Abstract
Purpose
The aim of this study was to assess patients’ preferences for efficacy, safety, and mode of administration in relation to available bone-targeted agents (BTA) for the prevention of skeletal-related events (SREs) associated with bone metastases in Europe.
Methods
Adults in France (n = 159), Germany (n = 166), and the United Kingdom (UK; n = 159) with a self-reported physician diagnosis of bone metastases secondary to a solid tumour completed an online discrete- choice experiment survey of ten questions, choosing between pairs of hypothetical BTA profiles. Profiles were defined by five treatment attributes: delay of first SRE, delay of worsening of pain, annual risk of osteonecrosis of the jaw (ONJ), annual risk of renal impairment, and mode of administration. Profiles were generated using an experimental design with known statistical properties. A main-effects random parameters logit (RPL) model was applied to relate participants’ choices to the characteristics of the BTA profiles.
Results
The most important treatment attributes for patients across all three countries were time until first SRE, annual risk of renal complications and time until pain worsening. For these attributes, better levels of outcomes were significantly preferred to worse levels (p < 0.05). A 120-minutes infusion every 4 weeks was the least preferred mode of administration. Risk of ONJ was judged by patients in the UK and Germany to be the least important attribute.
Conclusions
Patients consider delaying SREs, avoiding renal impairment and delaying pain worsening as the most important goals to consider when selecting treatment to prevent the bone complications commonly associated with bone metastases.
Electronic supplementary material
The online version of this article (doi:10.1007/s00520-014-2309-x) contains supplementary material, which is available to authorized users.
Keywords: Patient preference, Bone metastases, Bone complications, Bone-targeted agents, Conjoint analysis, Discrete- choice experiment
Introduction
Bone is one of the most common sites for metastatic spread in advanced cancer, occurring in 65–75 % of patients with advanced breast and prostate cancer and 30–40 % of patients with advanced lung, kidney or thyroid cancer [1]. Bone metastases-associated morbidity can be debilitating and is often characterised by severe pain, reduced health-related quality of life and bone complications (skeletal-related events, SREs) defined as pathologic fracture, spinal cord compression or the need for radiation or surgery to bone. If left untreated, two thirds of patients with bone metastases will develop a SRE [2], and on average, they will experience a SRE every 3–6 months [1].
Bisphosphonates (predominantly zoledronic acid) were the mainstay of treatment to prevent SREs. Recently, the fully- human anti-RANKL monoclonal antibody, denosumab, demonstrated superiority versus zoledronic acid in preventing SREs in patients with bone metastases secondary to solid tumours [3]. Although the phase 3 head-to-head studies evaluated clinical efficacy of the two treatments, patient and physician preference could not be assessed given the double-dummy, double-blind design. Patient-reported preferences incorporate multiple aspects of treatment elements from a patient’s perspective, including, but not limited to, clinical endpoints.
The primary objective of this study was to quantify patients’ preferences associated with currently available bone-targeted agents (BTAs) to prevent SREs in patients with bone metastases secondary to solid tumours. Best practices [4] were followed in designing and administering a discrete- choice experiment (DCE) to elicit preferences over attributes associated with each BTA.
Patients and methods
Discrete- choice experiments
DCEs are being increasingly used to quantify preferences for treatments and health outcomes [4–6] as they offer a systematic way to elicit trade-offs and quantify the relative importance that patients and physicians place on treatment characteristics or outcomes [7]. Treatment-related characteristics or outcomes can be used in a DCE to describe different aspects of the healthcare system. The relative value of a treatment to a particular individual is therefore described as a function of them [8, 9]. These treatment-related characteristics or outcomes, referred to as ‘attributes’ [10–12], are assigned levels to define the severity, likelihood, or timing of each. The premise behind DCEs was developed using psychology and economic (welfare and consumer) theories combined with statistical methodology. DCEs were first used in the field of marketing and have since been extended to health economics.
Study sample
Eligible participants were at least 18 years of age from France, Germany, or the United Kingdom (UK) with a self-reported physician diagnosis of at least one bone metastasis from a solid tumour. An online research firm recruited members from existing panels in these countries and administered the 25-minute survey (January–February 2013).
The survey and protocols for pretesting and final administration were reviewed by the Office of Research Protection and Ethics at RTI International (the responsible study organisation) and approved by their Institutional Review Board (IRB).
