Abstract
Purpose
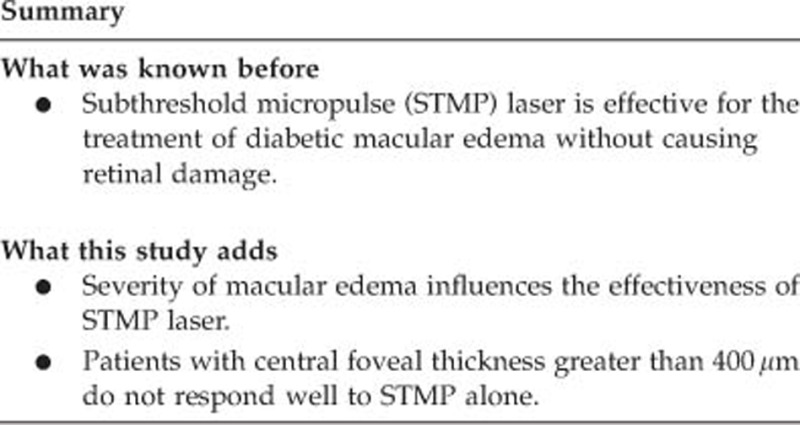
To determine if the severity of diabetic macular edema influences the effectiveness of subthreshold micropulse (STMP) laser treatment.
Methods
A total of 63 eyes of 58 patients with diabetic macular edema were divided into two groups based on their initial central foveal thickness (CFT). Group 1 had CFT ≤400 μm, group 2 had CFT >400 μm. The change from baseline in CFT and visual acuity were compared at 3, 6 and 12 months follow-up. Patients were considered for retreatment with micropulse laser at 3 months if macular edema had not improved. Patients were considered for rescue anti-VEGF injections if there was clinically significant macular edema at 6 months follow-up. Number of laser retreatments, injections, and any adverse effects from STMP laser were recorded.
Results
Group 1 (n=33) experienced an average of 55 μm reduction in CFT and 0.2log MAR gain in visual acuity at 12 months (P<0.001). No patient required rescue anti-VEGF injections. Group 2 (n=30) experienced no significant change in CFT or visual acuity by 6 months despite retreatment with STMP in 19 eyes. From 6 to 12 months follow-up, all the patients in group 2 received rescue Bevacizumab injections that resulted in 307 μm reduction in CFT and 0.3log MAR improvement in visual acuity (P<0.001). No adverse effects from STMP laser were recorded.
Conclusion
Severity of edema can influence the effects of STMP laser. STMP monotherapy is safe and effective in treating edema of mild to moderate severity.
Introduction
Macular edema is the leading cause of vision loss in patients with diabetes.1 An important component of the treatment for clinically significant diabetic macular edema (CSME) is visible end point laser photocoagulation proposed by the Early Treatment Diabetic Retinopathy Study (ETDRS).2 This treatment proved to be an effective method in decreasing the risk of moderate visual loss in patients with CSME. However, the beneficial effect of conventional laser photocoagulation is associated with the destruction of retinal photoreceptors, progressive enlargement of laser retinal scars, and the risk of developing choroidal neovascularization and subfoveal fibrosis.3, 4, 5
Unlike conventional laser photocoagulation, where a steady continuous wave laser output is applied, subthreshold micropulse (STMP) laser treatment delivers laser energy by dividing the beam into a train of short laser pulses. Each pulse has an on and off duration. The ratio of on to off time is defined as the duty cycle. A lower duty cycle reduces the laser energy from being delivered, thus diminishing the overall thermal effect and allowing tissue temperature to decrease to the baseline prior to the arrival of the next pulse. This method of laser delivery limits the overall laser-induced heat spread to the adjacent tissues and provides a means to deliver the laser energy without the detectable tissue damage associated with standard photocoagulation.6, 7, 8
Several prospective randomized trials have reported equal improvement in BCVA and retinal thickness between STMP and conventional ETDRS laser photocoagulation for CSME.9, 10, 11, 12 In those trials, the average CFT prior to STMP treatment was <400 μm and the subgroup analysis specifically looking at patients with large CFT was not reported. Retinal thickness may affect the tissue distribution of energy delivered by the STMP laser in a manner that may affect the clinical outcome. To our knowledge, there are no published data comparing the efficacy of STMP laser in accordance with the anatomical severity of macular edema. This is a pilot study to investigate whether retinal thickness has a role in response to 810-nm STMP.
