Abstract
For determination of possible neurotransmitters synthesized by photoreceptor cells, turtle retinas were dissociated into single cells with proteolytic enzymes. These cells were partially separated by velocity sedimentation to yield a fraction rich in photoreceptors. Individual photoreceptor cells were then sucked into a micropipette and incubated with labeled precursors of known or suspected neurotransmitters. After incubation, the radioactive products were analyzed by high-voltage electrophoresis. Of all the chemicals tested, turtle photoreceptor cells synthesized only acetylcholine, suggesting that these cells may be cholinergic.
Keywords: Pseudemys scripta elegans, cell dissociation, cell separation, neurotransmitters, sensory neurons
Full text
PDF
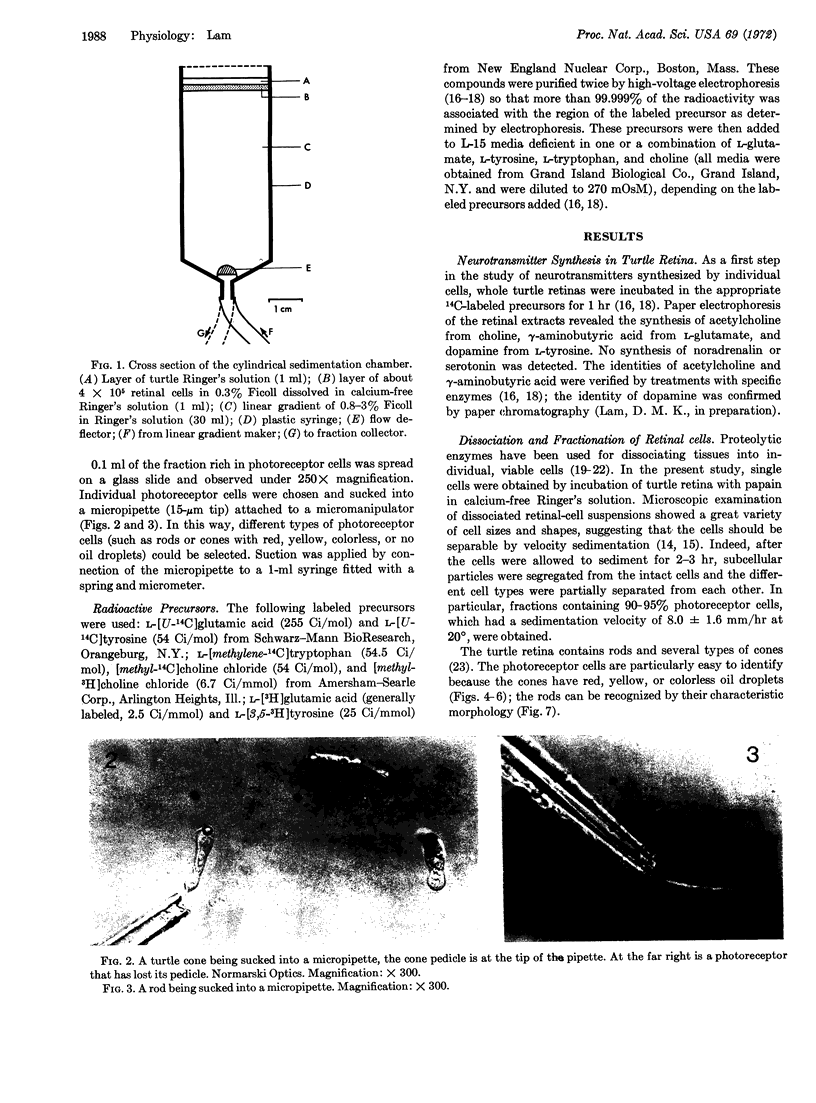
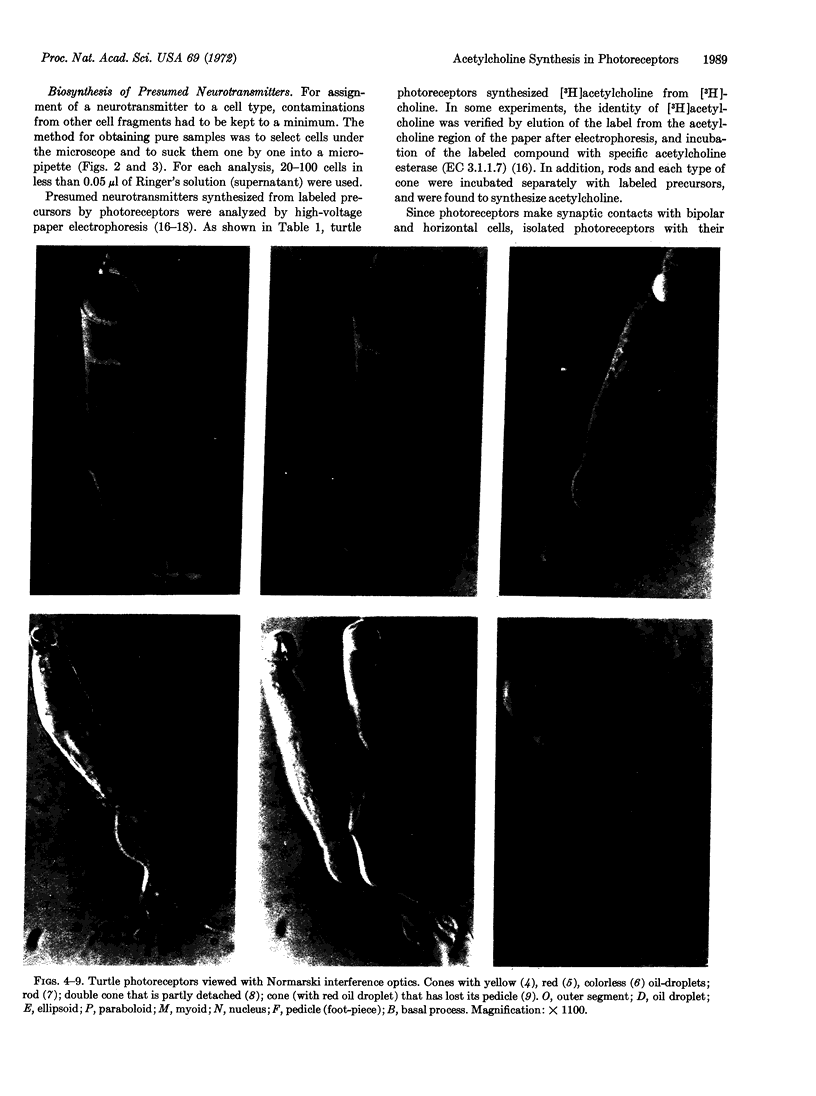


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Pollen D. A. Neurotransmission in central nervous tissue: a study of isolated rabbit retina. J Neurophysiol. 1969 May;32(3):424–442. doi: 10.1152/jn.1969.32.3.424. [DOI] [PubMed] [Google Scholar]
- Banks B. E., Banthorpe D. V., Lamont D. M., Pearce F. L., Redding K. A., Vernon C. A. Dissociation of sensory ganglia from the embryonic chick by pronase and other dispersing agents. J Embryol Exp Morphol. 1970 Apr;23(2):519–530. [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. H., Flumerfelt B. A., Hellenberg M. J., Gwyn D. G. Ultrastructural localization of cholinesterase activity in the outer plexiform layer of the newt retina. Brain Res. 1971 Dec 10;35(1):299–303. doi: 10.1016/0006-8993(71)90623-8. [DOI] [PubMed] [Google Scholar]
- Dowling J. E. Organization of vertebrate retinas. Invest Ophthalmol. 1970 Sep;9(9):655–680. [PubMed] [Google Scholar]
- FLOREY E., BIEDERMAN M. A. Studies on the distribution of factor I and acetylcholine in crustacean peripheral nerve. J Gen Physiol. 1960 Jan;43:509–522. doi: 10.1085/jgp.43.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCIS C. M. Cholinesterase in the retina. J Physiol. 1953 May 28;120(3):435–439. doi: 10.1113/jphysiol.1953.sp004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey E. Neurotransmitters and modulators in the animal kingdom. Fed Proc. 1967 Jul-Aug;26(4):1164–1178. [PubMed] [Google Scholar]
