Abstract
Introduction
Greater sympathetic drive has been established in the early stages of essential hypertension, suggesting that neurohormonal dysregulation may be key to its aetiology and progression. The aims of this review are to discuss evidence of the role of autonomic dysfunction in essential hypertension and proposed mechanisms, and also some applications of this knowledge to current management strategies of essential hypertension.
Methods
A computer search was performed using the PUBMED database for peer reviewed original articles comparing autonomic function tested via heart rate variability (HRV), muscle sympathetic nerve activity (MSNA) or plasma noradrenaline levels in normotensive (mean blood pressure (BP) of ≤140/90 mmHg or ≤135/85 mmHg if measured via home BP measurements) and hypertensive groups (mean resting BP of ≥140/90 mmHg (or ≥135/85 mmHg if measured via home BP measurements). Subjects were excluded with secondary causes of hypertension or autonomic dysfunction.
Results
A total of 17 studies were included for discussion. The main findings of this study include that of reduced baroreflex sensitivity, believed to be secondary to increased arterial stiffness, is hypothesised to be implicated in the pathogenesis of essential hypertension. Also, angiotensin converting enzyme inhibitors were not as effective on markers of autonomic control of blood pressure when compared with alternative anti-hypertensive drugs.
Conclusions
Consistent research is needed to establish the effectiveness of pharmacotherapies at each of stage of hypertension, and on markers of autonomic dysfunction. Consistent study designs will enable more accurate accumulation of data across multiple studies, and appropriate application of such data into clinical practice.
Keywords: Hypertension, Autonomic dysfunction, Heart rate variability
1. Introduction
Essential hypertension is the world's most prevalent cardiovascular disorder, affecting approximately 26% of the worldwide population and still rising [1,2]. However, our understanding of the underlying causal mechanism behind the pathology remains somewhat unclear. Greater sympathetic drive has been established in the early stages of essential hypertension, suggesting that neurohormonal dysregulation may be key to its aetiology [3], the progression of hypertension and subsequent end-organ damage, such as raised arterial stiffness and left ventricular hypertrophy [3]. However, the specific mechanisms by which this occurs are still unknown, as are the implications of anti-hypertensive pharmacotherapies on such dysfunction. The aims of this review are to discuss evidence of the role of autonomic dysfunction in essential hypertension and proposed mechanisms, and also some applications of this knowledge to current management strategies of essential hypertension.
2. Methodology
An electronic database search was performed using the PUBMED database (years 1975–2012). The following search terms were used in combinations, restricted to human studies only: autonomic dysfunction, baroreflex sensitivity, hypertension, sympathetic and randomised controlled trial. The reference lists of analysed articles were also searched. Additional articles were included from the author's own library, screened to meet the aforementioned criteria [4–7]. Inclusion criteria included: i) peer-reviewed publications reporting original data; ii) a minimum of 10 subjects tested to maximise reliability; iii) subjects had autonomic function tested via heart rate variability (HRV), muscle sympathetic nerve activity (MSNA) or plasma noradrenaline levels; iv) studies with hypertensive groups had a mean resting blood pressure (BP) of ≥140/90 mmHg (or ≥135/85 mmHg if measured via home BP measurements) [8] or normotensive groups must have a mean BP of ≤140/90 mmHg (or ≤135/85 mmHg if measured via home BP measurements); v) studies must have provided details of at least one of: epidemiology, pathophysiology, mechanism of action or long-term progression. Publications with titles or abstracts appearing to meet these inclusion criteria were selected for detailed review. Studies including patients with secondary causes of either hypertension (such as renal disease or endocrine disorders) or of autonomic dysfunction were excluded (such as subjects with Parkinson's disease, spinal trauma, and inherited or acquired peripheral neuropathy such as amyloidosis, diabetes mellitus, acute porphyria, infectious disease, connective tissue disease and vitamin deficiency and nutrition-based neuropathy). The articles listed were reviewed by the author only. Due to the variability in study designs and of the outcome measures used, a narrative of the collected data will be reported rather than quantitative analysis. Where possible, data is presented as mean ± SD, percentages and confidence intervals (CI).
