Abstract
Fetal interventions to diagnose and treat congenital anomalies are growing in popularity but often lead to preterm labor. The possible contribution of the maternal adaptive immune system to post-surgical pregnancy complications has not been explored. We recently showed that fetal intervention in mice increases maternal T cell trafficking into the fetus and hypothesized that this process may also lead to increased maternal T cell recognition of the foreign conceptus and subsequent breakdown in maternal-fetal tolerance. Here, we show that fetal intervention in mice results in accumulation of maternal T cells in the uterus and that these activated cells can produce effector cytokines. In adoptive transfer experiments, maternal T cells specific for a fetal alloantigen proliferate after fetal intervention, escape apoptosis, and become enriched compared to endogenous T cells in the uterus and uterine-draining lymph nodes. Finally, we demonstrate that such activation and accumulation can have a functional consequence: in utero transplantation of hematopoietic cells carrying the fetal alloantigen leads to enhanced demise of semiallogeneic fetuses within a litter. We further show that maternal T cells are necessary for this phenomenon. These results suggest that fetal intervention enhances maternal T cell recognition of the fetus and that T cell activation may be a culprit in post-surgical pregnancy complications. Our results have clinical implications for understanding and preventing complications associated with fetal surgery such as preterm labor.
Keywords: maternal immune response, fetal intervention, T cell activation, indirect reactivity, direct reactivity, preterm labor
Introduction
Fetal surgery is a promising strategy to treat fetuses with severe or fatal congenital anatomic anomalies such as diaphragmatic hernias or spina bifida (1). Beyond these conditions, fetal stem cell transplantation has the potential to cure congenital immunodeficiencies and hematopoietic stem cell disorders (2). However, fetal intervention is often associated with preterm labor (PTL), a complication that severely limits the widespread use of this approach (3). Clinical trials of fetal surgery have consistently demonstrated that frequent and severe PTL dampens much of the therapeutic benefit of the fetal intervention (4, 5). While PTL is a complication that curtails our ability to offer fetal treatments for congenital anomalies, the precise mechanisms that lead to PTL after surgery are poorly understood.
Pregnancy is the most robust form of allograft tolerance and multiple mechanisms protect the semi-allogeneic fetus from the maternal immune system (reviewed in (6-8)). The fetus is specifically protected from maternal effector T cells (Teff) by a unique combination of biological mechanisms that impede Teff function (reviewed in (9)). For example, it has been demonstrated that maternal T cells recognize the fetal allograft primarily using the relatively inefficient “indirect” pathway of antigen presentation (in which fetal antigen is presented by maternal antigen presenting cells, APCs) and that these indirectly-reactive T cells undergo clonal deletion after activation (10). Directly-reactive T cells (which recognize antigen presented by fetal APCs) represent a higher percentage of alloreactive T cells (11) but are not activated in normal pregnancy. Pregnancy is also associated with an increase in maternal regulatory T cells (Tregs)(12-19) whose loss leads to elimination of the semi-allogeneic fetus (13-15, 17-19). However, it is not known whether these maternal-fetal tolerance mechanisms are thwarted during after fetal intervention, leading to recognition and rejection of the fetus by maternal T cells. Since fetal surgery can trigger PTL without overt infection, it is possible that inflammation from surgical trauma can activate maternal T cells.
While patients experience preterm labor after fetal surgery, murine fetal intervention instead results in resorption of some of the fetuses in the litter. Resorption has also been observed during T cell-mediated rejection early in pregnancy in mice (15, 20, 21) but the possible contribution of maternal T cells to resorption after fetal intervention has not been examined. We have previously reported that fetal stem cell transplantation increases maternal T cell trafficking into the fetus and that these T cells limit the engraftment of transplanted cells in mice (22). Given that fetal injection also causes resorption, we hypothesized that maternal T cell activation after fetal intervention could perturb maternal-fetal tolerance, leading to enhanced maternal T cell recognition of the semi-allogeneic fetus and, possibly, increased fetal loss. We therefore tested whether various complementary methods of fetal intervention result in maternal T cell activation and used transgenic tools to study the antigen-specificity of such activation. We demonstrate that after fetal intervention, maternal T cells become activated and accumulate in the uterus, where they assume an effector phenotype. Furthermore, maternal T cells can exacerbate selective demise of allogeneic fetuses when triggered by an additional dose of paternal antigen. These results suggest that medical interventions to inhibit maternal T cells could be beneficial in treating pregnancy complications after fetal intervention.
Materials and Methods
Reagents and Antibodies
The following reagents were used: Invitrogen Vybrant® CFDA SE Cell Tracer Kit (CFSE, Invitrogen), Ficoll-Paque Plus (GE Healthcare), Annexin V (BD Pharmingen), Qdot 605 Strepavidin conjugate (Invitrogen), Foxp3 staining buffer set (eBiosciences), 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Invitrogen), Lipopolysaccharide from Salmonella abortus equi S-form (TLR-grade™) (LPS, Alexis Biochemicals), DAPI vector shield (Vector Laboratories), Paraformaldehyde Aqueous Solution (Electron Microscopy Science), LIVE/DEAD Cell Viability Dye (Invitrogen), DNase I (Roche), Collagenase D (Roche), Alexa Fluor 488 Goat Anti-Rat IgG Antibody (Life Technologies), Triton-x 100 (Sigma), Bovine Serum Albumin (Fisher Scientific), Goat serum (Jackson ImmunoResearch Laboratories, Inc), Sucrose (Fisher Scientific), Tissue-Tek OCT Compound (VWR), Phorbol 12-myristate 13-acetate (PMA) (Sigma), Ionomycin (Sigma), and Brefeldin A (Sigma). The following antibodies for flow cytometry were purchased from Becton Dickinson: CD3 (145-2C11), CD8 (53-6.7), CD19 (1D3), CD45 (30-F11), CD45.1 (A20), CD45R/B220 (RA3-6B2), H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), H-2Kk (36-7-5), I-Ad (AMS-32.1), Thy1.1 (HIS51), Vβ8 (F23.1), Vβ13 (MR12-3), Ki67 (B56), NK1.1 (PK136); EBiosciences: CD4 (RM4-5), CD8 (53-6.7), CD45.1 (A20), CD45.2 (104), CD25 (PC61.5), Foxp3 (FJK-16s), IL-17A (eBio17B7), TNF-α MP6-XT22), IFN-y (XMG1.2), CD11c (N418), IgG2a,k isotype control (eBM2a); Southern Biotech: CD8 (53-6.7); UCSF Hybridoma Core: Gr-1 (RB6-8C5), Fc receptor (2.4G2); Biolegend: CD25 (3C7), H2-Ld (28-14-8), IgG2a,k isotype control (MOPC-173); Abcam: CD3 (RM0027-3B19).
