Abstract
Purpose
Among some local side effects of prostaglandin-associated periorbitopathy (PAP), deepening of the upper eyelid sulcus (DUES) is the most prominent clinical feature, and is one of the most significant adverse cosmetic events. Here, we prospectively investigated the incidence of DUES in Japanese open-angle glaucoma patients initially treated with latanoprost (Xalatan 0.005%) ophthalmic solution.
Methods
This was an open-label prospective study. Facial photographs and subjective reports of the recognition of DUES were obtained at the beginning of latanoprost treatment and at 2, 4, and 6 months thereafter. Intraocular pressure (IOP) was measured at three consecutive visits before and after treatment with latanoprost. The incidence of DUES was evaluated objectively by three blinded investigators who compared the series of photographs.
Results
A total of 52 eyes of 52 newly diagnosed open-angle glaucoma Japanese patients (28 males, 24 females) were evaluated. The objective rate of DUES was 1/52 (2% 95% CI 0.05 to 10.7%) at 2 months, 2/52 (4% 95% CI 0.5 to 13.9%) at 4 months, and 3/52 (6% 95% CI 1.2 to 16.9%) at 6 months. During this period, no patient self-reported an occurrence of DUES. Mean IOPs before and after treatment were 16.5±2.9 and 13.8±3.0 mm Hg, respectively. Latanoprost reduced the IOP significantly (P<0.0001, paired t-test).
Conclusions
Latanoprost caused DUES rarely and had a robust IOP-lowering effect in Japanese glaucoma patients.
Introduction
Latanoprost (Xalatan 0.005% Pfizer, Tokyo, Japan; ‘LAT') was the first prostaglandin analog (PGA) to be launched and is currently a first-line drug for reducing intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension.1, 2 Systemic side effects are rarely observed after the topical use of PGAs, including three PGAs developed after LAT (travoprost, bimatoprost, and tafluprost); however, some apparent side effects involving the ocular surroundings have been noted.3 Among these local side effects, prostaglandin-associated periorbitopathy (PAP),4 which includes periorbital fat atrophy, deepening of the upper eyelid sulcus (DUES), ptosis, enophthalmos, and involution of dermatochalasis, has been reported after the use of prostaglandin F2α and prostamide analogs.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
DUES, which has been closely monitored for several years, is the most prominent clinical feature of PAP. Since the first case report of bimatoprost (BIM)-induced DUES,5 many cases induced by PGAs have been reported.6, 8, 9, 10 Although LAT has been used clinically for more than a decade, there are some case reports of DUES caused by long-term use in a glaucoma patient who used LAT unilaterally.15, 18 Subsequent retrospective studies reported that DUES occurred in association with the use of all PGAs.19, 22 These case reports and cross-sectional and retrospective studies may not be suitable for determining the incidence rates of DUES because they did not consider differences in the duration of treatment with each PGA and differences between both upper eyelids before PGA treatment. Thus, we reported the frequency of DUES occurrence associated with each PGA in 6-month prospective studies of Japanese glaucoma patients.12, 16, 20, 21 The rate of occurrence of DUES at 6 months was 60% for BIM 0.03%,12 53% for travoprost 0.004% (TRV),21 and 16% for tafluprost 0.0015% (TAF).20 However, LAT has remained the standard comparative target for other PGAs regarding the frequency of DUES occurrence.
In the present study, we prospectively monitored the occurrence of DUES in Japanese glaucoma patients using LAT as an initial drug treatment.
Materials and methods
This was an open-labeled, prospective study. All procedures were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent (including for the use of photographs) was obtained from all patients for inclusion in the study. The study protocol was approved by the local Ethics Committee of Miyata Eye Hospital (Miyazaki, Japan). We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
A total of 52 consecutive Japanese patients diagnosed initially with OAG were enrolled at the Yotsuya Shirato Eye Clinic (Tokyo, Japan) from September 2010 to July 2012. OAG was diagnosed by the presence of glaucomatous optic nerve head damage with corresponding visual field damage, an unoccludable normal open angle, and a lack of other ocular abnormalities or history of other ocular diseases leading to IOP elevation. The exclusion criteria included the use of eye drops within the previous 12 months or the use of an oral agent such as acetazolamide, a previous history of intraocular surgery (including laser treatment), and the presence of other ocular diseases affecting IOP.
