 compared to untreated mice (P < 0.0001). In contrast there was no change in the number of circulating T cells (Fig. 6A). To be able to quantify the tumor burden in the tissue we determined the number of live malignant B cells relative to the total number of nucleated cells in single cell suspensions prepared from spleens. The majority of these nucleated cells are murine cells that have been used successfully as internal reference in other studies (40, 41). The tumor burden in spleens decreased on average by 64.9 ± 9.7 on treatment with the Fcμ-Sec-MMAF conjugate (P <0.0001) but again there was no effect on T cell numbers (Fig. 6B). The unconjugated Fcμ-Sec carrier protein alone had no effect on the viability of CLL cells nor T cells (Supplementary Fig. S3A and B). Taken together, these in vivo studies revealed substantial reductions of the total tumor burden in both blood (~74%) and spleens (~65%) of mice treated with Fcμ-drug conjugate (Fig. 6C). Consistent with the desired selectivity of this targeted approach, there was no effect on T cells neither in blood nor spleen (Fig. 6C). Notably, none of the mice showed signs of toxicity, and the viability of murine blood cells was not reduced in treated as compared to control mice (data not shown).
Figure 6.
In vivo cytotoxicity of the Fcμ-Sec/drug conjugate. A total of thirty NSG mice were injected i.v. on day 1 with 5 × 107 PBMC from 4 different CLL patients and treated on days 4, 6, and 8 with 10 mg/kg Fcμ-Sec/drug conjugate in PBS or with PBS alone (control) by i.v. injection. On day 11, the absolute numbers of live (TO-PRO-3-negative) CD19+ malignant B cells and autologous CD3+ T-cells in blood and spleen were quantified by flow cytometry. Two-way ANOVA was used to compare cell numbers in treated and control mice. A, the leukemic cell count in the blood of the xenografted NSG mice is significantly reduced by Fcμ-Sec/drug, while there was no change in T cells numbers. B, live CD19+ malignant B cells relative to the total number of live nucleated cells in the spleen are also greatly reduced by Fcμ-Sec/drug. Again T-cells were unchanged. Note that while the human cells engraft in the spleen, the majority of nucleated cells are of murine origin. C, The column graph summarizes the effect of the Fcμ-Sec/drug conjugate on tumor burden (left panel) and T cells (right panel). Shown is the mean +/− SEM.
Discussion
FcμR is overexpressed in CLL and mediates the rapid internalization of IgM by malignant B cells and its trafficking to lysosomes (24-26). In this study, we used an Fcμ-drug conjugate to exploit FcμR for targeted drug delivery. In contrast to conventional ADCs our targeting platform is a recombinant protein scaffold designed to mimic binding of the natural ligand and not a mAb. In other respects our Fcμ-drug conjugate is built on important design principles shared with the recently FDA approved ADCs brentuximab vedotin and trastuzumab emtansine (1-8). Specifically, the target antigen FcμR is overexpressed on tumor cells and rapidly internalized upon ligand binding; the Fcμ carrier protein was engineered with a C-terminal Sec residue for site-specific conjugation to the potent anti-tubulin agent MMAF; finally, use of a non-cleavable linker that can minimize systemic drug release is possible because internalized FcμR travels to the lysosome where the carrier protein is degraded releasing the cytotoxic payload. The resulting Fcμ-Sec-MMAF conjugate selectively killed FcμR-expressing malignant B cells in vitro, ex vivo, and in vivo. Accordingly, our study provides proof-of-concept for FcμR as a therapeutic target in CLL and for Fcμ carrier proteins as new targeting devices.
As the carrier protein we tested two formats of the Fc fragment of IgM carrying a C-terminal Sec residue. Both Cμ3-Cμ4-Sec and Cμ2-Cμ3-Cμ4-Sec were expressed, purified, and conjugated in high yield, and both mediated selective drug delivery in vitro. However, for the subsequent ex vivo and in vivo studies we focused on the larger Cμ2-Cμ3-Cμ4-Sec as it more closely resembled IgM with respect to the formation of stable pentamers and hexamers, which are required for high affinity binding to the target molecule (23). Despite their differences in molecular weight and composition, Cμ2-Cμ3-Cμ4-Sec and IgM shared similar circulatory half-lives (~1 day), which are relatively short compared to IgG (>7 days), but long compared to scFv and other antibody fragments (<1 h). While providing sufficient time for on-target toxicity, a circulatory half-life of ~1 day may diminish off-target toxicity. We conclude that the pharmacokinetic properties of Cμ2-Cμ3-Cμ4-Sec are suitable for targeted drug delivery.