Survey instrument
DCEs rely on survey instruments to collect the information to elicit preferences. In addition to the survey, participants provided demographic information and described disease and treatment experience to facilitate interpretation of results. The survey consisted of a series of choices between hypothetical BTA profiles defined by five treatment attributes: how long the treatment delays the first SRE, how long the treatment delays a clinically- relevant worsening of pain (2-point increase in the brief pain inventory worst pain score), annual treatment-related risk of osteonecrosis of the jaw (ONJ), annual treatment-related risk of renal impairment, and mode of administration. Product inserts for available BTAs and the literature were consulted to determine the treatment attributes, attribute definitions and the attribute levels included. The levels of the attributes in the survey were designed to encompass the range observed in current clinical practice.
Draft survey instruments were pretested using face-to-face, semi-structured interviews in the United States of America (USA) to assess clarity and the appropriateness of the descriptive information, to evaluate the salience of the attributes and levels, to confirm that no other attributes were missing and to assess participants’ willingness to accept trade-offs among BTA treatment attributes. Additional face-to-face interviews were conducted in Europe (four in France, four in Germany, and three in the UK) to test translation and cultural relevance. These data were not included in the study results as these interviews were used to validate the instrument prior to study start.
An algorithm that maximised the amount of statistical information obtained from a given number of choice questions was used to develop an experimental design of 40 choice questions [13–17]. The experimental design ensured that preferences for all attribute-level combinations were statistically identifiable. To avoid participant fatigue, the experimental design was divided into four versions, each with ten questions. Participants were then randomly assigned to one version (Supplemental Table S1).
Participants chose between pairs of hypothetical BTA profiles (Supplemental Fig. S1), with varying levels of the attributes (Supplemental Table S2). For each question, participants indicated the BTA they would choose if only the two options presented each time were available. To help patients understand the choices, a written explanation was provided for each attribute in the first part of the survey (Supplemental Table S3).
Statistical analysis
Responses were modelled using a random parameters logit (RPL) model whereby treatment choice is explained by the attribute levels of the available treatments and a parameter is estimated for each attribute level. Parameter estimates reflect the outcome’s marginal influence on treatment choice [9, 18, 19]. The dependent variable in an RPL model is a dichotomous variable set to be 1 when a participant chooses a treatment and 0 otherwise. All explanatory variables were modelled as categorical variables and effects-coded, reflecting participants’ preference weights relative to the mean BTA profile in the experimental design. Because the parameter estimates reflect the outcomes’ marginal influence on treatment choice, they can be interpreted as relative preference weights, indicating participants’ intensities of preference for each attribute level [9, 18, 19]. More preferred outcomes and features resulted in greater relative preference weights.
The 95 % confidence interval was calculated and reported for each preference weight estimate to help determine the significance of differences in the preferences for attribute levels. When confidence intervals did not overlap, the mean estimates were statistically different from each other at the 5 % level of significance. P values were estimated using Wald tests.
We characterised the differences between preference weights within an attribute as the relative importance of treatment-related changes between two levels of the same attribute. The importance of treatment-related changes was comparable across attributes. With this in mind, we compared overall attribute importance by comparing treatment-related changes that evaluated the least and most preferred level within each attribute [20, 21]. Data were analysed by country and were not intended to be pooled.
The secondary endpoint of the study was to estimate the predicted proportion of participants who would choose given treatment profiles. This was done using the model results for a product with characteristics similar to denosumab, zoledronic acid, clodronate, and pamidronate (Supplemental Table S4). Other available products (e.g. ibandronic acid) were not specifically included since their attributes values would fall within the parameters estimated for the products included, thus allowing extrapolation of results.
Results
Participants
Members of patient panels completed a screening test to corroborate eligibility. Of the 629 eligible patients, 506 (80.4 %) completed the survey (France, 166; Germany, 175; UK, 165). Twenty-two participants always selected the same answer, i.e. Medication A or B, and were excluded from the final sample given that such lack of variation in response was a strong indication that they were not paying attention to the questions [21]. Thus, the final sample of 484 patients included 159 French patients, 166 German patients and 159 UK patients (Supplemental Fig. S2). In Germany and the UK, a large proportion of patients were younger than 45 years of age (58 and 42.8 %, respectively; Table 1), whereas French patients were mostly aged 46–65 years (44.2 %).