3. Results
Fig. 1 summarises the number of articles included for analysis based on the aforementioned search criteria. A total of 203 citations were identified with 8 articles excluded as duplicates. 171 articles were excluded on the basis of reviewing the title and abstract only. Of the remaining 24 articles, 1 study was excluded on the basis of inappropriate outcome measures of autonomic nervous system (ANS) function [4], 1 excluded due to low sample sizes [5] and 5 excluded on the basis of data not including data conducive to the aims of this review [6,7,9–11]. A total of 17 studies were included for discussion. Of these, 4 studies demonstrate evidence of autonomic dysfunction's implication in essential hypertension and its development (2 prospective studies [12,13] and 2 cohort studies [14,15]), 3 discuss the role of reduced baroreflex sensitivity in the pathogenesis of essential hypertension (prospective, population-based cohort studies) [16–18], 2 studies discuss the use of baroreceptor stimulation for drug-resistant hypertension (1 randomised controlled trial (RCT) [19] and 1 non-randomised trial [20]), 6 studies compare the effectiveness of two anti-hypertensive medications on markers of autonomic dysfunction (2 randomised cross-over studies [21,22] and 4 RCTs [23–26]), and 2 discuss the use of anti-hypertensives and their peripheral effects (1 RCT [27] and 1 meta-analysis [28]).
Fig. 1.
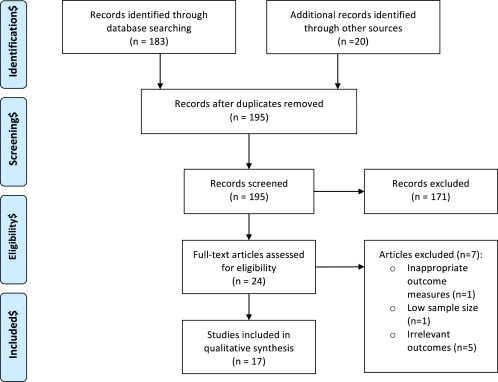
PRISMA flow diagram.
4. Discussion
4.1. Essential hypertension: evidence of autonomic dysregulation
Autonomic dysfunction has been demonstrated before hypertension is established as well as in its early stages (Table 1), characterised by increased cardiac dynamics manifesting in raised cardiac output and plasma catecholamine levels [12]. The excessive sympathetic tone and/or vagal withdrawal that define autonomic dysfunction is still relatively unknown in its aetiology and mechanism, but there are associated pathophysiological changes which highlight the relationship between the ANS and essential hypertension, including baroreflex sensitivity (BRS) that will be discussed later in this text. There are several methods by which autonomic modulation of the cardiovascular system can be assessed. Methods include plasma noradrenaline levels, MSNA, and HRV – the variations in heart rate established by comparing the beat-to-beat intervals between successive cardiac cycles, assessed via standard electrocardiogram recordings and inter-pulse intervals from systolic BP. There are many parameters of HRV used to establish the degree of cardiac sympathetic or parasympathetic modulation (Table 2). Ultimately, reduced HRV is associated with greater sympathetic tone, and higher variability with vagal tone. The Framingham Heart Study [13] is one the major studies which found reduced HRV in men and women with systemic hypertension (p < 0.05), and that LF power of HRV was associated with new-onset hypertension in men (95% CI: 1.04–18.3).
Table 1.
Studies analysis autonomic dysfunction in essential hypertension. Methods of analysis include plasma noradrenaline levels, heart rate variability and muscle sympathetic nerve activity.