After three consecutive IOP measurements in both eyes, we initiated LAT treatment in eyes with the more severe glaucomatous visual field damage when both eyes met the entry criteria, and the same medication was continued for 6 months. However, if medication became necessary in the control eye during the observation period, LAT was applied.
A single examiner (SS) measured IOP, checked the condition of the ocular surface and optic disc by slit-lamp examination, and took photographs at the beginning of LAT treatment and after every 2 months up to 6 months. In total, four photographs were taken over the entire observation period, including at the start of treatment. Facial photographs were taken using a 9.1-megapixel digital camera (EX-FC 100; Casio, Tokyo, Japan) to capture the eyebrow and lower eyelid without using the frontalis muscle.
The mean IOPs of three consecutive visits before and after the initiation of LAT treatment (IOPbase and IOPLAT) were calculated. The change in IOP was calculated as ΔIOP=IOPLAT−IOPbase. The IOP was measured using a Goldman applanation tonometer (Haag Streit, Koeniz, Switzerland), and slit-lamp microscopy examination was conducted at every outpatient visit at approximately the same time of day after applying one drop of topical oxybuprocaine hydrochloride anesthesia (0.4% Benoxil; Santen, Tokyo, Japan). In addition, during the observation period, refraction was measured using an automatic refractor/keratometer (ARK-900; Nidek, Tokyo, Japan), and the visual field was measured using a Humphrey Field Analyzer (Zeiss-Humphrey, San Leandro, CA, USA) Swedish interactive threshold algorithm (SITA) standard 30-2 program. Reliable test results (<30% fixation loss, false-negative, and <15% false-positive rate) were adopted.
Three ophthalmologists independently assessed DUES by evaluating a series of four photographs (at the starting point and after 2, 4, and 6 months). The three photographs taken after starting LAT were displayed on a 21-inch liquid-crystal display (FlexScan L997; Eizo, Tokyo, Japan) in order and were compared with the initial (starting point) photograph. When changes to the upper eyelid sulcus, even a slight deepening, were recognized, the photograph was marked as positive regardless of the degree of deepening in comparison with the initial photograph. When the three observers concurred on the deepening of the palpebral in the same photograph, the patient was judged as DUES positive. They stipulated the initiation of DUES as the date of the earliest DUES-positive photograph, which was confirmed in subsequent (second and third) pictures. The patients were asked to self-assess changes in the deepening of the palpebral at each visit. This series of methodologies for appraising DUES was the same as has been applied previously, using the same camera and visualization process.12, 16, 20
Analyses were performed using JMP software (ver. 9.0.2; SAS, Cary, NC, USA) and MedCalc (ver. 13.3.3, MedCalc Software bvba, Ostend, Belgium). Two-sided P-values of<0.05 were considered to indicate significance. The data are presented as means±SD.
Results
All 52 patients (52 eyes) completed the 6-month study with no severe ocular or systemic adverse effects. There were 47 eyes with normal-tension glaucoma (NTG) and 5 eyes with primary open-angle glaucoma (POAG). Demographic data are presented in Table 1. No medication except LAT was administered in the target eyes and no medication was administered in the control eyes during the observation period.
Table 1. Background of enrolled Japanese glaucoma patients.
| Characteristic | Value |
|---|---|
| Age (years) | 49.7 (10.9) |
| Number of patients | 52 |
| NTG/POAG | 47/5 |
| Male/female | 28/24 |
| Spherical equivalent refraction (diopter) | −5.1 (5.4) |
| HFA 30-2 SITA standard mean deviation (dB) | −4.4 (3.2) |
| IOPbase=mean IOP over the last three consecutive visits before treatment with LAT (mm Hg) | 16.5 (2.9) |
| IOPLAT= mean IOP over the first three consecutive visits after treatment with LAT (mm Hg) | 13.8 (3.0) |
Abbreviations: HFA, Humphrey field analyzer; IOP, intraocular pressure; LAT, latanoprost ophthalmic solution; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma; SITA, Swedish Interactive Threshold Algorithm. All data are numbers or means (SD).