Mice
The inbred strains, BALB/c, C3H-HeJCr, C57BL/6.CD45.2 (B6), and the F1 hybrid strain, B6 x BALB/c (F1), were obtained from either NCI or Jackson Laboratories. BALB/c.CD45.1 were obtained from Dr. Michelle Hermiston (UCSF) and B6. Thy1.1.2C (2C), B6.Thy1.1.4C (4C), and B6.Thy1.1.TCR75 (TCR75) mice were obtained from Dr. Sang-Mo Kang (UCSF). All mice were bred and maintained in a specific pathogen-free facility at UCSF. All mouse experiments were performed according to the UCSF Institutional Animal Care and Use Committee approved protocol. B6 females used were all nulliparous.
In utero injections
Fetal mice were injected with PBS, LPS, or hematopoietic cells (5 ul/fetus in all experiments) directly into the fetal liver using pulled glass micropipettes as previously described on embryonic day (E) 13.5-14.5 (22, 23). The pregnant dam was anesthetized, a laparotomy was performed, and the fetuses were injected through the intact uterus. For LPS injections, a dose of 0.5ug was diluted among the total fetuses in the litter. We based our LPS concentration on a reported model of intrauterine injection of LPS (24) and performed titration experiments to achieve a dose that resulted in the loss of the litter within 40 hours without maternal death (0.5 ug, data not shown). For allogeneic stem cell transplantation experiments, hematopoietic cells were harvested from fetal livers of E13.5-E14.5 donor fetuses as previously described (22) and 2.5x106 cells were injected into each fetus. For experiments involving survival of the semi-allogeneic and syngeneic fetuses, peripheral blood leukocytes were stained for H-2b and H-2d to genotype the surviving BALB/c (H-2d) and F1 (H-2b/H-2d) pups at the time of weaning.
Tissue harvesting and processing
Tissues at the maternal-fetal interface were harvested by separating the uterus from the fetus and placenta; deciduas could be further separated from the placentas in live fetuses. Fetal livers were harvested from E13.5–E14.5 fetuses in PBS. Single-cell suspensions were made by gently pipetting the fetal livers and filtering through a 70-μm Nitex filter. Tissues surrounding each fetus were processed separately with DNase I (5 ug/ml) and Collagenase D (400 U/ml) to make a single cell suspension. Cells were also harvested from spleens, uterine draining lymph nodes (udLNs; para-aortic), and non-draining lymph nodes (ndLNs; inguinal, axillary, brachial and mesenteric). For surface staining experiments, tissues surrounding individual fetuses were each analyzed separately whereas for intracellular cytokine staining and adoptive transfer experiments, all resorbed or all non-resorbed uterine segments within a dam were pooled to obtain adequate cell numbers. After staining with the indicated antibodies, samples were analyzed on a LSRII flow cytometer using FACS Diva or FlowJo software.
Intracellular cytokine stain
Maternal lymphocytes were stimulated with PMA (70 ng/ml) and Ionomycin (70 ng/ml) for three hours, treated with Brefeldin A (200 mg/ml) for two hours, then stained for flow cytometry.
Proliferation of adoptively transferred fetal antigen-specific lymphocytes
Whole lymphocytes were harvested from the spleen and lymph nodes of TCR75, 2C or 4C mice and labeled with CFSE. 1-5x106 T cells were adoptively transferred intravenously into pregnant females at E12.5 followed by injection of PBS or LPS into the fetuses one day later. Five days after in utero injection, the dams were sacrificed and the adoptively transferred T cells were identified in the maternal and fetal tissues by their congenic marker, Thy1.1. Positive controls for 2C and 4C proliferation were B6 females sensitized with 5x106 BALB/c splenocytes intravenously prior to adoptive transfer for 2C or 4C cells.
Statistics
Differences between two groups were evaluated using either a Chi-square test (χ2, for changes in survival) or a Student's t test (or Mann-Whitney test, for non-normally distributed data) and those among more than two groups were evaluated using ANOVA with Tukey's multiple comparison test (or Kruskal-Wallis with Dunn's post-test, for non-normally distributed data) using Graphpad Prism. P values of less than 0.05 were considered to be significant. Data are summarized as means ± SEM.
Results
Fetal PBS injection leads to increased resorption in allogeneic matings compared to syngeneic
We used our established method of fetal intervention (injection into the fetal liver through an intact uterus (22)) to study maternal T cell activation. We bred B6 females to B6 (syngeneic) or BALB/c (allogeneic) males and injected the fetuses with phosphate-buffered saline (PBS) to study the effect of surgical trauma alone, or with lipopolysaccharide (LPS), to study the effect of trauma along with a strong inflammatory stimulus on fetal survival (Table 1). Baseline resorption in this allogeneic strain combination is low and we observed increased fetal loss with PBS injection in syngeneic matings compared to no intervention, indicating there is some fetal loss secondary to the trauma of the intervention. However, there was a significantly higher rate of resorption in allogeneic matings compared to syngeneic (χ2=0.04) with PBS injection, suggesting the contribution of an adaptive immune response to this process. With LPS injection, which provides a stronger innate inflammatory stimulus, there was near-total resorption in most experiments, which precluded discerning a difference between syngeneic and allogeneic matings (χ2=0.16). We therefore proceeded to define whether T cells become activated in the PBS injection model and to devise other experimental breeding schemes to read out a possible functional effect of such activation.
Table 1. Complementary models of fetal intervention in mice.
| Models of Fetal Intervention | Rationale | % Resorption | # of litters (L)/# of fetuses (F) |
|---|---|---|---|
| Uninjected | Baseline | Syngeneic - 3.2±2 Allogeneic - 1.5± 1 |
7L/58F 12L/114F |
| PBS injection | Sterile inflammation | Syngeneic - 34±7 Allogeneic - 44±6* |
20L/155F 43L/355F |
| LPS injection | Strong inflammatory stimulus | Syngeneic - 90±6 Allogeneic - 79±4 |
10L/74F 32L/252F |
χ2 =0.04 between Syngeneic and Allogeneic matings undergoing fetal PBS injection;
χ2=NS between Syngeneic and Allogeneic matings in uninjected and LPS injected groups.
n≥4 independent experiments in each group.