The rationale for using our Sec technology (34, 35) was to enable site-specific as opposed to random attachment of the drug payload. Site-specific conjugation minimizes interference with functional domains of the Fcμ scaffold and has been shown to increase the therapeutic index compared to conventional drug conjugation strategies (42, 43). Given a Sec insertion rate of ~10% (35), the dominant fraction of the purified Fcμ-Sec pentamers and hexamers contains one C-terminal Sec residue, affording a drug to carrier ratio of 1:1. Although this proved to be sufficient for killing malignant B cells in vitro, ex vivo, and in vivo, it is lower than the ratio of 4:1 considered ideal for ADCs generated using non site-specific techniques (2). While the lower drug to carrier ratio may decrease the potency of the current Fcμ-drug conjugate, improvements in site-specific conjugation technologies that increase the number of drug molecules per carrier could further increase the potency of the Fcμ-drug conjugate. For example, we have recently shown that proteins carrying two C-terminal Sec residues can be conjugated with two drug molecules (44).
The linker between carrier protein and payload has to be stable to minimize systemic toxicity while at the same time ensuring effective intracellular drug release. Drug release from non-cleavable linkers such as the alkyl linker used here and in trastuzumab emtansine requires antibody degradation within lysosomes (38, 45). The use of this technology in the targeting of FcμR is made possible by the effective delivery of the internalized complex to the lysosome where it is degraded (26, 46).
FcμR is the only Ig receptor that exclusively binds IgM (23). In addition, the polymeric Ig receptor (PIGR) and the Fcα/μ receptor (FCAMR) bind and internalize both IgM and IgA. Whereas binding of IgA and IgM to PIGR requires the J chain, which is not present in our Fcμ-drug conjugates, FCAMR can mediate IgA and IgM binding in the absence of the J chain (47, 48). As is the case for FcμR, the expression of FCAMR is virtually restricted to specialized immune cells, including follicular B cells and follicular dendritic cells of germinal centers (49). Nonetheless, an assessment of on-target and off-target toxicities of Fcμ-drug conjugates in preclinical and clinical investigations will have to take both FcμR and FCAMR expression by normal cells into consideration.
For the in vivo studies we chose a recently established adoptive transfer model of human primary PBMC from patients with CLL injected into NSG mice, which recapitulates key aspects of tumor biology as seen in patients (40, 41). Both malignant B cells and the corresponding autologous T cells of the patient engraft in the blood and spleen of the mouse, the latter demonstrating tumor cell aggregates reminiscent of human lymph nodes. Thus the model allows testing of potency and selectivity of the Fcμ-drug conjugate in a partially humanized environment. Furthermore, variability in tumor biology among individual patients is, at least partially, reproduced by injecting cells from different patients into separate cohorts of mice. A caveat of this model is that the survival of the mice is not determined by tumor progression and that over time T-cell expansion dominates and can lead to the demise of the animals. We therefore chose the impact of the Fcμ-drug conjugate on tumor burden as the clinical endpoint.
Here we demonstrated effectiveness and selectivity of Fcμ-drug conjugates by comparing the effect on malignant B cells and normal T cells from patients with CLL side-by-side ex vivo and in vivo. With only three injections of the Fcμ-Sec-MMAF over one week we consistently obtained objective responses quantified as >60% reduction in tumor burden. Notably, the tumor samples studied here were primarily from patients with high risk disease characterized by advanced Rai stage and the expression of unmutated IGHV genes, an indicator of more rapid disease progression and reduced benefit from standard treatment. The potent activity of Fcμ-Sec-MMAF against tumor cells from these high risk patients is promising. Using this partially humanized xenograft model, we could also verify a degree of selectivity in vivo; while CLL B cells were killed, we did not observe a decrease in the viability of the autologous T cells. This is consistent with the fact that the malignant B cells overexpress the FcμR while the normal T cells of the CLL patients as well as the normal B and normal T cells of healthy donors have considerably lower expression levels (24, 26). This suggests that FcμR targeting with ADCs will be less damaging to normal cells and tissues than current treatment options in CLL.