Incidence of objective DUES
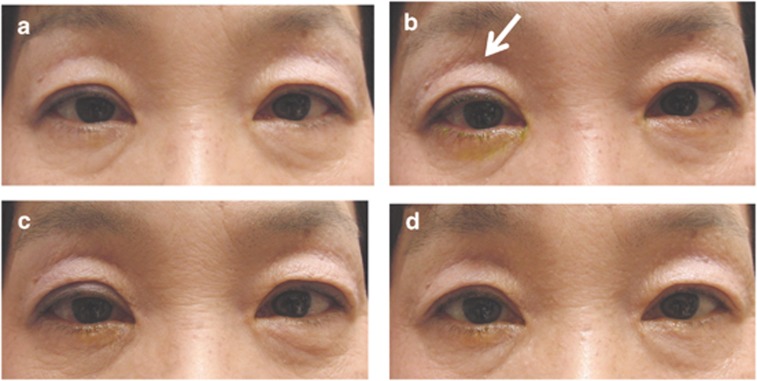
The objective rate of DUES was 1/52 (2% 95% CI 0.05 to 10.7%) at 2 months, 2/52 (4% 95% CI 0.5 to 13.9%) at 4 months, and 3/52 (6% 95% CI 1.2 to 16.9%) at 6 months. All three ophthalmologists attained consensus on all judgments at these three time points. Demographic data of the three DUES-positive patients are presented in Table 2. Figure 1 shows the development of DUES in one patient (right eye) that appeared 2 months after starting LAT.
Table 2. Background of the patients positive to deepening of the upper eyelid sulcus.
| Characteristic | Value |
|---|---|
| Age (years) | 50.0 (7.0) |
| Number of patients | 3 |
| NTG/POAG | 3/0 |
| Male/female | 1/2 |
| Spherical equivalent refraction (diopter) | −5.3 (1.4) |
| HFA 30-2 SITA standard mean deviation (dB) | −6.8 (5.5) |
| IOPbase=mean IOP over the last three consecutive visits before treatment with LAT (mm Hg) | 13.1 (0.5) |
| IOPLAT=mean IOP over the first three consecutive visits after treatment with LAT (mm Hg) | 10.7 (1.2) |
Abbreviations: HFA, Humphrey field analyzer; IOP, intraocular pressure; LAT, latanoprost ophthalmic solution; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma; SITA, Swedish Interactive Threshold Algorithm. All data are numbers or means (SD).
Figure 1.
A case of deepening of the upper eyelid sulcus caused by latanoprost ophthalmic solution. This 58-year-old female showed deepening of the upper eyelid sulcus in her right eye 2 months after starting latanoprost ophthalmic solution (b, white arrow). (a–d) Respectively, 0 (starting point), 2, 4, and 6 months after starting latanoprost.
Subjective symptoms
No patient reported DUES during the full observation period.
IOP measurements
The mean IOPs at three consecutive visits before (IOPbase) and after starting LAT (IOPLAT) were 16.5±2.9 and 13.8±3.0 mm Hg, respectively. LAT reduced the IOP significantly (ΔIOP=−2.6 mm Hg, P<0.0001, paired t-test).
Discussion
This is the first prospective study to investigate the rate of LAT-induced DUES, a noticeable symptom of PAP. Originally, PAP was associated with five major clinical symptoms (ie, periorbital fat atrophy, DUES, ptosis, enophthalmos, and involution of dermatochalasis),4 but has not as yet been formally defined. Thus, there may be some overlapping clinical symptoms noted in a single patient, or as-yet-unrecognized configurations that will appear in the future. However, for this study, we monitored only DUES, as in preceding reports.12, 16, 20 To date, there is no quantitative method for defining PAP with the inclusion of DUES. Thus, it should be noted that DUES-positive cases represent a broad range of the severity of DUES, from slight changes recognized by photographs to obvious deepening noticed at a glance.
In this 6-month study of LAT treatment, only 3 of 52 patients expressed slight changes in DUES by objective examination. Because none of these three patients noticed the DUES by themselves despite the fact that LAT induced the DUES, the degree of deepening may have been mild in this short-term study. Our results indicate that LAT-induced DUES did not become widely known until recently, and this is in contrast to the fact that LAT is the first launched PGA and has been in long-term use for more than a decade. As previously noted, there have been many case reports and clinical studies of the later PGAs such as BIM and TRV that have caused DUES.5, 6, 8, 9, 10, 12, 21 The rate of TAF-induced DUES was demonstrated recently.17, 20
The first case report of LAT-induced PAP (including DUES) focused on periocular changes in the treated eye with chronic unilateral administration of LAT.15 It involved three late elderly patients, and the mean duration of LAT usage was 24 months (range, 12–48 months). The observed symptoms of PAP were dermatochalasis, DUES, and loss of eyelid fullness, but no patient demonstrated enophthalmos. These patients continued LAT treatment even after the emergence of these changes.