Maternal effector T cells accumulate in the uterus after fetal intervention
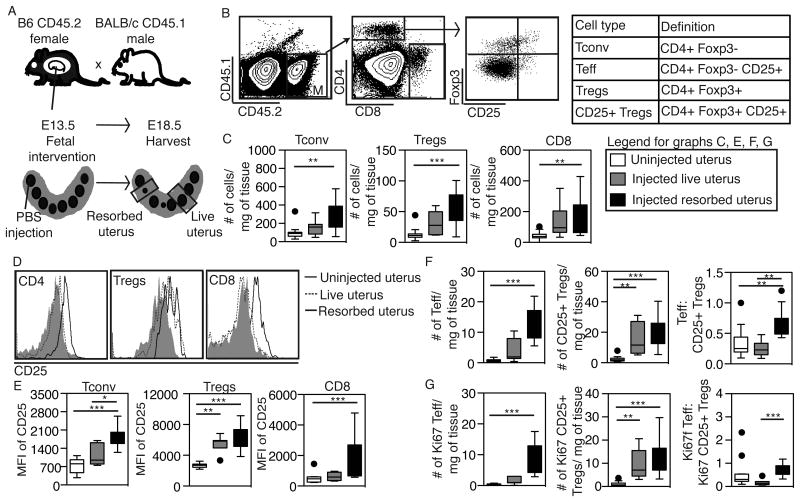
To determine whether fetal intervention results in expansion and proliferation of maternal lymphocytes at the maternal-fetal interface, we bred B6.CD45.2 females to BALB/c.CD45.1 males, injected fetuses with PBS on E13.5, and phenotyped the maternal lymphocytes in the uterus on E18.5 using flow cytometry (Figure 1A). Since some fetuses are resorbed after injection while littermates are not, we analyzed the uterus surrounding resorbed fetuses (“resorbed uterus”) separately from the uterus surrounding live fetuses (“live uterus”) (Figure 1A). To further define the maternal T cell population, we used congenic alleles of CD45 to distinguish maternal and fetal cells when harvesting tissues at the maternal-fetal interface by flow cytometry as previously described (22) (Figure 1A,B).
Figure 1. Maternal T cells accumulate in the uterus after fetal intervention.
(A) Breeding scheme to identify maternal T cells in uterine segments surrounding fetuses (some of which are resorbed) after fetal intervention. Maternal tissues were harvested 5 days after fetal PBS injection. (B) Gating strategy for identifying subsets of maternal CD4 (defined in table) and CD8 T cells. Representative plot of CD25 and Foxp3 expression in an injected resorbed uterus is shown. (C) The absolute number of Tconv cells, Tregs, and CD8 T cells per mg of tissue in various experimental conditions. (D) Representative histograms and (E) the mean fluorescence intensity (MFI) of CD25 on Tconv, Tregs, and CD8 T cells in uninjected, live and resorbed uteri. (F-G) The absolute numbers of total (F) or Ki67+(G) Teff and CD25+ Tregs per mg of tissue and the ratio of these two cell types in uninjected, injected live, and injected resorbed uteri. Data in C-F represent n≥7 uterine segments in ≥2 independent experiments and each point represents a uterine segment surrounding one fetus. *P < 0.05, ** P ≤ 0.01, ***P ≤ 0.001, by Kruskal-Wallis test with Dunn's post-hoc comparison.
We first analyzed the uterine T cell composition to detect changes in effector and regulatory T cell subsets (Figure 1B). The numbers of conventional Foxp3- CD4 T cells (Tconv) and CD8 cells increased after fetal intervention, with significant increases in resorbed uteri compared to uninjected (Figure 1C). We also detected an increase in the number of Foxp3+ CD4 T cells (Tregs) in the resorbed uterus, as has been reported in other models of inflammation (25, 26). In addition, CD25 expression increased on all of these T cells subsets after fetal intervention (Figure 1D,E). CD25 expression on CD4 T cells further increased in resorbed compared to live uteri, suggesting increased activation of effector cells in this setting (Figure 1E). When we enumerated CD25+ effector and regulatory CD4 cells in the uterus, we found increases in the number of CD25+ T cells (Teff) in resorbed uteri compared to live uteri, such that the overall Teff/Treg ratio was significantly increased in resorbed uteri (Figure 1F). Given these increases in cell numbers, we next asked whether proliferation of certain T cell subsets increased after fetal intervention using Ki67 staining. We noted an increase in the proliferation of both Teff and CD25+ Tregs, with a higher proportion of cycling Teff to CD25+Tregs in resorbed uteri (Figure 1G). Collectively, these results indicate that fetal intervention leads to inflammation in the uterus, with an increase in local T cell and Treg activation and proliferation. In resorbed uterine segments, the net effect is a shift in the effector to regulatory T cell balance.
We also analyzed other leukocyte populations in the uterus and found increases in the percentage of Gr-1+ myeloid cells (both Gr1low (monocytes) and Gr1high (neutrophils)) after fetal intervention (Figure S1). There were no differences in the percentages of NK cells, B cells, or dendritic cells between groups (Figure S1).