| Author(s) | GRADE | Year published | Group BP (SBP/DBP; mmHg) and sample size (n=) | Methods | Follow-up | Main findings |
|---|---|---|---|---|---|---|
| Masuo et al. [12] | Moderate | 2003 | 123 ± 8/70 ± 5 (n = 433) | Plasma noradrenaline | 5 yrs | Plasma noradrenaline was a sig. determining factor of change in mean BP over 5 yrs. |
| Smith et al. [14] | Low | 2002 | N: 129 ± 1.7/82 ± 2.1 (n = 12) WH: 157 ± 4.3/95 ± 1.1 (n = 12) H: 155 ± 3.4/93 ± 1.4 (n = 12) |
MSNA | – | MSNA was greater in white coat hypertensive than normotensive subjects, and greater still in the hypertensive group. |
| Singh et al. [13] | Moderate | 1998 | N: 120 ± 0.5/77 ± 0.3 (n = 1570) H: 143 ± 0.7/89 ± 0.5 (n = 472) |
HRV | 4 yrs | HRV is reduced in those with hypertension. Lower HRV is associated with the development of hypertension. |
| Grassi et al. [15] | Low | 1998 | N: 134 ± 3.1/79 ± 2.9 (n = 10) H: 138 ± 3.2/96.8 ± 1.9 (n = 10) |
MSNA | – | MSNA was sig. greater in hypertensive subjects. |
HRV = heart rate variability; MSNA = muscle sympathetic nerve activity; BP = blood pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; N = normotensive group; H = hypertensive group; WH = white-coat hypertensive group. WH was diagnosed as a sustained clinic BP of ≥140/90 mmHg with a daytime ambulatory BP of <130/80 mmHg. GRADE: The Grades of Recommendation, Assessment, Development and Evaluation.
Table 2.
Frequency-domain and corresponding time-domain measures of heart rate variability, including the definitions of time-domain variables, and the overall physiological interpretation of these variables.
| Frequency-domain measure | Time-domain measure & definition | Physiological interpretation | |
|---|---|---|---|
| Total power (TP) | SDNN | Standard deviation of all normal-to-normal (NN) intervals | Total HRV |
| High frequency (HF) power | NN50 | The number of NN intervals differing by >50 ms from the preceding interval | Parasympathetic modulation |
| rMSSD | Average change in the NN intervals | ||
| pNN50 | The percentage of intervals >50 ms different from the preceding interval | ||
| SDΔNN | Standard deviation of the differences between adjacent NN intervals. | ||
| Low frequency (LF) power | n/a | n/a | Baroreceptor function |
| LF/HF ratio | n/a | n/a | Sympathovagal interactions on heart rate |
Information obtained from the Task Force of The European Society of Cardiology and The Society of Pacing and Electrophysiology [38]. HRV = heart rate variability.
Plasma noradrenaline (normal range: 80–498 pg/mL) is used as a biochemical marker of the degree of systemic sympathetic activity. Masuo et al. [12] assessed plasma noradrenaline levels in 433 young healthy subjects, finding that plasma noradrenaline levels predicted subsequent development of high BP over 5-year follow-ups (R2 = 0.1014, p = 0.0048). The use of microneurography to directly assess sympathetic nerve traffic towards a specific skeletal muscle can be assessed clinically, known as muscle sympathetic nerve activity. Smith et al. [14] used MSNA to assess sympathetic tone in those with white coat hypertension, compared to normotensive and essential hypertensive subjects. The study found that those with white coat hypertension had greater MSNA than normotensive subjects, with the essential hypertension group having greater MSNA still. Grassi et al. [15] found that MSNA was significantly higher in untreated essential hypertensive subjects than in normotensive subjects (p < 0.01). This adds further evidence that the phenomenon of excessive sympathetic tone may manifest itself before blood pressure itself is within hypertensive range, and that white coat hypertension may prove not only as a risk factor for the development of established essential hypertension but could also be used as a clinical indicator of autonomic dysfunction.
Autonomic dysfunction has been demonstrated in hypertensive groups in a multitude of previous studies. However, there appears to be no data on what proportion of hypertensive subjects are believed to exhibit signs of excessive sympathetic tone. It is therefore possible that not all patients have sympathetic over-activity, as hypertension itself is a multifactorial pathology. However, this review will focus on the role of autonomic dysfunction in identified groups of hypertensive subjects exhibiting such signs, both in terms of pathophysiology and on the role of pharmacotherapies on these markers.
4.2. The role of the baroreflex
It has been suggested that the complex pathophysiology of essential hypertension may also be influenced by altered baroreflex responses. This is an area that has become a highly studied and is a somewhat controversial topic. Hesse et al. [17] (Table 3) found that BRS was inversely correlated with 24-hour mean arterial pressure (MAP; R = 0.49; p < 0.001) and positively with HRV (R = 0.33; p = 0.02) demonstrating that there is an established association between the two. In particular, the baroreceptors provide excitatory input the cardiac vagal preganglionic neurones, and hence HRV is reduced in those with low BRS.