Various cross-sectional and retrospective clinical record analyses have investigated the rate of LAT-induced PAP. Inoue et al19 showed that 24% of Japanese glaucoma patients undergoing LAT treatment incurred DUES (mean age, 62.1 years; mean administration period, 5.0 years), and this incidence was the second lowest among four PGAs, followed by TAF (18%). However, there are some concerns about the protocol used in that cross-sectional study. DUES was evaluated by comparing the depth between both eyelid sulci without considering the original difference in both eyelid sulci. Moreover, the duration of use of LAT was significantly longer than that of TAF (5.0 and 0.9 years, respectively). Therefore, the incidence rates in the study were not evaluated precisely. Although Kucukevcilioglu et al22 demonstrated that PAP had occurred in 41.4% of Caucasian patients, the rate of DUES was 15.7% (mean age, 66 years; mean administration period, 48 months). In that study, the incidence of DUES by LAT was the lowest among LAT, TRV, and BIM. Although the study design, enrolled subjects, and duration of drug use were different, our incidence (6%) was not comparable with these previous reports.
However, we are now able to discuss the objective rates of DUES occurrence among four types of PGAs (BIM, TRV, TAF, and LAT) according to our previous three reports using the same methodology in Japanese patients.12, 20, 21 Our prospective studies have demonstrated that approximately half of patients treated with BIM or TRV will develop DUES; the rate was relatively low for TAF treatment. In the present study, DUES barely developed with LAT use (Table 3). These studies indicate that the first 4 months after starting treatment may be particularly important because the incidence rates remained virtually unchanged after that time. The best method to avoid overlooking DUES might be by taking photographs before and after starting PGAs and evaluating them as still images. Previous comparative studies have (retrospectively) also demonstrated similar trends.19, 22 In each, the frequency of DUES was always higher in BIM and TRV treatment, followed by TAF or LAT. This is also corroborated by the fact that a much greater number of reports of BIM- or TRV-induced DUES have been published. In contrast, TAF-17, 20 or LAT-induced cases15 have been reported only rarely.
Table 3. Summary of the objectively evaluated rate of deepening of the upper eyelid sulcus treated with four prostaglandin analogs by prospective studies in Japanese glaucoma patients.
| Eye drop | 2 Months | 4 Months | 6 Months |
|---|---|---|---|
| Bimatoprost (na =25)12 | 44b | 60b | 60 |
| Travoprost (n =32)21 | 34 | 53 | 53 |
| Tafluprost (n =43)20 | 9 | 14 | 14 |
| Latanoprost (n =52) this study | 2 | 4 | 6 |
All data are given as the percentage (%).
Number of subjects.
In the original paper, 44 and 60% were obtained at 1 month and at 3 months.
We previously investigated the recovery rate from BIM-induced DUES after switching therapy treatment to LAT.16 After reverting to LAT, DUES was reduced or disappeared in most patients (85%). The fact that the rate of DUES induced by LAT was much lower than that seen with BIM in this study may explain the reversal of the condition after changing treatment from BIM to LAT.
Several contributing factors related to this side effect, such as age, sex, refraction, mean deviation, and IOP reduction, have been discussed,12, 16, 17, 21 but common risk factors have not yet been elucidated because of small sample sizes. Randomized long-term prospective clinical trials including all four PGAs with large numbers of patients are needed to identify the relevant ocular and systemic factors in play.
The proposed mechanism for DUES has changed repeatedly over time. It was first believed that DUES was derived from either fibrosis or atrophy of Müller's muscle.5 Later, atrophy of the preaponeurotic and deep orbital fat pads was thought to be the causative mechanism.6 PGA-induced lipolysis may play a role in DUES that is dependent upon the stimulation of the prostaglandin F (FP) receptor in orbital tissue.10 We recently reported that the activated form of all PGAs and PGF2α dose-dependently suppressed adipogenesis in differentiated adipocytes, but did not suppress adipogenesis in the adipocytes of FP receptor knockout mice.23 An in vivo histological analysis indicated that the density of adipocytes obtained from preaponeurotic fat biopsies was lowest in BIM-treated patients among those treated with BIM, TRV, or LAT. Cell density in the LAT group was not significantly different from that in the control group.14 The difference in the action of each PGA on adipose cells may be one reason behind the different phenotypic alterations.