Patients and methods
Institutional review board's approval was obtained for the study through the Western Institutional Review Board, Olympia, Washington. All data were collected in accordance with the Health Insurance Portability and Accountability Act of 1996. We retrospectively reviewed the office charts of patients with the diagnosis of diabetic macular edema (DME) treated with 810 nm STMP laser from January 2012 to March 2013.
Patients with a history of conventional laser application <6 months prior to STMP laser, anti-VEGF injection <3 months prior to STMP, subtenon or intravitreal steroid injection <6 months prior to STMP laser were excluded. Patients with documented follow-up of <12 months or missing follow-up appointments were excluded. Patients with simultaneous retinal diseases affecting visual acuity, such as age related macular degeneration, vein occlusions, and epiretinal membranes were also excluded.
All the patients were treated with the 810-nm diode micropulse laser (IRIDEX Corporation, Mountain View, CA, USA) with the following laser settings: power 950 mw; 300 ms duration; and 5% duty cycle and slit lamp (aerial) spot size of 125 μm. Area centralis contact lens (spot magnification factor of 1.06) was used to deliver the laser resulting in retinal laser spot size of 132 μm. Laser was applied in a confluent fashion to the entire area of the macular edema and leakage guided by OCT and fluorescein angiography. ETDRS visual acuity, spectral domain OCT (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany) measurements of central foveal thickness (CFT) and follow-up intervals were recorded from patient records. Visual acuities were converted to Log MAR values for analysis. Any adverse effects of the laser including subjective reports of scotoma, evidence of retinal tissue damage per fluorescein angiography, OCT, or clinical examination were recorded.
The decision to retreat with laser was to the discretion of the treating physician. Generally, if macular edema had not improved by 3 months after the initial laser, decision was made to retreat with laser. If by 6 months after the initial laser there was clinically significant macular edema, decision was made to treat with rescue Bevacizumab injection.
Patients were divided into two groups based on their initial CFT. Group 1 composed of patients with CFT ⩽400 μm, group 2 composed of patients with CFT >400 μm. The change from baseline in CFT, and visual acuity were compared between the groups. Analysis of variance was used to compare the change in CFT and visual acuity. Student's t-test was used to compare the patient's baseline characteristics. A P-value <0.05 was considered to be significant.
Results
One hundred and thirty-four consecutive patients with DME who were treated with STMP laser were identified. Of these, 63 eyes of 58 patients met the inclusion/exclusion criteria. Group 1 composed of 33 eyes of 30 patients and group 2 composed of 30 eyes of 28 patients. Patients' baseline characteristics are shown in Table 1.
Table 1. Patient baseline characteristics.
| Group 1 | Group 2 | |
|---|---|---|
| Age, years, mean (SD) (Range) P=0.87 | 58.9 (7) (47–71) | 58.7 (6.15) (48–72) |
| Percent female | 47 | 46 |
| HgA1C %, mean (SD) (Range) P=0.54 | 8.0 (0.53) (7.2–9.2) | 7.9 (0.72) (6.8–9.1) |
| Duration of diabetes, years, mean (SD) (Range) P=0.55 | 9.6 (3.51) (5–18) | 9.1 (3.1) (5–17) |
| Percent type II diabetes | 97 | 100 |
| CFT, μm, mean, (SD) (Range) P<0.001 | 331 (39.4) (276–400) | 605 (67.8) (485–731) |
| Visual acuity, Log MAR, (SD) (Range) P<0.001 | 0.3 (0.11) (0.1–0.5) | 0.54 (0.12) (0.3–0.7) |
Abbreviation: CFT, central foveal thickness.