Increased IFN-γ production by uterine T cells after fetal intervention
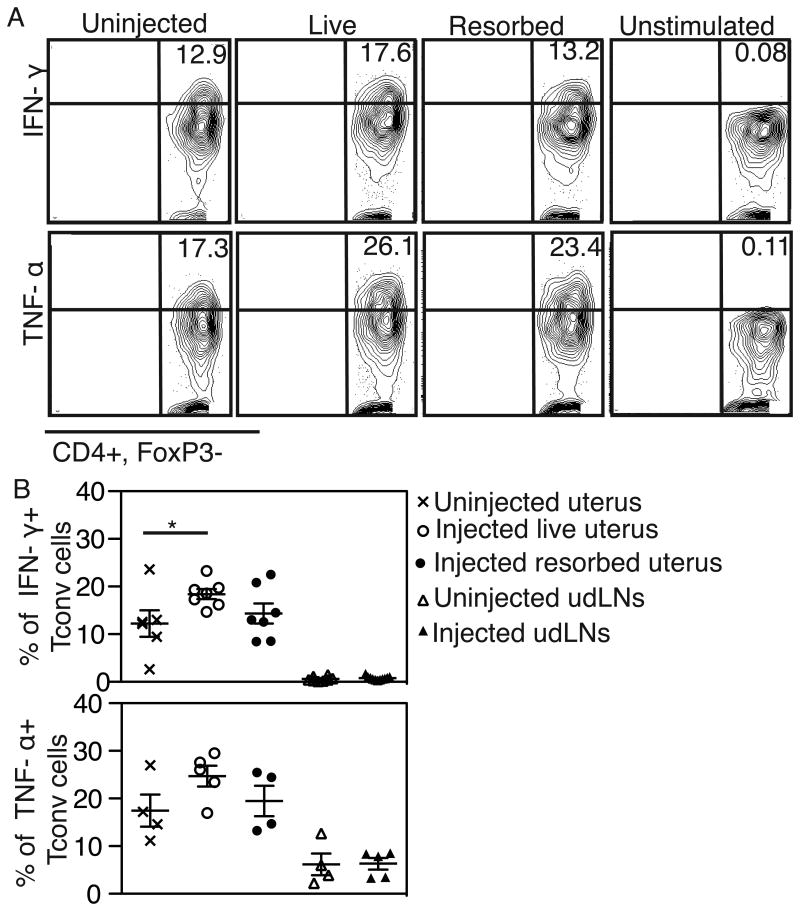
To determine whether the increased CD4 T cells in the uterus have functional significance, we next asked whether they produce effector cytokines. We harvested lymphocytes from the maternal uterus and uterine draining lymph nodes (udLNs) after fetal PBS injection, stimulated them with PMA and ionomycin, and stained for the intracellular cytokines IFN-γ, TNF-α and IL-17 (Figure 2A, IFN-γ and TNF-α in a representative experiment shown). We found a significant increase in the percentage of IFN-γ producing CD4 T cells and a trend for increased percentage of TNF-α producing cells in the uterus after fetal intervention (Figure 2B). Interestingly, for both cytokines, the percentage of cytokine-producing cells was significantly higher in the uterus than in the udLNs even in normal pregnancies (Figure 2B). There were no changes in IL-17 production with fetal intervention (Figure S2). Thus, maternal T cells resident in the uterus can assume an effector phenotype after fetal intervention.
Figure 2. Increased IFN-γ production by uterine T cells after fetal intervention.
(A) Representative flow cytometry plots of uterine CD4 Tconv cells after stimulation with PMA and ionomycin to detect the production of IFN-γ (top) and TNF-α (bottom). Maternal tissues were harvested 5 days after fetal PBS injection in an allogeneic mating. Percentages obtained in one representative experiment shown. (B) The percentage of Tconv cells producing a given cytokine in uterine segments and uterine draining lymph nodes (udLNs) harvested from pregnant dams with or without fetal injection of PBS. Each point represents tissues from one dam. N ≥ 4 dams per group in n≥4 independent experiments. *P < 0.05 by ANOVA with Tukey's multiple comparison test.
We also examined cytokine production (IFN-γ, TNF-α and IL-17) by Tregs to determine whether they also assume an effector phenotype in the context of inflammation. We detected no changes in cytokine production by Tregs for any of the cytokines examined (Figure S2).
Fetal intervention results in activation, proliferation, and accumulation of antigen-specific maternal T cells
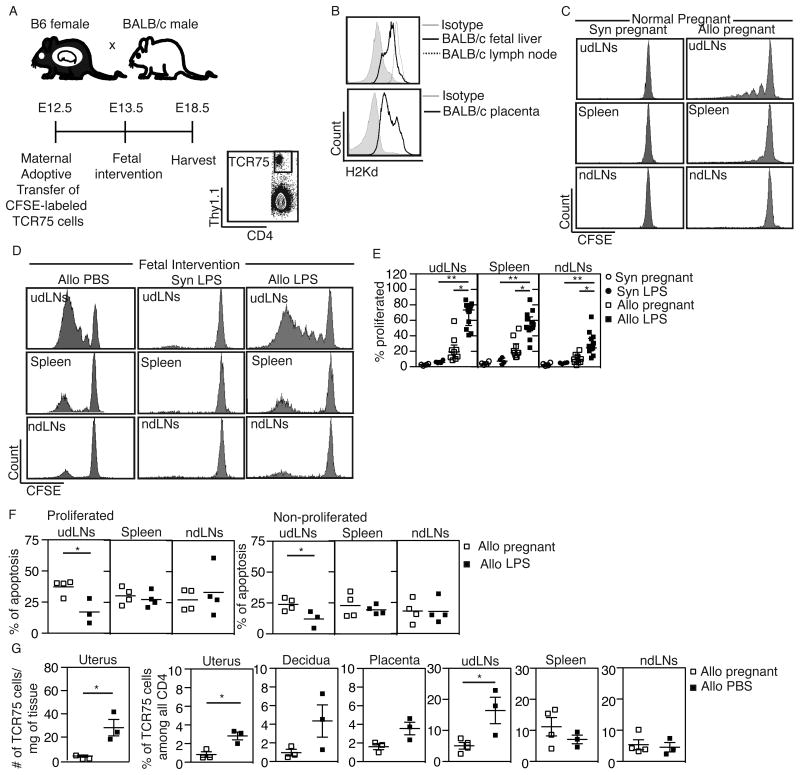
It is possible that the global T cell infiltration and activation we observed after fetal intervention is secondary to inflammation and that these T cells are not specific for fetal or placental antigens. To determine whether maternal T cell activation after fetal intervention is antigen-specific, we adoptively transferred fetal antigen-specific T cells from T cell receptor transgenic mice and analyzed their proliferation and accumulation in maternal tissues after fetal intervention. Maternal T cells may recognize fetal antigen presented by maternal APCs (indirect pathway) or by fetal APCs (direct pathway). Previous reports showed the predominance of the indirect pathway in normal pregnancy (10), using a model in which the β-actin promoter drives the expression of the fetal antigen (10, 17). To quantify maternal T cell activation to a fetal alloantigen that is endogenously expressed, we used TCR 75 mice (27), which have CD4 T cells that recognize fetal BALB/c class I antigen (H-2Kd) presented by B6 (maternal) APC. We mated B6 females to BALB/c males (or to B6 fathers, as syngeneic controls), adoptively transferred CFSE-labeled TCR75 cells into the dams on E12.5, injected the fetuses with PBS or LPS on E13.5, harvested maternal lymphoid organs on E18.5, and analyzed the proliferation of the transferred TCR75 cells using flow cytometry (Figure 3A). We first confirmed that the antigen recognized by TCR75 T cells is expressed in BALB/c fetal liver and placenta at E13.5-E14.5 (Figure 3B), consistent with the detailed analysis of H-2K and H-2D reported previously at E8.5 (28). In normal allogeneic pregnancies without fetal intervention, T cell proliferation was low but detectable in the udLNs and spleens, and even lower in the non-draining lymph node (ndLNs) (Figure 3C). Interestingly, in normal allogeneic pregnancies, the levels of T cell proliferation varied between litters, with some showing no proliferation and others showing detectable but abortive proliferation; this variation was not dependent on litter size (Figure S3) but may instead represent occasional spontaneous resorption.