Table 3.
Summary of the studies discussed regarding the role of baroreflex sensitivity in the pathophysiology of essential hypertension.
| Author | GRADE | Year published | Sample size (n=) | BP (Mean ± SD; mmHg) | BRS analysis method | Findings |
|---|---|---|---|---|---|---|
| Okada et al. [16] | Low | 2012 | 61 | SBP/DBP: 124 ± 3/73 ± 2 | Calculated using DBP and MSNA | Lower BRS may predispose to hypertension |
| Hesse et al. [17] | Low | 2007 | 50 | SBP/DBP: 117 ± 7/70 ± 6 | Calculating using BP and HRV. | BRS was inversely correlated with MAP (p < 0.001; R = 0.49) |
| Mattace-Raso et al. [18] | Moderate | 2007 | 4573 | MAP available only: 106.7 ± 12.6 | Calculating using BP and HRV. | Arterial stiffness was an independent determinant of impaired BRS |
BP = blood pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial blood pressure; BRS = baroreflex sensitivity; HRV = heart rate variability; MSNA = muscle sympathetic nerve activity. GRADE: The Grades of Recommendation, Assessment, Development and Evaluation.
It is also well established that the baroreflex is crucial in short-term control of BP. However, it has been widely debated as to whether it is important in long-term BP control. Arguments against its importance appear to be based on studies using low sample sizes of patients who have undergone baroreceptor denervation secondary to carotid surgery or high levels of radiation exposure [29]. However, current thinking appears to be that in favour of BRS being involved in long-term BP control. The proposed theory is that of reduced vascular compliance in baroreceptive areas, causing reduced afferent firing and an increase in sympathetic outflow [16,18]. The Rotterdam Study [18] (Table 3) observed the association between arterial stiffness (via aortic pulse wave velocity and the carotid distensibility coefficient) and BRS, as measured via spontaneous changes in BP and HRV. Aortic stiffness was negatively association with BRS (CI: −0.040, −0.019), which was found to be an independent determinant of impaired BRS. More recently, Okada et al. [16] (Table 3) studied BRS using MSNA, finding that sympathetic BRS was inversely association with carotid artery stiffness in elderly men (R = 0.54, p < 0.001). There is therefore increasing evidence that increased aortic stiffness is associated with reduced BRS and autonomic dysfunction in essential hypertension.
Smith et al. [14] found that when assessing MSNA and BRS in a groups that had normotension, white coat hypertension and essential hypertension, that the normotensive and white coat hypertensive groups had similar BRS despite a greater MSNA in the latter group. In the essential hypertensive group MSNA was significantly greater still and BRS significantly lower. This suggests that the rise in sympathetic tone may occur before any pathological lowering of BRS. Therefore, reduced BRS secondary to raised arterial stiffness in the baroreceptor regions could be a major pathophysiological mechanism by which autonomic dysfunction is associated with essential hypertension. However, it is still unclear whether excessive sympathetic tone is a cause or effect of BRS changes. Low BRS along with low HRV could also be measurable parameters to identify those groups who may be predisposed to developing essential hypertension.
With our increasing knowledge of such a mechanism, there is greater scope for new therapeutic approaches for subgroups of hypertensive patients such as those with drug-resistant hypertension, where current therapeutic options are limited. Methods such as baroreflex stimulation are being successfully studied for this reason. Wustmann et al. [20] studied chronic carotid baroreflex stimulation in 21 patients with drug-resistant hypertension. The findings included sustained changes in frequency-domain parameters of HRV consistent with inhibition of sympathetic tone and increased vagal activity (p < 0.001), with a significant positive correlation between the LF/HF ratio and the decrease in SBP (R = 0.47, p = 0.006). Similarly, Bisognano et al. [19] investigated the effect of baroreflex stimulation on SBP in resistant hypertensive patients, finding that such stimulation can reduce SBP following 6 months of therapy (p = 0.03).