- Graham L. T., Jr, Baxter C. F., Lolley R. N. In vivo influence of light or darkness on the GABA system in the retina of the frog (Rana pipiens). Brain Res. 1970 Jun 15;20(3):379–388. doi: 10.1016/0006-8993(70)90168-x. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. G., Barker D. L., Herbert E., Kravitz E. A. Screening for neurotransmitters: a rapid radiochemical procedure. J Neurobiol. 1971;2(3):231–246. doi: 10.1002/neu.480020305. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T. Roles of collagenases and other proteolytic enzymes in the dispersal of animal tissues. Biochim Biophys Acta. 1969 Apr 22;178(2):397–400. doi: 10.1016/0005-2744(69)90410-0. [DOI] [PubMed] [Google Scholar]
- Kramer S. G. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol. 1971 Jun;10(6):438–452. [PubMed] [Google Scholar]
- Kuriyama K., Sisken B., Haber B., Roberts E. The gamma-aminobutyric acid system in rabbit retina. Brain Res. 1968 Jun;9(1):165–168. doi: 10.1016/0006-8993(68)90269-2. [DOI] [PubMed] [Google Scholar]
- Lam D. M., Furrer R., Bruce W. R. The separation, physical characterization, and differentiation kinetics of spermatogonial cells of the mouse. Proc Natl Acad Sci U S A. 1970 Jan;65(1):192–199. doi: 10.1073/pnas.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M. The biosynthesis and content of gamma-aminobutyric acid in the goldifsh retina. J Cell Biol. 1972 Aug;54(2):225–231. doi: 10.1083/jcb.54.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Granda A. M. Microspectrophotometric measurements of visual pigments in two species of turtle, Pseudemys scripta and Chelonia mydas. Vision Res. 1971 Feb;11(2):105–114. doi: 10.1016/0042-6989(71)90227-6. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M. H. Comparison of aggregation of embryonic cells dissociated with trypsin or versene. Exp Cell Res. 1967 Jan;45(1):239–243. doi: 10.1016/0014-4827(67)90129-2. [DOI] [PubMed] [Google Scholar]
- Val'tsev V. B. Role of cholinergic structures in outer plexiform layer in the electrical activity of frog retina. Fed Proc Transl Suppl. 1966 Sep-Oct;25(5):765–766. [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]