At 3 months follow-up, group 1 experienced 41 μm reduction in CFT to 290 μm (P=2.6E-5), whereas group 2 experienced no significant change in CFT (605–608 μm, P=0.41). Mean visual acuity in group 1 increased from Log MAR 0.3 (20/40) to Log MAR 0.133 (∼20/27) (P=6.2E-10), whereas visual acuity in group 2 remained unchanged at Log MAR 0.54 (∼20/70) (P=0.43). At 3 months follow-up, 19 patients in group 2 were retreated with STMP laser versus none in group 1. No patient in either group received intravitreal injections.
At 6 months follow-up, group 1 experienced an additional 9 μm reduction in CFT to 281 μm. No patient in group 1 had clinically significant macular edema. Group 2 experienced no significant change in CFT (608–611 μm). Mean visual acuity in group 1 increased to Log MAR 0.1 (20/25). Mean visual acuity in group 2 remained unchanged at Log MAR 0.55 (∼20/70) (Table 2). Changes in CFT and visual acuity between 3 months follow-up and 6 months follow-up were not statistically significant in either group.
Table 2. Mean (range) CFT and visual acuity at baseline and various follow-up intervals in two groups.
| Baseline | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Group 1 CFT(μm) | 331 (292–400) | 290 (265–332) | 281 (255–298) | 276 (258–296) |
| Group 2 CFT(μm) | 605 (442–858) | 608 (445–897) | 611 (478–883) | 298 (247–329) |
| Group 1 visual acuity (Log MAR) | 0.3 | 0.13 | 0.1 | 0.1 |
| Group2 visual acuity (Log MAR) | 0.54 | 0.54 | 0.55 | 0.24 |
Abbreviation: CFT, central foveal thickness.
No patient in group 1 had persistent CSME at any time from 6 months follow-up until 12 months follow-up and received no intravitreal injections. At 12 months follow-up, group 1 had experienced a total of 55 μm reduction in CFT from baseline (331 μm) to 276 μm (P=5.6E-10). Mean visual acuity in group 1 was Log MAR 0.1 (20/25). This represented a total of 0.2log MAR increase compared with baseline (P=4.2E-14).
At 6 months follow-up, all the patients in group 2 had clinically significant macular edema and received monthly rescue Bevacizumab injections until CSME resolved. On an average, group 2 received 3.1 (range of 2–5) injections from 6 months follow-up until 12 months follow-up. At the final follow-up, group 2 experienced a significant reduction in CFT to 298 μm (P=5.1E-30). Mean visual acuity in group 2 increased significantly to Log MAR 0.24 (∼20/34) (P=6.7E-16).
No adverse effect from STMP laser was recorded in either group. There was no evidence of tissue damage from STMP laser per fluorescein angiography, OCT images, or clinical findings at any follow-up visit.
Discussion
STMP laser has gained increasing interest in the treatment of DME with promising results. STMP has been shown to be an effective treatment option comparable to the modified ETDRS macular photocoagulation without causing chorioretinal scarring or inducing visual field scotomas. Better transmission of 810-nm light through retina can imply that retinal thickness is not a determining factor for a response to 810 nm STMP laser. Our goal was to investigate the influence of macular thickness on the response to 810-nm STMP laser treatment.
Patients investigated in prior studies9, 10, 11, 12 had mean CFTs in the range of 250–350 μm and standard deviations in the range of 50–90. This implies that most patients in these studies had CFT <400 μm and therefore resembled patients in group 1 of our study. We chose 400 μm to stratify patients into two groups based on the authors' own observation that patients with CFT >400 μm do not respond well to STMP monotherapy.
Our results show an average CFT reduction of 55 μm and two lines of visual gain at 12 months in group 1. No patient in group 1 had retreatment with STMP laser or required rescue anti-VEGF injections. Vujosevic et al11 showed an average CFT reduction of 47 μm in eyes treated with STMP alone. Of note, in that study, the eyes treated with STMP had an average baseline CFT of 358 μm, which is close to that of our patients in group 1.
In studies done by Laursen et al9 and Lavinski et al12 patients continued to improve for up to 12 months post laser; however, more than half the reduction in macular edema was already achieved by 3 months post laser. Luttrull et al13 have shown in their prospective OCT measurement study that the majority of patients respond in 3 months post STMP laser. Figueria et al10 showed no further reduction in the macular edema between 4 months post STMP laser and 12 months post STMP laser. Therefore, if there is no improvement in macular edema by 6 months, it is unlikely that waiting longer would result in a significant improvement in non-responders to STMP. In our study, if there was persistent CSME at 6 months, rescue Bevacizumab was given.