Table 1.
Participant and disease characteristics
| Category | France | Germany | UK |
|---|---|---|---|
| (n = 159) | (n = 166) | (n = 159) | |
| (%) | (%) | (%) | |
| Gender | |||
| Female | 46.8 | 62.0 | 56.6 |
| Male | 53.2 | 38.0 | 43.4 |
| Age | |||
| < 45 years | 37.2 | 58.0 | 42.8 |
| 46–65 years of age | 44.2 | 32.9 | 37.0 |
| > 65 years | 18.6 | 9.1 | 20.2 |
| Primary cancer types | |||
| Breast | 36.1 | 23.2 | 36.5 |
| Lung | 20.9 | 20.7 | 13.2 |
| Prostate | 14.6 | 17.7 | 21.4 |
| Kidney | 8.2 | 7.9 | 4.4 |
| Colon | 6.3 | 7.9 | 5.0 |
| Thyroid | 2.5 | 4.3 | 5.0 |
| Melanoma | 1.9 | 4.3 | 3.1 |
| Other | 9.5 | 14.0 | 11.3 |
| Time since primary cancer diagnosis | |||
| < 2 years | 69.1 | 79.5 | 67.8 |
| Time since bone metastases diagnosis | |||
| < 1 year | 73.0 | 67.2 | 65.4 |
| Among those currently taking treatment to delay complications of bone metastases | 68.4 | 75.8 | 70.9 |
| Oral (pills or tablets) | 22.7 | 32.2 | 30.8 |
| Intravenously | 67.3 | 46.2 | 51.5 |
| Subcutaneously | 9.1 | 20.3 | 13.8 |
| Currently receiving chemotherapy | 73.0 | 68.5 | 53.5 |
| Among those currently receiving chemotherapy: How is chemotherapy given | |||
| Intravenously | 80.9 | 67.3 | 63.1 |
| Oral (pills and tablets) | 17.4 | 26.5 | 32.1 |
| Other | 1.7 | 6.2 | 4.8 |
| Losing ability to move around affected the patient the most in the past 2 weeks | 30.6 | 46.7 | 53.5 |
| Had a complication because of bone metastases | 50.9 | 57.4 | 59.5 |
| Severity of worst pain in the past week for any reason | |||
| No pain | 1.9 | 1.8 | 5.7 |
| Mild | 9.4 | 22.4 | 28.9 |
| Moderate | 45.3 | 50.3 | 44.7 |
| Severe | 43.4 | 25.5 | 20.8 |
| Severity of average pain in the past week for any reason | |||
| No pain | 2.5 | 3.0 | 6.3 |
| Mild | 19.0 | 29.9 | 32.3 |
| Moderate | 64.6 | 51.2 | 53.2 |
| Severe | 13.9 | 15.9 | 18.2 |
Preference weights
Figures 1, 2, and 3 show estimated preference weights for all attribute levels for the French, German, and UK patients, respectively. Across all countries, mean preference weights were consistent with the natural ordering of the level they represented in an attribute. Thus, better clinical outcomes were preferred to worse clinical outcomes.
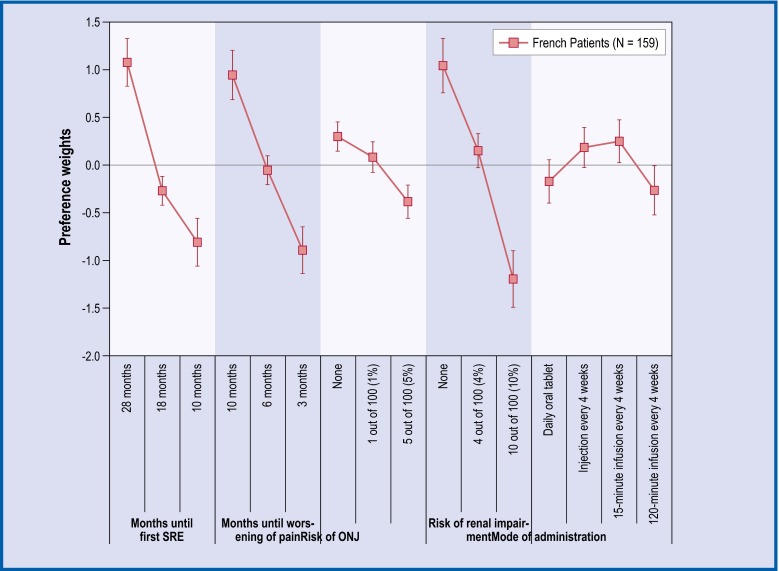
Fig. 1.