Figure 3. Fetal intervention results in activation, proliferation, and accumulation of antigen-specific maternal T cells.
(A) Experimental design and gating strategy to track proliferation of adoptively transferred fetal antigen-specific TCR75 (CD4+ Thy1.1+) cells after fetal intervention. TCR75 proliferation is detected as dilution of CFSE. (B) Expression of MHC class I antigen H-2Kd in BALB/c fetal liver (top) and placenta (bottom) on E13.5-E14.5. Adult BALB/c lymph nodes (top) and an isotype antibody (top and bottom) were used as controls. N ≥ 3 in each group in 3 separate experiments. (C, D) Representative proliferation profiles of TCR75 cells in the uterine draining lymph nodes (udLNs, top row), spleens (middle row), and non-draining lymph nodes (ndLNs, bottom row) of dams carrying syngeneic (Syn) or allogeneic (Allo) pregnancies at baseline (C) or after fetal injection of PBS or LPS (D). (E) The percentage of proliferated TCR75 cells among total TCR75 cells after LPS injection. N ≥ 4 dams in each group in 4 separate experiments.*P < 0.05, ** P ≤ 0.01 by Kruskal-Wallis test with Dunn's post-hoc comparison. (F) The percentage of apoptotic cells (Annexin V+DAPI-) among proliferated (CFSElow) and non-proliferated (CFSEhigh) TCR75 cells in the uterine draining lymph node (udLNs), spleens, and non-draining lymph nodes (ndLNs) (Allo pregnant, n = 4, Allo LPS, n ≥ 3 dams in 3 separate experiments).*P < 0.05 by Student's t-test. (G) Increase in the absolute number (first graph) and in the proportion of TCR75 cells among all CD4 cells after fetal intervention (PBS). For uterus, decidua and placenta, each data point represents an average of all such segments per dam. N≥3 dams in each group in 3 separate experiments.*P < 0.05 by Student's t-test.
We next asked whether antigen-specific maternal T cells become activated during fetal intervention. Fetal injection of PBS or LPS consistently led to a significant increase in the percentage of proliferated TCR75 cells (Figure 3D). Proliferation was antigen-specific, since there was no proliferation in syngeneic pregnancies even in the presence of LPS (Figure 3D,E). This TCR model was very sensitive in detecting any fetal antigen release after fetal intervention: TCR75 proliferation was robust with both PBS and LPS injection (Figure 3E and Figure S4) and these cells proliferated even when no fetuses resorbed (data not shown), suggesting that the injection procedure alone can expose maternal T cells to fetal and placental antigens. Unlike the proliferation pattern observed in the udLNs, TCR75 cells in spleens and ndLNs were either not proliferated or highly proliferated (Figure 3D), suggesting migration of TCR75 cells that were initially activated and proliferated in the udLNs.
In normal pregnancy, fetal antigen-specific T cells proliferate but fail to become activated or to accumulate, suggesting clonal deletion (10). Given the robust proliferation and expansion we observed, we asked whether apoptosis of TCR75 cells decreases after fetal intervention. We stained TCR75 cells for Annexin V and DAPI at the time of harvest. We found that 37% of the proliferated and 24% of the non-proliferated TCR75 cells in the udLNs of mothers with normal pregnancy were apoptotic (Annexin V+, DAPI-) but this percentage decreased significantly during fetal intervention (Figure 3F). Apoptosis decreased only in TCR75 cells in the udLNs, but not in other lymphoid organs (Figure 3F).
We next asked whether the adoptively transferred antigen-specific T cells accumulate at the maternal-fetal interface after fetal intervention. In normal pregnancy, we detected few TCR75 cells in the uterus. However, both the absolute numbers and the percentage of TCR75 cells among CD4 cells in the uterus increased significantly after fetal PBS injection, indicating preferential recruitment or expansion of these antigen-specific cells over endogenous CD4 cells (Figure 3G). Similar increases in the proportion of TCR75 cells were seen in the udLNs, but not in placentas, ndLNs or spleens (Figure 3G). We also detected some TCR75 cells in the decidua, which is normally protected from maternal T cell infiltration (29) (Figure 3G). Thus, fetal intervention leads to an enrichment of antigen-specific cells in the uterus and udLNs. Collectively, our results suggest that fetal intervention leads to changes in normal tolerance mechanisms that usually prevent proliferation, migration, and accumulation of antigen-specific T cells.