4.3. Treatment implications for essential hypertension
Studies have shown that changes in autonomic regulation of the cardiovascular system tend to occur before the manifestation of raised BP12. This concept in itself could be utilised as a screening tool for identifying populations at risk of developing hypertension. This would allow appropriate BP monitoring, and preventative measures to be implemented as early as possible to reduce the incidence of established hypertension and it's associated complications. Such measures may include lifestyle interventions such as appropriate nutrition from an earlier stage, and increased volumes of physical activity, but it may also include more regular BP checks by clinicians for prompt diagnosis. There is no specific treatment approach for essential hypertensive patients with markers of autonomic dysfunction. Recent studies have focused on the impact of these established anti-hypertensive pharmacotherapies on various markers of ANS function. Table 4 summarises the studies for discussion, which compare the effect of two anti-hypertensive medications on markers of autonomic dysfunction.
Table 4.
Summary of the studies comparing two anti-hypertensive medications for their effect on autonomic dysfunction.
| Author(s) | GRADE | Year published | Group 1 (druga, sample size, mean group SBP/DBP (mmHg)) | Group 2 (druga, sample size, mean group SBP/DBP (mmHg)) | Length of therapy | ANS findings | BP findings |
|---|---|---|---|---|---|---|---|
| Lewandowski et al. [21] | High | 2008 | ACE-I (enalapril; n = 16; 132 ± 7/73 ± 6) | ARB (telmisartan; n = 16; 129 ± 8/75 ± 8) | 4 weeks | ARB attenuated HRV (LF/HF ratio) more effectively (p < 0.008). | Both reduced mean BP (p < 0.0001). No sig. difference between groups. |
| Chern et al. [23] | High | 2006 | β-blocker (atenolol; n = 15; SBP 145 ± 15. DBP not reported) | ARB (losartan; n = 15; SBP 143 ± 16. DBP not reported) | 6 months | Losartan superior on baroreflex sensitivity and HRV (p < 0.05). | Both reduced mean BP (p < 0.05). No sig. difference between groups. |
| Bilge et al. [26] | High | 2005 | CCB (amlodipine; n = 14; 151 ± 15/101 ± 6) | ACE-l (fosinopril; n = 13; 161 ± 16/103 ± 7) | 6 months | No sig. change in HRV. | Both reduced mean BP (p < 0.0001). No sig. difference between groups. |
| Krum et al. [22] | High | 2005 | ARB (eprosartan; n = 19; 153 ± 14/95 ± 7) | ARB (losartan; n = 19; 153 ± 13/95 ± 8) | 4 weeks | No sig. change in MSNA or plasma noradrenaline. | Both reduced mean BP (p < 0.05). No sig. difference between groups. |
| Sakata et al. [24] | High | 1999 | CCB (amlodipine; n = 24; 158 ± 12/104 ± 25) | CCB (cilnidipine; n = 23; 161 ± 13/106 ± 22) | 3 months | There was no change in plasma noradrenaline in either group | Both reduced mean BP (p < 0.001). No sig. difference between groups. |
| Vesalainen et al. [25] | High | 1998 | β-blocker (metoprolol; n = 12; 140 ± 11/90 ± 7) | ACE-I (ramipril; n = 12; 140 ± 11/90 ± 7) | 4 weeks | Only metoprolol increases vagal control (HF power of HRV). | Both reduced mean BP (p < 0.001). No sig. difference between groups. |
All drugs were administered at standard therapeutic dosages [32]. ANS = autonomic nervous system; BP = blood pressure; CCB = calcium channel blocker; ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin-II receptor blocker; HRV = heart rate variability; SBP = systolic blood pressure; DBP = diastolic blood pressure. GRADE: The Grades of Recommendation, Assessment, Development and Evaluation.