Preference weights for French patients. The vertical bars surrounding each mean preference weight denote the 95 % CI about the point estimate. If the CIs do not overlap for adjacent levels in a particular attribute, the mean estimates are statistically different from each other at the 5 % level of significance. ONJ osteonecrosis of the jaw
Fig. 2.
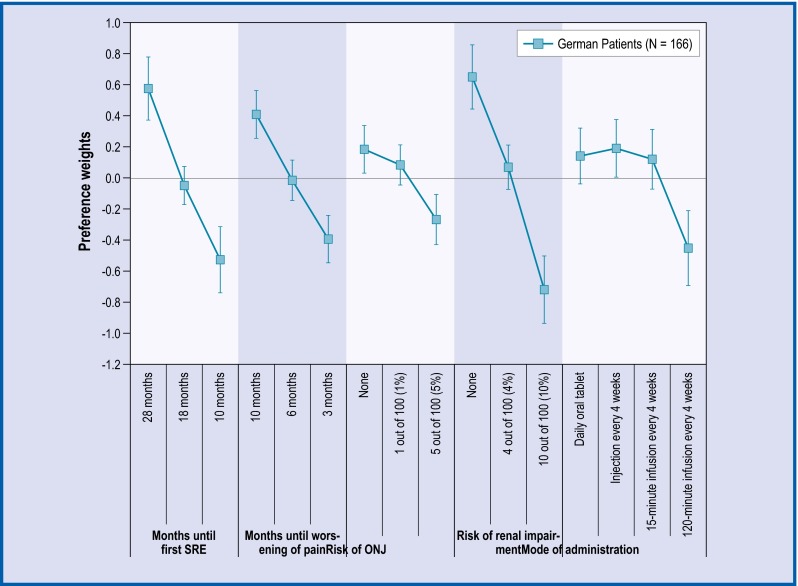
Preference weights for German patients. The vertical bars surrounding each mean preference weight denote the 95 % CI about the point estimate. If the CIs do not overlap for adjacent levels in a particular attribute, the mean estimates are statistically different from each other at the 5 % level of significance. ONJ osteonecrosis of the jaw
Fig. 3.
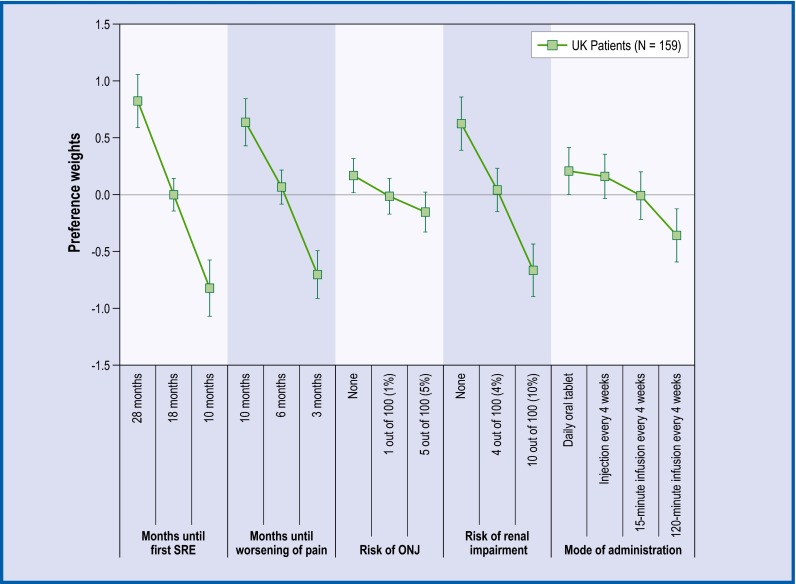
Preference weights for UK patients. The vertical bars surrounding each mean preference weight denote the 95 % CI about the point estimate. If the CIs do not overlap for adjacent levels in a particular attribute, the mean estimates are statistically different from each other at the 5 % level of significance. UK United Kingdom, ONJ osteonecrosis of the jaw
Across all countries, the levels for time until first SRE, time until worsening of pain, and risk of renal impairment followed the natural order from better clinical outcomes to worse, and the mean preference weight estimates were statistically different from each other. Among French and German patients, preference weight estimates for no annual risk versus a 1 % annual risk of ONJ were not statistically different from each other. In the UK, none of the adjacent levels in annual risk of ONJ were statistically different.