Fetal intervention does not lead to activation of directly-reactive maternal T cells
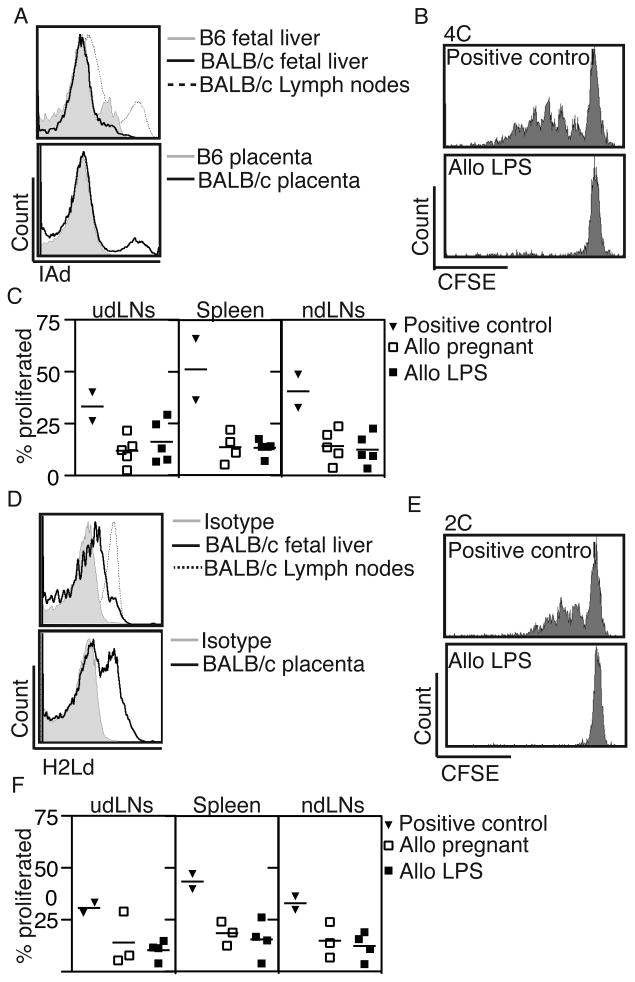
Maternal T cells can also recognize alloantigen presented on donor (fetal) APCs using the “direct” pathway. Although such directly alloreactive T cells constitute a significantly higher percentage of alloantigen-specific T cells compared to indirectly reactive T cells (11), they are not activated in normal pregnancy and the fetus is therefore protected from the majority of maternal antigen-specific T cells (10). We asked whether fetal intervention can increase antigen presentation by fetal APC and thus enhance maternal recognition of the fetus by directly-reactive T cells, using adoptive transfer of T cells from 4C (30) and 2C (31) mice. 4C mice have CD4 T cells that recognize the BALB/c class II antigen I-Ad while 2C mice have CD8 T cells that recognize BALB/c class I antigen H-2Ld, both presented “directly” by fetal BALB/c APC. We adoptively transferred T cells from 4C and 2C mice into B6 mothers bred to BALB/c fathers on E12.5, performed fetal injections on E13.5, harvested lymphoid organs on E18.5, and analyzed the proliferation of antigen-specific T cells using flow cytometry. To maximize the possibility of detecting fetal antigen exposure, we used the fetal LPS injection model, which leads to higher rates of resorption (Table 1). We first analyzed the expression of the antigens recognized by these transgenic T cells in fetal tissues on E13.5-E14.5 and determined that I-Ad (recognized by 4C) is present in the placenta but not in the fetal liver (Figure 4A), while H-2Ld (recognized by 2C) is present both in the placenta and in the fetal liver (Figure 4D). We found no increase in the proliferation of 4C (Figure 4B,C) or 2C (Figure 4E,F) T cells with fetal intervention over baseline in allogeneic matings in any lymphoid organ. Thus, fetal intervention does not result in direct antigen presentation, either because immature murine fetal APCs lack sufficient MHC expression (32, 33), or because maternal exposure to these antigens is not high enough to trigger activation in this TCR model, even when the majority of the litter is resorbed.
Figure 4. Maternal T cells that recognize the fetal alloantigen via the direct pathway do not proliferate after fetal intervention.
(A) Expression of MHC class II antigen I-Ad, the antigen recognized by 4C mice, in BALB/c fetal liver (top) and placenta (bottom) on E13.5-E14.5 compared to that found in adult BALB/c lymph nodes (positive control) or B6 fetal tissues (negative control). (B) 4C (CD4+ Thy1.1+) lymphocytes were adoptively transferred into B6 females bred to BALB/c males and their proliferation was examined 5 days after fetal intervention with LPS. The positive control represents splenocytes harvested from a B6 mouse sensitized with BALB/c lymphocytes prior to adoptive transfer. (C) The percentage of proliferated Thy1.1+ 4C cells among total 4C cells in the uterine draining lymph node (udLNs), spleens, and non-draining lymph nodes (ndLNs). (D) Expression of MHC Class I antigen H-2Ld (recognized by 2C mice) in BALB/c fetal liver (top) and placenta (bottom) on E13.5-E14.5 compared to that found in adult BALB/c lymph nodes (positive control) or isotype control. (E) 2C (CD8+ Thy1.1+) lymphocytes were adoptively transferred using the same experimental design indicated above for 4C mice. (F) The percentage of proliferated 2C cells among total 2C cells in lymph nodes and spleens. Positive control n=2; Allo pregnant n≥3, Allo LPS n≥4 dams in ≥2 experiments for all experiments.
Maternal T cells exacerbate selective loss of semi-allogeneic pups after in utero transplantation of additional paternal antigen
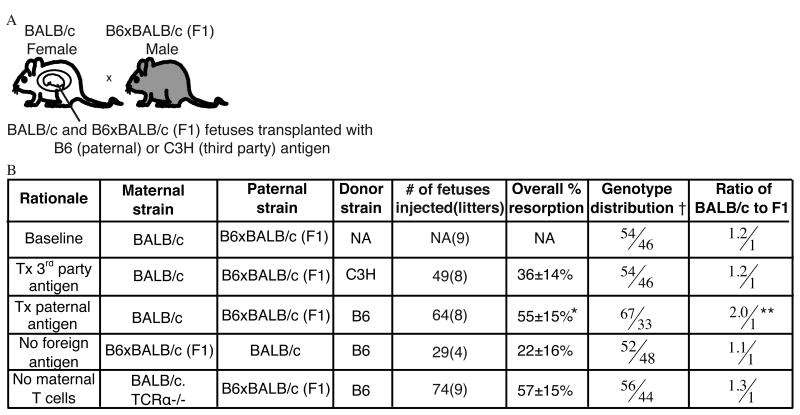
Given our findings that maternal T cells are activated and proliferate in the uterus after fetal intervention, we questioned whether these activated maternal T cells could be functional and cause resorption of the fetus in allogeneic matings. To address this question, we used our model of fetal hematopoietic cell transplantation, since we have previously shown that this intervention generates a maternal immune response to transplanted allogeneic cells (22). We reasoned that selective maternal rejection of the fetus could best be triggered by in utero transplantation of the same alloantigen carried by the fetus, which exposes the mother to a higher dose of the antigen in the context of surgical inflammation. To minimize experimental variability secondary to differences between individual litters, we designed an F1 breeding scheme in which a maternal anti-fetal immune response could be read out as decreased survival of semi-allogeneic pups compared to syngeneic littermates. We bred BALB/c females to B6 x BALB/c (F1) males such that half of the resulting fetuses expressed the foreign paternal antigen H2-Kb and were semi-allogeneic to the mother, while half were syngeneic to the mother (F1 and BALB/c pups, respectively) (Figure 5A). We recorded both the overall survival to birth as well as the genotype distribution (BALB/c or F1) of surviving pups at baseline and after in utero transplantation of hematopoietic cells testing various experimental conditions (Figure 5B). Uninjected litters had the expected equivalent survival of BALB/c and F1 pups, with a slight but consistent preference for survival of syngeneic over semi-allogeneic pups (1.2/1 ratio). Transplantation of allogeneic cells from a third party donor (C3H) resulted in some resorption but a conserved 1.2/1 ratio of syngeneic and semi-allogeneic pups. However, after in utero transplantation with hematopoietic cells from B6 mice, which carry the paternal antigen, we observed a striking decrease in the percentage of surviving semi-allogeneic fetuses, resulting in a 2:1 ratio of syngeneic to semi-allogeneic pups. As another control, we performed breedings in which the mother was B6 x BALB/c F1, such that neither the fetuses nor not the transplanted B6 cells are allogeneic to the mother. Consistent with our hypothesis, resorption rates were significantly lower in this group compared to the experimental (paternal antigen transplantation) group, and there was no skewing in the genotype of the litter. These results indicate that there is enhanced resorption of pups expressing the foreign paternal antigen only when the same paternal antigen is transplanted in utero, suggesting that in utero transplantation triggers a maternal adaptive immune response that ultimately results in fetal demise.
