Abstract
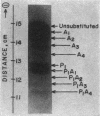
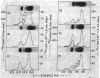
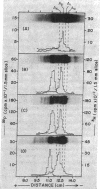
During spermatogenesis in trout testis, histone IV is extensively modified by acetylation and phosphorylation. To examine the relationship of synthesis of histone IV to its modification, histone IV labeled with [3H]aminoacids and inorganic [32P]phosphate was prepared from testis cells by acid extraction and column chromatography. Purified histone IV was resolved by starch gel electrophoresis into 10 bands, of which nine are modified by acetylation and/or phosphorylation. In the first 4 hr of labeling, the diacetyl-histone IV band showed the highest proportion of [3H]aminoacid label. After 12 hr of incorporation, more label was found in the triacetyl and tetraacetyl bands. A significant amount of amino-acid label in the two major bands (the unsubstituted and monoacetyl bands) of histone IV was not seen until 16 hr of incubation. From 1 to 12 days, the proportion of label in the unsubstituted and monoacetylated bands increased, while that in the tetra-, tri-, and monoacetyl bands decreased. Very little [3H]aminoacid was found in the phosphorylated bands of histone IV in the first 12 hr. However, after 16 hr about 20% of the total 3H was found in the phosphorylated bands. The proportion increased to 33% and remained at this level between 1 and 8 days, but, by 16 days, had decreased to 12% of the total.
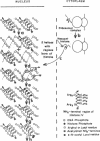
These data suggest that an “obligatory” acetylation of recently synthesized histone IV is involved in the correct binding of newly synthesized histone IV to DNA. We propose that ε-amino acetylation of lysyl residues 5, 8, 12, and 16 neutralizes their positive charges and allows the NH2-terminal region of histone IV to assume the correct conformation (in this case, an α-helix), and fit into the major groove of DNA. Deacetylation then “locks” histone IV to DNA by ionic linkages. The biological significance of phosphorylation of histone IV is not known.
Keywords: starch gels, kinetics of labeling, histone-DNA binding
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Boublík M., Bradbury E. M., Crane-Robinson C. An investigation of the conformational changes in histones F1 and F2a1 by proton magnetic resonance spectroscopy. Eur J Biochem. 1970 Jul;14(3):486–497. doi: 10.1111/j.1432-1033.1970.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Bustin M., Rall S. C., Stellwagen R. H., Cole R. D. Histone structure: asymmetric distribution of lysine residues in lysine-rich histone. Science. 1969 Jan 24;163(3865):391–393. doi: 10.1126/science.163.3865.391-a. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Sites of in vivo acetylation in trout testis histone IV. J Biol Chem. 1971 May 25;246(10):3182–3188. [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. 3. Complete amino acid sequence of pea seedling histone IV; comparison with the homologous calf thymus histone. J Biol Chem. 1969 Oct 25;244(20):5669–5679. [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- Gallwitz D. Extraction and partial purification of two histone-specific transacetylases from rat liver nuclei. Biochem Biophys Res Commun. 1970 Jul 13;40(1):236–242. doi: 10.1016/0006-291x(70)91072-7. [DOI] [PubMed] [Google Scholar]
- Gallwitz D. Organ specificity of histone Acetyltransferases. FEBS Lett. 1971 Mar 22;13(5):306–308. doi: 10.1016/0014-5793(71)80247-8. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. I. Synthesis of histone fractions during the life cycle of mammalian cells. Arch Biochem Biophys. 1968 Nov;128(2):285–292. doi: 10.1016/0003-9861(68)90034-9. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Dixon G. H. Phosphorylation of protamine during spermatogenesis in trout testis. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1011–1018. doi: 10.1073/pnas.58.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles C. J., Trevithick J. R., Smith M., Dixon G. H. Biosynthesis of protamine during spermatogenesis in salmonoid fish. Biochem Biophys Res Commun. 1966 Mar 22;22(6):627–634. doi: 10.1016/0006-291x(66)90192-6. [DOI] [PubMed] [Google Scholar]
- Inoue A., Fujimoto D. Histone deacetylase from calf thymus. Biochim Biophys Acta. 1970 Nov 11;220(2):307–316. doi: 10.1016/0005-2744(70)90015-x. [DOI] [PubMed] [Google Scholar]
- Itzhaki R. F. The arrangement of proteins on the deoxyribonucleic acid in chromatin. Biochem J. 1971 Nov;125(1):221–224. doi: 10.1042/bj1250221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Trevithick J. R., Dixon G. H. The biosynthesis of protamine in trout testis. I. Intracellular site of synthesis. Can J Biochem. 1969 Jan;47(1):51–60. doi: 10.1139/o69-009. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Fractionation of liver chromatin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2941–2944. doi: 10.1073/pnas.68.12.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Transformation of trout testis chromatin. J Biol Chem. 1971 Sep 25;246(18):5799–5805. [PubMed] [Google Scholar]
- Marushige K., Ling V., Dixon G. H. Phosphorylation of chromosomal basic proteins in maturing trout testis. J Biol Chem. 1969 Nov 10;244(21):5953–5958. [PubMed] [Google Scholar]
- Prothero J. W. Correlation between the distribution of amino acids and alpha helices. Biophys J. 1966 May;6(3):367–370. doi: 10.1016/S0006-3495(66)86662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT P. J., MITCHELL B. S., SMITH M., TSYUKI H. PITUITARY HORMONES OF THE PACIFIC SALMON. I. RESPONSE OF GONADS IN IMMATURE TROUT (SALMO GAIRDNERII) TO EXTRACTS OF PITUITARY GLANDS FROM ADULT PACIFIC SALMON (ONCORHYNCHUS). Gen Comp Endocrinol. 1965 Apr;5:197–206. doi: 10.1016/0016-6480(65)90114-0. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. R., Noland B. J., Hardin J. M. Histone acetylation in synchronized mammalian cell cultures. Biochim Biophys Acta. 1971 Jan 28;228(2):544–549. doi: 10.1016/0005-2787(71)90060-8. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Thermal denaturation and template properties of DNA complexes with purified histone fractions. J Mol Biol. 1970 Mar;48(3):469–487. doi: 10.1016/0022-2836(70)90059-8. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Circular dichroism studies of deoxyribonucleic acid complexes with arginine-rich histone IV (f2al). Biochemistry. 1971 Apr 27;10(9):1675–1683. doi: 10.1021/bi00785a027. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Interaction of a repotter molecule with chromatin. Evidence suggesting that the proteins of chromatin do not occupy the minor groove of deoxyribonucleic acid. Biochemistry. 1970 Nov 24;9(24):4814–4819. doi: 10.1021/bi00826a028. [DOI] [PubMed] [Google Scholar]
- Sung M. T., Dixon G. H. Modification of histones during spermiogenesis in trout: a molecular mechanism for altering histone binding to DNA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1616–1623. doi: 10.1073/pnas.67.3.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M., Smithies O. Differential elution of histones from gel-trapped nuclei. Biopolymers. 1969;7(1):39–58. doi: 10.1002/bip.1969.360070105. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. A serum-free nutrient solution sustaining rapid and continuous proliferation of strain L (Earle) mouse cells. J Natl Cancer Inst. 1956 Sep;17(3):315–327. [PubMed] [Google Scholar]