For French patients, administration via 120-minutes infusion every 4 weeks was statistically significantly less preferred than an injection or a 15-minutes infusion. Among German patients, administration via 120-minutes infusion every 4 weeks was the least preferred method of administration and statistically significantly different from all other administration modes. Finally, for the UK patients, administration via 120-minutes infusion was statistically less preferred than a daily oral tablet and injection.
The most important attributes for patients across all three countries were time until first SRE, annual risk of renal complications, and time until pain worsening (Table 2). Among the French patients, the least important attribute appeared to be the mode of administration and, for the German and UK patients, it was treatment-related risk of ONJ. Of note, in all the three countries, patients’ preferences for 8-month increase in time until SRE and a 3-month improvement in the delay of pain worsening were statistically significant.
Table 2.
Relative importance of product characteristics
| Relative Importance | UK | France | Germany |
|---|---|---|---|
| 1 | Time until first SRE | Risk of renal impairment | Risk of renal impairment |
| 2 | Time until pain worsening | Time until first SRE | Time until first SRE |
| 3 | Risk of renal impairment | Time until pain worsening | Time until pain worsening |
| 4 | Mode of administration | Risk of ONJ | Mode of administration |
| 5 | Risk of ONJ | Mode of administration | Risk of ONJ |
ONJ osteonecrosis of the jaw, UK United Kingdom, SRE skeletal-related event
In all the three countries, according to the predicted choice probabilities for the attributes and levels included in the survey, the majority of patients would choose a treatment profile with characteristics similar to denosumab (91.2–94.6 %; Table 3).
Table 3.
Predicted choice probabilities
| Mean, % (95 % CI) | Characteristics similar to denosumab | Characteristics similar to zoledronic acid | Characteristics similar to clodronate | Characteristics similar to pamidronate |
|---|---|---|---|---|
| French patients | 91.2 (85.4, 94.9) | 3.6 (1.8, 6.3) | 5.0 (2.9, 8.5) | 0.4 (0.1, 0.9) |
| German patients | 94.6 (90.7, 96.9) | 3.0 (1.5, 5.4) | 2.3 (1.3, 4.1) | 0.2 (0.1, 0.5) |
| UK patients | 91.8 (86.8, 94.9) | 3.1 (1.6, 5.3) | 5.0 (2.9, 8.1) | 0.3 (0.1, 0.7) |
UK United Kingdom
Discussion
Patient’s views and preferences are often given minimal consideration in the selection of treatments they will receive; yet, in some cases, they endure a high impact on their lives at a time when they may already feel more vulnerable. It is important, especially in this palliative setting, that the patient is seen as a key stakeholder and is actively involved in selecting the appropriate disease management strategy, incorporating his/her situation and preferences. Thus, DCEs are important to assess and understand patients’ priorities in choosing from available treatment options based on their basic attributes related to efficacy, safety and mode of administration. DCE methodology has been applied previously in other therapeutic areas (i.e. HIV, osteoarthritis, cardiovascular, and hepatitis) in order to elicit patient preferences and better inform clinical practice [4, 20, 21]. To our knowledge, this is the first such patient preference or DCE study in this treatment setting and thus plays an important role for helping to define future treatment strategies and fostering a more collaborative approach between the patient and their physicians.
DCEs have some limitations, and perhaps the most important is that preferences are inferred from choices over hypothetical treatment profiles. Although choice questions are intended to simulate clinical decisions, they do not have the same clinical or emotional consequences of actual decisions. Thus, differences can arise between stated and actual choices. With this in mind, the hypothetical scenarios were prepared to mimic real-world trade-offs as closely as possible in an effort to minimise any hypothetical bias. Also, the participant rate for this study was <5 %, which may have introduced selection bias. Without data on the characteristics of non-participants (i.e. those who were not eligible or did not consent to participate), it is not possible to determine whether the recruitment procedure used in this study resulted in any bias.