Figure 5. Maternal T cells exacerbate selective loss of the semi-allogeneic fetus after in utero hematopoietic cell transplantation.
(A) Breeding scheme to detect a functional maternal immune response. BALB/c mothers are bred to F1 (BALB/c x B6) fathers and the survival of BALB/c and F1 littermates is compared after in utero transplantation with hematopoietic cells from B6 mice which expressing the paternal antigen or from third party mice (C3H) which express a different allogeneic antigen. (B) Overall litter resorption rate and the relative survival of the BALB/c and F1 fetuses at baseline and in various experimental conditions explained under “rationale.”†: Ratio between the percentage of live BALB/c pups born and percentage of live F1 pups born. The percentage is calculated as the # of live pups born of the specific genotype divided by all live pups born. NA: not applicable (no fetal intervention). *= p< 0.0001 and **= α < 0.0005 by χ2 test compared to no foreign antigen group.
We next asked whether maternal T cells, which we have determined to be activated after fetal intervention, were mediating the selective loss of the semi-allogeneic pups after fetal intervention. We bred BALB/c. TCRα-/- females (which lack T cells) to B6 x BALB/c F1 males and performed in utero transplantation with B6 hematopoietic cells. We found that the survival of semi-allogeneic fetuses was not affected by in utero transplantation when the mother lacks T cells (1.3/1 ratio, Figure 5B). We have noted high overall rates of surgical complications in immunodeficient dams (including RagKO and B cell deficient mice, data not shown) and therefore do not expect the overall rate of resorption in this maternal strain to be comparable to the other experimental groups. These results are consistent with our observations of increased T cell activation and accumulation in the uterus with fetal surgery and suggest a functional role for maternal T cells in enhancing the loss of pups carrying the foreign transplanted antigen.
Discussion
In this study, we tested the hypothesis that fetal intervention perturbs maternal-fetal tolerance, leading to activation of fetal antigen-specific maternal T cells. We first showed that fetal PBS injection leads to higher rate of fetal resorption in allogeneic matings compared to syngeneic. We then demonstrated that this intervention results in expansion and proliferation of maternal T cells in the uterus, with an increase in local production of IFN-γ. Using an adoptive transfer model, we demonstrated that fetal intervention results in activation and proliferation of antigen-specific maternal T cells, which escape apoptosis and accumulate in the uterus. We also showed that maternal T cells can exacerbate demise of semi-allogeneic fetuses after in utero transplantation of additional paternal antigen. The finding that maternal T cells become activated after fetal intervention and can contribute to an adverse outcome suggests that treatments aimed at blocking the maternal adaptive immune response may be useful to treat complications of fetal surgery.
In normal pregnancy, multiple overlapping mechanisms keep maternal T cells in check such as lack of direct antigen presentation (10), physical entrapment of dendritic cells in the uterus (34), chemokine gene silencing (29), and dominant suppression by regulatory T cells (12-19). Fetal intervention may increase antigen presentation to maternal T cells through the release of fetal antigen into the maternal circulation. We have also observed increased trafficking of maternal cells into the fetus after fetal surgery in both this mouse model (22) and in patients (35) and such trafficking may facilitate maternal T cell activation. In addition, bleeding in the uterus after surgical trauma might release the physical entrapment of maternal dendritic cells (34) and upregulate Class II expression on these cells, in addition to recruiting other inflammatory cells such as macrophages. Finally, inflammatory signals may hinder Treg function, as has been demonstrated during Listeria infection (36) or render effector T cells less sensitive to Treg suppression (37).
In many models of pregnancy complications, it is difficult to distinguish the effects of non-specific inflammation from a true antigen-specific immune activation. Resorption in our fetal intervention model is multifactorial and includes a component of non-specific inflammation since there is some baseline resorption in syngeneic matings after PBS injection. We have used several experimental settings to show that fetal alloantigens are critical to the maternal T cell response. First, we noted a significantly higher rate of fetal loss in the allogeneic setting compared to syngeneic in our fetal PBS injections. Second, in our adoptive transfer experiments, we detected TCR75 proliferation only when fetal alloantigen is present, and not in the syngeneic setting. Finally, with the stem cell transplantation experiments, we detected fetal loss only when the fetus carries the same alloantigen that is transplanted into the fetus. These latter experiments were designed to also tease out the possible functional contribution of maternal T cells in enhancing fetal loss after the intervention. Although the degree of inflammation induced by surgery may vary between animals, comparing the survival of syngeneic and semi-allogeneic pups within the same litter allows a more accurate quantification of an allospecific immune response. It is interesting that in spite of the maternal T cell activation and enhanced loss of some of the pups in the litter, the entire litter is not lost, highlighting the importance of local tolerance mechanisms in this complex biological system.
We used several TCR transgenic mice to study maternal T cell recognition of the fetus in the context of fetal intervention. Our results are complementary to the studies of maternal T cell activation against fetal ovalbumin observed at baseline (10) and after infection (36), and provide further information regarding the response to an MHC antigen expressed physiologically. We have also performed a detailed analysis of immune cells in the uterus and udLNs to show that there is antigen-specific T cell infiltration locally after fetal intervention, supporting the concept of maternal rejection of the foreign conceptus. During fetal intervention, maternal T cells can be exposed to fetal antigens that are released from the fetal liver at the time of injection as well as those present in resorbed fetal and placental tissues. We devised the LPS injection model to mimic a more severe inflammatory insult, such as that seen with a microbial infection after fetal intervention. Chorioamnionitis has been reported in after fetal surgery (4) and its true incidence is likely higher than the reported rate since preterm premature rupture of membranes, seen commonly after fetal intervention, can represent a subclinical infection (38). Although we did not detect activation of directly-reactive T cells, the question of whether this pathway is relevant after clinical fetal surgery remains open. While it has been suggested that the indirect pathway of antigen presentation may be predominant for human pregnancies (39), the gestation period after fetal surgery is longer in patients than in mice and it is possible that human fetal APCs may have enough time to mature and stimulate directly-reactive maternal T cells after surgery.