Collectively, our study provides proof-of-concept for the therapeutic targeting of the recently identified FcμR with a novel IgM derived protein-drug conjugate. We established the utility of lead components and compositions of Fcμ-drug conjugates that provide opportunities for further optimization of FcμR-targeted drug delivery and translation of this approach into the clinic.
Supplementary Material
Précis.
Findings identify a novel strategy for targeted cytotoxic therapy of Chronic Lymphocytic Leukemia through IgM receptor-mediated drug delivery.
Acknowledgments
We thank Dr. William K. Gillette and his team at the Protein Expression Laboratory, SAIC-Frederick, for custom expression and purification of Fcμ-Sec, and Dr. David J. Fitzgerald for helpful comments on the manuscript.
Financial support: This work was funded by the Intramural Research Programs of the National Heart, Lung, and Blood Institute and the National Cancer Institute, National Institutes of Health, and by National Institutes of Health U01 grant CA174844.
Footnotes
Conflict of interest: The authors have no competing interests.
Author contributions: B.V., M.S., J.D.T., C.G.N., A.D., and G.A. designed, performed, and analyzed experiments and contributed to the manuscript. T.R.B. Jr., C.R., and A.W. designed, analyzed, and oversaw experiments and edited the manuscript. C.R. and A.W. conceived the project and wrote the manuscript.
Data and materials availability: The Fcμ-Sec constructs can be obtained under a Materials Transfer Agreement from the National Institutes of Health.
References
- 1.Adair JR, Howard PW, Hartley JA, Williams DG, Chester KA. Antibody-drug conjugates - a perfect synergy. Expert Opin Biol Ther. 2012;12:1191–206. doi: 10.1517/14712598.2012.693473. [DOI] [PubMed] [Google Scholar]
- 2.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 3.de Claro RA, McGinn K, Kwitkowski V, Bullock J, Khandelwal A, Habtemariam B, et al. U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18:5845–9. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
- 4.Baron JM, Boster BL, Barnett CM. Ado-trastuzumab emtansine (T-DM1): a novel antibody-drug conjugate for the treatment of HER2-positive metastatic breast cancer. J Oncol Pharm Pract. 2014 doi: 10.1177/1078155214527144. [DOI] [PubMed] [Google Scholar]
- 5.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30:631–7. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 6.LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17:6437–47. doi: 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]
- 7.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–9. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 9.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 10.Gribben JG, O'Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–50. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 12.Tam CS, Keating MJ. Chemoimmunotherapy of chronic lymphocytic leukemia. Nat Rev Clin Oncol. 2010;7:521–32. doi: 10.1038/nrclinonc.2010.101. [DOI] [PubMed] [Google Scholar]
- 13.Jaglowski SM, Alinari L, Lapalombella R, Muthusamy N, Byrd JC. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood. 2010;116:3705–14. doi: 10.1182/blood-2010-04-001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tembhare PR, Marti G, Wiestner A, Degheidy H, Farooqui M, Kreitman RJ, et al. Quantification of expression of antigens targeted by antibody-based therapy in chronic lymphocytic leukemia. Am J Clin Pathol. 2013;140:813–8. doi: 10.1309/AJCPYFQ4XMGJD6TI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47:187–98. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–91. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia Vela J, Delgado I, Benito L, Monteserin M, Garcia Alonso L, Somolinos N, et al. CD79b expression in B cell chronic lymphocytic leukemia: its implication for minimal residual disease detection. Leukemia. 1999;13:1501–5. doi: 10.1038/sj.leu.2401511. [DOI] [PubMed] [Google Scholar]
- 21.Trentin L, Zambello R, Sancetta R, Facco M, Cerutti A, Perin A, et al. B lymphocytes from patients with chronic lymphoproliferative disorders are equipped with different costimulatory molecules. Cancer Res. 1997;57:4940–7. [PubMed] [Google Scholar]
- 22.Deutsch YE, Tadmor T, Podack ER, Rosenblatt JD. CD30: an important new target in hematologic malignancies. Leuk Lymphoma. 2011;52:1641–54. doi: 10.3109/10428194.2011.574761. [DOI] [PubMed] [Google Scholar]
- 23.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med. 2009;206:2779–93. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallasch CP, Schulz A, Kutsch N, Schwamb J, Hagist S, Kashkar H, et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood. 2008;112:4213–9. doi: 10.1182/blood-2008-05-157255. [DOI] [PubMed] [Google Scholar]