The results from this study suggest that patients are able to make informed decisions about currently available treatment options and that there were generally well-defined preferences for the efficacy, safety and different modes of administration associated with them. Patients considered delaying SREs, avoiding renal impairment and delaying pain worsening as the most important treatment goals to consider when selecting treatment to prevent the bone complications associated with bone metastases, thus confirming that for patients as well as physicians, clinical endpoints such as avoiding SREs [3] and pain prevention [22] are the most important treatment goals. Results suggest that patients in fact place value on differences of different magnitudes between attribute levels, for example, 3 months of additional delay in pain or 8 months of increased delay of SREs. Thus, certain differences may be meaningful to patients although they may not reach the level of ‘clinical significance’ based on traditional definitions. Because of the nephrotoxicity associated with chemotherapy and the general health of patients with advanced cancer, patients may fear that if they develop renal impairment, then it could prevent them from receiving full-dose chemotherapy regimens and therefore prevent them from an optimal management of their disease. Considering all of these points, patients are essentially more frightened by bone complications, the thought of severe and uncontrollable pain and concerns about renal impairment than a risk of ONJ. Such patient acceptance may be related to the fact that today, ONJ is more proactively managed by primary caregivers and that patients are willing to accept an increasing risk of ONJ in order to prevent SREs and pain.
For all participants, 120-minutes infusion was the least preferred mode of administration. Factors, such as additional contact with the primary caregiver and co-administration of a BTA with chemotherapy, may influence patient preference relating to the modes of administration and therefore make this outcome difficult to interpret. For this study, treatment attributes were taken from the available prescribing information/summary of product characteristics for each treatment option. This allowed us to compare across the different treatment options; however, it did not take into consideration time spent by patients having additional laboratory tests (i.e. renal function) prior to receiving an infusion nor did it consider that in the real-world setting, an infusion may take some time to set up, and, therefore, total drug administration time may in fact exceed that reported in the product information, as demonstrated in time and motion studies in this setting [23]. In addition, the possibility of home administration of an injection was not specifically considered nor was it explained that the injection was subcutaneous and not intravenous. This may explain why clearer preferences between an injection and a 15-minutes infusion were not seen.
The patient population surveyed here is more representative of the real-world setting than the clinical trial population, and it can be considered that these results are reflective of the real-world setting. In this study, only 25–30 % of patients had been diagnosed with bone metastases for longer than 1 year. Thus, it could be considered that their opinion may be conservative as they have not yet been living with the long-term effects of bone complications. Despite this, two-thirds of patients already reported moderate-to-severe pain, suggesting that current pain management was suboptimal. This also highlights the fact that prevention of pain and pain worsening is a critical consideration for patients.
Although data were not pooled and there was no formal analysis between the countries, it is clear that there is a high consistency between the reported outcomes across all countries. Of note, no difference was observed between countries where all products are available versus those where only some are available. This suggests that this kind of evaluation is useful and perhaps in the future should be considered by reimbursement agencies as another critical part of the decision-making process. Understanding and acknowledgement of what patients consider to be important provides ‘added value’ to the decision-making process and should be incorporated using robust methods such as this type of study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Study Experimental Design (DOCX 286 kb)
Attributes and Levels for the Choice Questions (DOCX 16.4 kb)
Explanation of Treatment Attributes (DOCX 22.7 kb)
Treatment Profiles (DOCX 20.8 kb)
Example of a Choice Question (PDF 113 kb)
Patient Flow and Sample Sizes (PDF 71 kb)
Acknowledgments
Joshua Posner and Juan Marcos Gonzalez of RTI Health Solutions provided support during the data analysis phase of this study. Medical writing support was provided by Emma Thomas of Amgen (Europe) GmbH. Editorial support was provided by Oxford PharmaGenesis™ Ltd (UK). Funding for this support was provided by Amgen Europe (GmbH).
Funding
This work was supported by Amgen Inc., who was involved in the study design, analysis and interpretation of data and in the writing of and the decision to submit this manuscript.