One limitation of our study is that we could not examine the activation of indirectly-reactive CD8 T cells, which may play a role in fetal rejection, since there is no BALB/c allospecific TCR transgenic model for these cells. CD8 T cells expressing markers of differentiated effector memory cells have been observed in human deciduas and may be controlled locally (40). Our analysis of uterine T cells showed an increase in CD8 cells and both CD4 and CD8 cells are likely involved in a maternal immune response.
Our experiments indicate that surgical inflammation can perturb maternal-fetal tolerance by shifting the Teff/Treg balance, similar to what has been observed after infection-induced immune activation (36). Although the mouse feto-placental unit is significantly different from the human, it is likely that clinical fetal surgery would also expose maternal T cells to fetal and placental antigens and enhance maternal T cell recognition of the fetus. Furthermore, maternal T cells have been observed in fetal tissues of patients with villitis of unknown etiology (41, 42), with coordinate changes in placental chemokines (43), suggesting that T cell activation may be a culprit in other human pregnancy complications.
Fetal intervention is becoming ever more frequent— in addition to surgeries for fatal anatomic abnormalities, it is now common to perform diagnostic and therapeutic interventions for a variety of reasons. Therefore, determining whether maternal T cells are activated in patients who undergo uterine manipulation has vital clinical significance. Our results suggest that inhibiting maternal T cells may be a therapeutic target for complications of fetal surgery such as preterm labor.
Supplementary Material
Acknowledgments
We would like to thank the Tang, Rosenblum, and Abbas labs, and Drs. Susan Fisher and Mike McCune for helpful discussions. We also thank Dr. Nancy Hills and Shannon Fleck for statistical analysis.
Footnotes
This work was supported by The California Institute for Regenerative Medicine (grants to AN and TCM), NIH/NIAID K08 AI085042 (TCM), National Science Foundation (MW), The March of Dimes (TCM), and the Pathology & Imaging Core of the UCSF Liver Center (P30 DK026743). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of CIRM or any other agency of the State of California.
Author Contributions: M.W., A.N., Q.T., and T.C.M. designed the research and analyzed the data. M.W., A.N., C.W., N.L., and T.L. performed the experiments. M.W., A.N., Q.T. and T.C.M. wrote the paper.
References
- 1.Adzick NS. Open fetal surgery for life-threatening fetal anomalies. Semin Fetal Neonatal Med. 2010;15:1–8. doi: 10.1016/j.siny.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Nijagal A, Flake AW, MacKenzie TC. In utero hematopoietic cell transplantation for the treatment of congenital anomalies. Clin Perinatol. 2012;39:301–310. doi: 10.1016/j.clp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Golombeck K, Ball RH, Lee H, Farrell JA, Farmer DL, Jacobs VR, Rosen MA, Filly RA, Harrison MR. Maternal morbidity after maternal-fetal surgery. Am J Obstet Gynecol. 2006;194:834–839. doi: 10.1016/j.ajog.2005.10.807. [DOI] [PubMed] [Google Scholar]
- 4.Adzick NS, Thom EA, Spong CY, Brock JW, 3rd, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Dabrowiak ME, Sutton LN, Gupta N, Tulipan NB, D'Alton ME, Farmer DL. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, Lee H, Filly RA, Farrell JA, Albanese CT. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- 6.Erlebacher A. Immunology of the Maternal-Fetal Interface. Annu Rev Immunol. 2013 doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 8.Zenclussen AC. Adaptive immune responses during pregnancy. Am J Reprod Immunol. 2013;69:291–303. doi: 10.1111/aji.12097. [DOI] [PubMed] [Google Scholar]
- 9.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 10.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 12.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 13.Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhao JX, Zeng YY, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75:71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 22.Nijagal A, Wegorzewska M, Jarvis E, Le T, Tang Q, MacKenzie TC. Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice. J Clin Invest. 2011;121:582–592. doi: 10.1172/JCI44907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nijagal A, Le T, Wegorzewska M, Mackenzie TC. A mouse model of in utero transplantation. J Vis Exp. 2011;(47) doi: 10.3791/2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honjo K, Xu X, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004;77:452–455. doi: 10.1097/01.TP.0000112937.12491.42. [DOI] [PubMed] [Google Scholar]
- 28.Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci. 2011;108:4012–4017. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan TV, Hoang V, Garrod KR, Liu FC, Hayden T, Kim J, Kang SM. A new T-cell receptor transgenic model of the CD4+ direct pathway: level of priming determines acute versus chronic rejection. Transplantation. 2008;85:247–255. doi: 10.1097/TP.0b013e31815e883e. [DOI] [PubMed] [Google Scholar]
- 31.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 32.Dakic A, Shao QX, D'Amico A, O'Keeffe M, Chen WF, Shortman K, Wu L. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 33.Muthukkumar S, Goldstein J, Stein KE. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J Immunol. 2000;165:4803–4813. doi: 10.4049/jimmunol.165.9.4803. [DOI] [PubMed] [Google Scholar]
- 34.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saadai P, Lee TH, Bautista G, Gonzales KD, Nijagal A, Busch MP, Kim CJ, Romero R, Lee H, Hirose S, Rand L, Miniati D, Farmer DL, Mackenzie TC. Alterations in maternal-fetal cellular trafficking after fetal surgery. J Peds Surg. 2012;47:1089–1094. doi: 10.1016/j.jpedsurg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe JH, Ertelt JM, Xin L, Way SS. Listeria monocytogenes cytoplasmic entry induces fetal wastage by disrupting maternal Foxp3+ regulatory T cell-sustained fetal tolerance. PLoS Pathog. 2012;8:e1002873. doi: 10.1371/journal.ppat.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 38.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg VDVMM, Roelen DL, van Rood JJ, Claas FH. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, Prins F, van Lith JM, van der Mast BJ, Roelen DL, Scherjon SA, Claas FH. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. 2010;185:4470–4477. doi: 10.4049/jimmunol.0903597. [DOI] [PubMed] [Google Scholar]
- 41.Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993;143:473–479. [PMC free article] [PubMed] [Google Scholar]
- 42.Myerson D, Parkin RK, Benirschke K, Tschetter CN, Hyde SR. The pathogenesis of villitis of unknown etiology: analysis with a new conjoint immunohistochemistry-in situ hybridization procedure to identify specific maternal and fetal cells. Pediatr Dev Path. 2006;9:257–265. doi: 10.2350/08-05-0103.1. [DOI] [PubMed] [Google Scholar]
- 43.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.