- 25.Proto-Siqueira R, Panepucci RA, Careta FP, Lee A, Clear A, Morris K, et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood. 2008;112:394–7. doi: 10.1182/blood-2007-11-124065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vire B, David A, Wiestner A. TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol. 2011;187:4040–50. doi: 10.4049/jimmunol.1100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–56. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 28.Choi SC, Wang H, Tian L, Murakami Y, Shin DM, Borrego F, et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J Immunol. 2013;190:987–96. doi: 10.4049/jimmunol.1202227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honjo K, Kubagawa Y, Jones DM, Dizon B, Zhu Z, Ohno H, et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcmuR). Proc Natl Acad Sci U S A. 2012;109:15882–7. doi: 10.1073/pnas.1206567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci U S A. 2012;109:E2699–706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 33.Mende I, Hoffmann P, Wolf A, Lutterbuse R, Kopp E, Baeuerle PA, et al. Highly efficient antigen targeting to M-DC8+ dendritic cells via FcgammaRIII/CD16-specific antibody conjugates. Int Immunol. 2005;17:539–47. doi: 10.1093/intimm/dxh232. [DOI] [PubMed] [Google Scholar]
- 34.Hofer T, Skeffington LR, Chapman CM, Rader C. Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry. 2009;48:12047–57. doi: 10.1021/bi901744t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofer T, Thomas JD, Burke TR, Jr., Rader C. An engineered selenocysteine defines a unique class of antibody derivatives. Proc Natl Acad Sci U S A. 2008;105:12451–6. doi: 10.1073/pnas.0800800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azuma Y, Ishikawa Y, Kawai S, Tsunenari T, Tsunoda H, Igawa T, et al. Recombinant human hexamer-dominant IgM monoclonal antibody to ganglioside GM3 for treatment of melanoma. Clin Cancer Res. 2007;13:2745–50. doi: 10.1158/1078-0432.CCR-06-2919. [DOI] [PubMed] [Google Scholar]
- 37.Tchoudakova A, Hensel F, Murillo A, Eng B, Foley M, Smith L, et al. High level expression of functional human IgMs in human PER.C6 cells. MAbs. 2009;1:163–71. doi: 10.4161/mabs.1.2.7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–24. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 39.Hughey CT, Brewer JW, Colosia AD, Rosse WF, Corley RB. Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol. 1998;161:4091–7. [PubMed] [Google Scholar]
- 40.Bagnara D, Kaufman MS, Calissano C, Marsilio S, Patten PE, Simone R, et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117:5463–72. doi: 10.1182/blood-2010-12-324210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman SE, Sun X, McAuley EM, Hsieh MM, Pittaluga S, Raffeld M, et al. Modeling tumor-host interactions of chronic lymphocytic leukemia in xenografted mice to study tumor biology and evaluate targeted therapy. Leukemia. 2013;27:1769–73. doi: 10.1038/leu.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, et al. Engineered thiotrastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res. 2010;16:4769–78. doi: 10.1158/1078-0432.CCR-10-0987. [DOI] [PubMed] [Google Scholar]
- 43.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–32. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Yang J, Rader C. Antibody conjugation via one and two C-terminal selenocysteines. Methods. 2014;65:133–8. doi: 10.1016/j.ymeth.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13:235–44. doi: 10.1016/j.cbpa.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov A, Beers SA, Walshe CA, Honeychurch J, Alduaij W, Cox KL, et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest. 2009;119:2143–59. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo EM, Trinh KR, Lim H, Wims LA, Morrison SL. Characterization of IgA and IgM binding and internalization by surface-expressed human Fcalpha/mu receptor. Mol Immunol. 2011;48:1818–26. doi: 10.1016/j.molimm.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 49.Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K, et al. Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci U S A. 2009;106:11230–5. doi: 10.1073/pnas.0809917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hopkins RF, Wall VE, Esposito D. Optimizing transient recombinant protein expression in mammalian cells. Methods Mol Biol. 2012;801:251–68. doi: 10.1007/978-1-61779-352-3_16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.