The studies summarised in Table 4 highlight the differences in effectiveness of various anti-hypertensive pharmacotherapies on markers of autonomic dysfunction. The studies show that angiotensin receptor blocker (ARB) therapy has been found to be more beneficial at effecting autonomic dysfunction than angiotensin converting enzyme-inhibitors (ACE-I) and β-blockers [21,23], despite the fact that ACE-I are first line therapies for essential hypertension. However, studies differed in the choice of drug in each class, the dosage used, length of treatment and the outcome measure used. This makes comparison between studies difficult, and prevents accurate interpretation in terms of clinical implications. It does highlight the need for more consistent research in this area to maximise the potential for application of this knowledge into establishing the effectiveness of anti-hypertensive pharmacotherapies in essential hypertensive patients with evidence of autonomic dysfunction. Also, the long-term effectiveness of these pharmacotherapies on autonomic dysfunction is still unknown, and hence research needs to focus on establishing this. This in turn could influence the approach by physicians to treat essential hypertension in those with established autonomic dysfunction. Eventually, this may mean that anti-hypertensive treatment may be as per separate guidance specifically for those with markers of autonomic dysfunction.
When discussing the role of autonomic control of BP, it is important to also discuss the role of not only direct sympathetic innervation of the vasculature and myocardium, but also the role of the renin–angiotensin–aldosterone system (RAAS). The sympathetic nervous system is known to cause an increase in renin release via direct simulation of the β-1 receptors on the juxtaglomerular apparatus. Therefore, anti-hypertensive medications such as β-blockers may have a role in reducing the amplitude of RAAS stimulation, as well as by reducing the inotropic and chronotropic effects of adrenergic stimulation on the myocardium. A Cochrane review on the use of β-blocker therapy in hypertension demonstrated that overall, these were inferior to calcium channel blockers (CCB), diuretics, ARB and ACE-I in terms of all-cause mortality, coronary artery disease and cardiovascular morbidity [28]. However, Blumenfeld et al. [27] found that following 1 week of β-blocker therapy, there was not only a reduction in plasma angiotensin II levels comparable with that of ACE-I therapy, but also a reduction in renin stimulation not seen in ACE-I and ARB therapy. Therefore, despite not causing a beneficial reduction in overall mortality and morbidity, the renin suppressing actions exhibited by β-blockers may lead to benefits in reducing hypertension in combination with the likes of ACE-I and ARB.
The lack of benefit of ACE-I on biomarkers of autonomic dysfunction opens up increased need for research in this area. However, the overwhelming evidence that ACE-I are more beneficial at reducing overall morbidity and mortality compared to other anti-hypertensive agents means that these drugs are still recommended as first-line therapy. It is recommended that future research focus on combination therapy of first-line medications beneficial for overall cardiovascular mortality such as ACE-I, in conjunction with ARB that are seemingly beneficial for markers autonomic dysfunction and direct autonomic modulating drugs such as β-blockers that may reduce renin release.
An increasingly studied area is the role or renal denervation in the management of resistant hypertension. Renal denervation has been found to substantially lower blood pressure without compromising kidney function [30] or the cardiac chronotropic response to exercise [31]. The Symplicity HTN-1 trial demonstrated an average BP reduction of 33/19 mmHg after a two-year follow-up [32], while the Symplicity HTN-2 trial has demonstrated a similar reduction of 32/12 mmHg after six months [33]. However, the long-term benefit (beyond the ≤1 year follow-up used in these studies) still needs to be assessed. Success with this intervention has been demonstrated in multiple studies [30–33]. However, the mechanism behind the sustained drop in BP is still unclear, with the authors of the Symplicity HTN-1 trial speculating that it may be due to a resetting of the baroreflex or vascular remodelling over-riding any sympathetic re-innervation [32]. Whether successful renal sympathetic ablation is indicative of autonomic dysfunction being a cause or effect of resistant hypertension remains unclear.