Conflicts of interest
GH, JA, IH and YQ are employed by Amgen and own stock. FG is a contractor employed by Amgen (Europe) GmbH. A. Brett Hauber is an employee of RTI Health Solutions, an independent scientific research organization. The study which is the subject of this manuscript was conducted by RTI Health Solutions and funded by Amgen. AFM was employed at RTI Health Solutions during the study and has no conflict of interest. AB has received honoraria for participating in Advisory Boards from Amgen, Sanofi, Astellas, Roche, Novartis and Bayer. RvM has received research grants from Amgen, Roche and Merck. He has participated in Advisory Boards for Amgen, Roche, Novartis, Merck, MSD, and Elly Lilly. He has received speaking honoraria from Amgen and GSK. JJB has received speaking honoraria and consulting fees from Amgen and Novartis.
Footnotes
Roger von Moos and Jean-Jacques Body shared last authorship.
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer: implications for management. Eur J Cancer. 2000;36:476–482. doi: 10.1016/S0959-8049(99)00331-7. [DOI] [PubMed] [Google Scholar]
- 3.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 4.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–172. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 5.Marshall D, Bridges JFP, Hauber AB, et al. Conjoint analysis applications in health—how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3:249–256. doi: 10.2165/11539650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Bridges JFP, Kinter E, Kidane L, Heinzen RR, McCormick C. Things are looking up since we started listening to patients. Patient. 2008;1:273–282. doi: 10.2165/1312067-200801040-00009. [DOI] [PubMed] [Google Scholar]
- 7.Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–411. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Hensher DA, Rose JM, Greene WH. Applied choice analysis. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 9.Louviere JJ, Hensher DA, Swait J. Stated choice methods: analysis and application. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 10.Hauber AB, Gonzalez JM, Schenkel B, Lofland J, Martin S. The value to patients of reducing lesion severity in plaque psoriasis. J Dermatol Treat. 2011;22:266–275. doi: 10.3109/09546634.2011.588193. [DOI] [PubMed] [Google Scholar]
- 11.Johnson FR, Hauber AB, Ozdemir S, Lynd L. Quantifying women’s stated benefit-risk tradeoff preferences for IBS treatment outcomes. Value Health. 2010;13:418–423. doi: 10.1111/j.1524-4733.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- 12.Hauber AB, Mohamed AF, Johnson FR, Oyelowo O, Curtis BH, Coon C. Estimating importance weights for the IWQOL-Lite using conjoint analysis. Qual Life Res. 2010;19:701–709. doi: 10.1007/s11136-010-9621-9. [DOI] [PubMed] [Google Scholar]
- 13.Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: Report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 14.Dey A. Orthogonal fractional factorial designs. New York, USA: Halstead Press; 1985. [Google Scholar]
- 15.Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Mark Res. 1996;33:307–317. doi: 10.2307/3152127. [DOI] [Google Scholar]
- 16.Kanninen B. Optimal design for multinomial choice experiments. J Mark Res. 2002;39:214–227. doi: 10.1509/jmkr.39.2.214.19080. [DOI] [Google Scholar]
- 17.Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Mark Res. 1994;31:545–557. doi: 10.2307/3151882. [DOI] [Google Scholar]
- 18.Train K. Discrete choice methods with simulation. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- 19.Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Applications of simulation methods in environmental and resource economics. Dordrecht (Netherlands): Springer Publisher; 2005. [Google Scholar]
- 20.Hauber AB, Mohamed AF, Beam C, Medjedovic J, Mauskopf J. Patient preferences and assessment of likely adherence for hepatitis C virus treatments. J Viral Hepat. 2011;18:619–627. doi: 10.1111/j.1365-2893.2010.01343.x. [DOI] [PubMed] [Google Scholar]
- 21.Hauber AB, Arden NK, Mohamed AF, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthr Cartil. 2013;221:289–297. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Von Moos R, Body JJ, Egerdie B, et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer. 2013;21:3497–3507. doi: 10.1007/s00520-013-1932-2. [DOI] [PubMed] [Google Scholar]
- 23.Richhariya A, Qian Y, Zhao Y, Chung K. Time associated with intravenous zoledronic acid administration in patients with breast or prostate cancer and bone metastasis. Cancer Manag Res. 2012;4:55–60. doi: 10.2147/CMAR.S27693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Experimental Design (DOCX 286 kb)
Attributes and Levels for the Choice Questions (DOCX 16.4 kb)
Explanation of Treatment Attributes (DOCX 22.7 kb)
Treatment Profiles (DOCX 20.8 kb)
Example of a Choice Question (PDF 113 kb)
Patient Flow and Sample Sizes (PDF 71 kb)