Before the use of PGAs in glaucoma treatment, adipocyte differentiation was reportedly inhibited by PGF2α through the activation of the FP receptor.24, 25, 26 Stimulation of the prostaglandin E (EP3) receptor is also thought to inhibit adipocyte differentiation.27 Thus, differences in the affinities of the FP and EP3 receptors for the various forms of PGA may influence events in vivo. PGAs may act not only on Müller's muscle,5 but may also influence collagen degradation in the levator muscle complex.9 Systemic prostaglandins are reported to change with high-density lipoprotein levels;28 accordingly, they may play a role in adipogenesis. Differences in the penetration rate or concentration of each PGA in the orbital tissue, including the eyelids, may also be involved in the differences in the clinical phenotypes of DUES. Such multiple mechanisms of action, which may also involve interactions between effectors and adipose cells, must be considered when DUES develops.
An improved formulation of benzalkonium chloride-free LAT (Monoprost) has recently been approved in Europe. In addition, many generic drug forms of LAT are on the market in Japan. We should continue to investigate to what extent these formulations may be involved in DUES.
LAT showed a good IOP-lowering effect, even in eyes with normal pressure. No IOP elevation or aggravation of the optic disc was seen in the noninstilled eye during 6 months of observation. In addition, we confirmed that there was no incidence of upper eyelid change on the noninstilled side.
The present study has some limitations. First, the small number of patients included in the study renders a detailed analysis difficult. Additional studies with larger populations are needed. Second, our follow-up period was relatively short, similar to previous studies. The follow-up duration could have been prolonged for >6 months, as the time of DUES occurrence has ranged up to 5 years. It is possible that LAT-induced DUES occurs gradually; thus, it could be overlooked, together with other symptoms of PAP. The assessment procedures for DUES are not currently standardized. Judgments are based on subjective measurements made by skilled observers. A quantitative, noninvasive method based on objective scientific measurements is needed. We believe that taking frontal photographs, including the eyebrow, using a standardized photographic technique is a minimal requirement at the present time. In this context, before a point-by-point numerical index is developed, we cannot monitor how DUES progresses through time. Although we examined and photographed the patients at approximately the same time and under identical conditions as much as possible, there may be exogenous or endogenous agents affecting the appearance of the upper eyelid. Another limitation is the semiquantitative measurement method used to evaluate DUES. A final drawback is that the subjects were almost all Japanese NTG patients. The incidence rate may differ in other races or ethnicities. Structural differences in the bone, orbit, or skin may also be related to the responses to PGAs. However, at least in Japanese OAG patients, it is clear that DUES was least common with the use of LAT among the four PGAs now available.12, 20, 21
In conclusion, LAT has a low probability of inducing DUES, but was effective in lowering IOP in Japanese OAG patients. LAT appears to have a favorable balance between efficacy and tolerability.
The authors declare no conflict of interest.
References
- Stjernschantz JW. From PGF(2alpha)-isopropyl ester to latanoprost: a review of the development of xalatan: the proctor lecture. Invest Ophthalmol Vis Sci. 2001;42:1134–1145. [PubMed] [Google Scholar]
- Perry CM, McGavin JK, Culy CR, Ibbotson T. Latanoprost: an update of its use in glaucoma and ocular hypertension. Drugs Aging. 2003;20:597–630. doi: 10.2165/00002512-200320080-00005. [DOI] [PubMed] [Google Scholar]
- Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53 (Suppl 1:S93–105. doi: 10.1016/j.survophthal.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Herndon LW, Alward WLM.State-of-the-Art Glaucoma Care for Today and Tomorrow Glaucoma 2011. Available at http://www.aao.org/pdf/Glaucoma-2011-Syllabus.pdf . Accessed 6 May 2014.
- Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574–577. doi: 10.1097/01.opx.0000141791.16683.4a. [DOI] [PubMed] [Google Scholar]
- Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, Grosskreutz CL. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg. 2008;24:302–307. doi: 10.1097/IOP.0b013e31817d81df. [DOI] [PubMed] [Google Scholar]
- Tappeiner C, Perren B, Iliev ME, Frueh BE, Goldblum D. [Orbital fat atrophy in glaucoma patients treated with topical bimatoprost—can bimatoprost cause enophthalmos?] Klin Monbl Augenheilkd. 2008;225:443–445. doi: 10.1055/s-2008-1027362. [DOI] [PubMed] [Google Scholar]
- Yam JC, Yuen NS, Chan CW. Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol Ther. 2009;25:471–472. doi: 10.1089/jop.2009.0019. [DOI] [PubMed] [Google Scholar]
- Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol. 2009;53:176–179. doi: 10.1007/s10384-008-0623-x. [DOI] [PubMed] [Google Scholar]
- Aydin S, Isikligil I, Teksen YA, Kir E. Recovery of orbital fat pad prolapsus and deepening of the lid sulcus from topical bimatoprost therapy: 2 case reports and review of the literature. Cutan Ocul Toxicol. 2010;29:212–216. doi: 10.3109/15569521003796860. [DOI] [PubMed] [Google Scholar]
- Jayaprakasam A, Ghazi-Nouri S. Periorbital fat atrophy - an unfamiliar side effect of prostaglandin analogues. Orbit. 2010;29:357–359. doi: 10.3109/01676830.2010.527028. [DOI] [PubMed] [Google Scholar]
- Aihara M, Shirato S, Sakata R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn J Ophthalmol. 2011;55:600–604. doi: 10.1007/s10384-011-0075-6. [DOI] [PubMed] [Google Scholar]
- Nakakura S, Tabuchi H, Kiuchi Y. Latanoprost therapy after sunken eyes caused by travoprost or bimatoprost. Optom Vis Sci. 2011;88:1140–1144. doi: 10.1097/OPX.0b013e3182231202. [DOI] [PubMed] [Google Scholar]
- Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011;55:22–27. doi: 10.1007/s10384-010-0904-z. [DOI] [PubMed] [Google Scholar]
- Ung T, Currie ZI. Periocular changes following long-term administration of latanoprost 0.005% Ophthal Plast Reconstr Surg. 2012;28:e42–e44. doi: 10.1097/IOP.0b013e31821d86a5. [DOI] [PubMed] [Google Scholar]
- Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013;57:179–184. doi: 10.1007/s10384-012-0219-3. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Tsuchisaka A, Sakamoto J, Shirato S, Goto H. Incidence of deepening of upper eyelid sulcus after topical use of tafluprost ophthalmic solution in Japanese patients. Clin Ophthalmol. 2013;7:1441–1446. doi: 10.2147/OPTH.S47783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Berke S.Latanoprost-induced prostaglandin-associated periorbitopathy Optom Vis Sci 201390e245–e247.discussion 1029. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shiokawa M, Wakakura M, Tomita G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22:626–631. doi: 10.1097/IJG.0b013e31824d8d7c. [DOI] [PubMed] [Google Scholar]
- Sakata R, Shirato S, Miyata K, Aihara M. Incidence of deepening of the upper eyelid sulcus on treatment with a tafluprost ophthalmic solution. Jpn J Ophthalmol. 2014;58:212–217. doi: 10.1007/s10384-013-0299-8. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Shirato S, Tsuchisaka A. Incidence of deepening of the upper eyelid sulcus after topical use of travoprost ophthalmic solution in Japanese. J Glaucoma. 2014;23:160–163. doi: 10.1097/IJG.0b013e31826a7e09. [DOI] [PubMed] [Google Scholar]
- Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Experiment Ophthalmol. 2014;42:126–131. doi: 10.1111/ceo.12163. [DOI] [PubMed] [Google Scholar]
- Taketani Y, Yamagishi R, Fujishiro T, Igarashi M, Sakata R, Aihara M. Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Invest Ophthalmol Vis Sci. 2014;55:1269–1276. doi: 10.1167/iovs.13-12589. [DOI] [PubMed] [Google Scholar]
- Casimir DA, Miller CW, Ntambi JM. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation. 1996;60:203–210. doi: 10.1046/j.1432-0436.1996.6040203.x. [DOI] [PubMed] [Google Scholar]
- Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology. 1996;137:5641–5650. doi: 10.1210/endo.137.12.8940395. [DOI] [PubMed] [Google Scholar]
- Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun. 1997;233:200–202. doi: 10.1006/bbrc.1997.6433. [DOI] [PubMed] [Google Scholar]
- Strong P, Coleman RA, Humphrey PP. Prostanoid-induced inhibition of lipolysis in rat isolated adipocytes: probable involvement of EP3 receptors. Prostaglandins. 1992;43:559–566. doi: 10.1016/0090-6980(92)90115-a. [DOI] [PubMed] [Google Scholar]
- Steinhauser SL. Decreased high-density lipoprotein serum levels associated with topical bimatoprost therapy. Optometry. 2006;77:177–179. doi: 10.1016/j.optm.2006.02.001. [DOI] [PubMed] [Google Scholar]