5. Study limitations
There appears to be no previous review articles to draw together the established association of autonomic dysfunction in essential hypertension, along with current applications to clinical practice and treatment. However, the findings are limited by several factors. There is enormous heterogeneity in study designs and in the outcome measures of autonomic dysfunction. This means it is very difficult to accurately compare and quantify groups of studies without making generalisations. Also, although studies using populations with comorbidity were excluded, it is very difficult to control for confounding influences such as smoking and alcohol consumption, both of which are risk factors for essential hypertension development, and also are themselves associated with autonomic dysfunction [34–36]. It is therefore very difficult to judge whether autonomic dysfunction was independent of lifestyle factors such as these. Also, studies addressing the effect of pharmacotherapies have used hypertensive groups with a no consistent cut-off value, including both those with stage 1 and stage 2 hypertension. It is established that sympathetic tone is high predominantly in early and borderline hypertension, and changes as hypertension becomes more established [37]. However, stage 2 hypertension is the level at which NICE guidelines suggest initiating pharmacotherapy. This highlights great inconsistencies between research study design and clinical practice. It is recommended that the precise role of autonomic dysfunction should be established in stage 1, stage 2 and severe hypertension, and the impact of treatments assessed at each of these severities using standard drug doses as per NICE guidelines. This will allow more accurate and clearer application of such work to clinical practice.
The criteria used for this study excluded the use of sample populations with comorbidities associated with inherent neuropathy, to avoid confounding influence to any autonomic dysfunction associated with essential hypertension only. The author acknowledges that diabetes mellitus frequently co-exists with essential hypertension under the umbrella term of ‘metabolic syndrome’. However, given the large array of literature studying autonomic dysfunction in diabetics with or without hypertension, it is beyond the scope of this review to discuss the role of coexistent diabetes with regards to autonomic dysfunction in essential hypertension. It is acknowledge that this group may exhibit alternative neurovascular features implicated with essential hypertension than those studies discussed in this text.
Other limitations of this study include the use of only one database for this review, and that the one author for this study was the only person responsible for selection and extraction of studies appropriate for analysis. The latter may be considered an advantage in allowing consistency in study selection. However, this may also be a source of selection bias limiting the number of studies used for analysis. Further studies may widen their search criteria used by multiple investigators. This may reduce possible selection bias, and increase the clinical relevance of these findings.
6. Conclusions
This review highlighted 3 main points. Firstly, sympathetic predominance is believed to precede the rise in BP itself to within hypertensive range. Secondly, reduced BRS, believed to be secondary to increased arterial stiffness is hypothesised to be implicated in the pathogenesis of essential hypertension. Also, ACE-I are seemingly not as effective at benefiting autonomic control of blood pressure when compared with its competitors, despite being first-line drugs for treatment. It must be emphasised that clinically, it is not routine to assess sympathovagal balance in those with diagnosed essential hypertension, and hence there is no recommended clinical method on how to do so in this circumstance. Nor is there any specific treatment approach for hypertensive patients with biomarkers of sympathetic overactivity. However, analysing such parameters using HRV, MSNA or plasma noradrenaline levels may be useful at enhancing our knowledge of the underlying pathophysiology, which in turn may improve therapeutic approaches for subsets of hypertensive patients with signs of autonomic dysfunction.
Acknowledgements
Thanks go to Dr Christopher Askew and Dr Fraser Russell for their guidance.
References