The decision to retreat with laser was left to the discretion of the treating physician. Generally, if macular edema had not improved by three months, decision was made to retreat with laser. In group 2, majority of eyes (19 out of 30) were retreated with STMP laser at 3 months. Despite this, all 30 eyes had persistent CSME at 6 months follow-up and required rescue injections of Bevacizumab. The significant reduction in the macular edema which was noted at the final follow-up for group 2 is most likely due to rescue injections rather than delayed effect of laser.
The exact cause of this lack of response to STMP alone in patients with severe anatomical disease is not clear. We are not aware of any study reporting on the treatment effects of STMP alone on the subgroup of patients with severe macular edema. It is possible that the effect of STMP on this subgroup is washed out in reporting averages for the entire group as is customary. Multiple factors could be in play. It is thought that RPE cell stimulation by laser results in the release of cytokines that decrease the edema and might be responsible for the beneficial effects of STMP.14, 15 Severe edema could possibly dilute the concentration of such cytokines or alter the distribution of laser energy throughout the retina and RPE. Perhaps different laser parameters are required in patients with greater edema. Another option might be to reduce the macular edema with anti-VEGF agents or steroids prior to the application of STMP laser.
The limitations of our study include its retrospective nature and small sample size. Prospective randomized control trials with larger sample sizes comparing different laser power settings and combination regimens are required to further elucidate the optimal role of STMP laser in the treatment of severe DME.
In summary, our pilot study indicates that the anatomical severity of DME can influence the treatment response to STMP laser. STMP laser monotherapy is safe and effective in treatment of mild to moderate DME.
The authors declare no conflict of interest.
References
- Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy XI. The incidence of macular edema. Ophthalmology. 1989;96:1501–1510. doi: 10.1016/s0161-6420(89)32699-6. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–1551. doi: 10.1001/archopht.1991.01080110085041. [DOI] [PubMed] [Google Scholar]
- Lewis H, Schachat AP, Haimann MH, Haller JA, Quinlan P, von Fricken MA, et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology. 1990;97:503–510. doi: 10.1016/s0161-6420(90)32574-5. [DOI] [PubMed] [Google Scholar]
- Guyer DR, D'Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113:652–656. doi: 10.1016/s0002-9394(14)74789-0. [DOI] [PubMed] [Google Scholar]
- Mainster MA. Decreasing retinal photocoagulation damage: principles and techniques. Semin Ophthalmol. 1999;14:200–209. doi: 10.3109/08820539909069538. [DOI] [PubMed] [Google Scholar]
- Luttrull JK, Sramek C, Palanker D, Spink CJ, Musch DC. Long-term safety, high-resolution imaging and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina. 2012;32:375–386. doi: 10.1097/IAE.0b013e3182206f6c. [DOI] [PubMed] [Google Scholar]
- Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular edema. Br J Ophthalmol. 2005;89:74–80. doi: 10.1136/bjo.2004.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–1179. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomized controlled trial comparing subthreshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–1344. doi: 10.1136/bjo.2008.146712. [DOI] [PubMed] [Google Scholar]
- Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorecence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010;30:908–916. doi: 10.1097/IAE.0b013e3181c96986. [DOI] [PubMed] [Google Scholar]
- Lavinsky D, Cardillo JA, Melo L, Dare A, Farah ME, Belfort R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52 (7:4314–4323. doi: 10.1167/iovs.10-6828. [DOI] [PubMed] [Google Scholar]
- Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2006;37:370–377. doi: 10.3928/15428877-20060901-03. [DOI] [PubMed] [Google Scholar]
- Ulbig MW, McHugh DA, Hamilton AM. Diode laser photocoagulation for diabetic macular edema. Br J Ophthalmol. 1995;79:318–321. doi: 10.1136/bjo.79.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser BM, D'Amore PA, Michels RG, Patz A, Fenselau A. Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol. 1980;84:298–304. doi: 10.1083/jcb.84.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]