- 1.Kearney P., Whelton M., Reynolds K., Muntner P., Whelton P., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Cushman W.C. Are there benefits to specific antihypertensive drug therapy? Am J Hypertens. 2003;16:31–35. doi: 10.1016/j.amjhyper.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Palatini P., Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Sci Inc. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 4.Wray D.W., Supiano M.A. Impact of aldosterone receptor blockade compared with thiazide therapy on sympathetic nervous system function in geriatric hypertension. Hypertension. 2010;55:1217–1223. doi: 10.1161/HYPERTENSIONAHA.109.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okano Y., Tamura K., Masuda S., Ozawa M., Tochikubo O., Umemura S. Effects of angiotensin ii receptor blockers on the relationships between ambulatory blood pressure and anti-hypertensive effects, autonomic function, and health-related quality of life. Clin Exp Hypertens. 2009;31:680–689. doi: 10.3109/10641960903407041. [DOI] [PubMed] [Google Scholar]
- 6.Fogari R., Zoppi A., Corradi L., Preti P., Malalamani G.D., Mugellini A. Effects of different dihydropyridine calcium antagonists on plasma norepinephrine in essential hypertension. J Hypertens. 2000;18:1871–1875. doi: 10.1097/00004872-200018120-00023. [DOI] [PubMed] [Google Scholar]
- 7.Grassi G., Seravalle G., Turri C., Bolla G., Mancia G. Short-versus long-term effects of different dihydropyridines on sympathetic and baroreflex function in hypertension. Hypertension. 2003;41:558–562. doi: 10.1161/01.HYP.0000058003.27729.5A. [DOI] [PubMed] [Google Scholar]
- 8.Parati G., Stergiou G.S., Asmar R., Bilo G., de Leeuw P., Imai Y. European society of hypertension guidelines for blood pressure monitoring at home: a summary report of the second international consensus conference on home blood pressure monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 9.Ruzicka M., Coletta E., Leenen F.H.H. Does blockade of the renin angiotensin system affect sympathetic and blood pressure responses to amlodipine in young hypertensive patients? Am J Hypertens. 2007;20:1202–1208. doi: 10.1016/j.amjhyper.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Minami J., Ishimitsu T., Kawano Y., Matsuoka H. Effects of amlodipine and nifedipine retard on autonomic nerve activity in hypertensive patients. Clin Exp Pharmacol Physiol. 1998;25:572–576. doi: 10.1111/j.1440-1681.1998.tb02254.x. [DOI] [PubMed] [Google Scholar]
- 11.Leenen F.H., Coletta E., White R. Sympatho-excitatory responses to once-daily dihydropyridines in young versus older hypertensive patients: amlodipine versus felodipine extended release. J Hypertens. 2006;24:177–184. doi: 10.1097/01.hjh.0000198032.07224.c3. [DOI] [PubMed] [Google Scholar]
- 12.Masuo K., Kawaguchi H., Mikami H., Ogihara T., Tuck M.L. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 13.Singh J.P., Larson M.G., Tsuji H., Evans J.C., O'Donnell C.J., Levy D. Reduced heart rate variability and new-onset hypertension. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 14.Smith P.A., Graham L.N., Mackintosh A.F., Stoker J.B., Mary D.A.S.G. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol. 2002;40:126–132. doi: 10.1016/s0735-1097(02)01931-9. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G., Colombo M., Seravalle G., Spaziani D., Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension. 1998;31:64–67. doi: 10.1161/01.hyp.31.1.64. [DOI] [PubMed] [Google Scholar]
- 16.Okada Y., Galbreath M.M., Shibata S., Jarvis S.S., VanGundy T.B., Meier R.L. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesse C., Charkoudian N., Liu Z., Joyner M.J., Eisenach J.H. Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension. 2007;50:41–46. doi: 10.1161/HYPERTENSIONAHA.107.090308. [DOI] [PubMed] [Google Scholar]
- 18.Mattace-Raso R.U., van den Meiracker A.H., Bos W.J., van der Cammen T.J., Westerhof B.E., Elias-Smale S. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam study. J Hypertens. 2007;25:1421–1426. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 19.Bisognano J.D., Bakris G., Nadim M.K., Sanchez L., Kroon A.A., Schafer J. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–773. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Wustmann K., Kucera J.P., Scheffers I., Mohaupt M., Kroon A.A., de Leeuw P.W. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54:530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 21.Lewandowski J., Abramczyk P., Dobosiewicz A., Bidiuk J., Sinski M., Gaciong Z. The effect of enalapril and telmisartan on clinical and biochemical indices of sympathetic activity in hypertensive patients. Clin Exp Hypertens. 2008;30:423–432. doi: 10.1080/10641960802279132. [DOI] [PubMed] [Google Scholar]
- 22.Krum H., Lambert E., Windebank E., Campbell D.J., Esler M. Effect of angiotensin ii receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1706–H1712. doi: 10.1152/ajpheart.00885.2005. [DOI] [PubMed] [Google Scholar]
- 23.Chern C., Hsu H., Hu H., Chen Y., Hsu L., Chao A. Effects of atenolol and losartan on baroreflex sensitivity and heart rate variability in uncomplicated hypertension. J Cardiovasc Pharmacol. 2006;47:169–174. doi: 10.1097/01.fjc.0000199225.17928.f5. [DOI] [PubMed] [Google Scholar]
- 24.Sakata K., Shirotani M., Yoshida H., Nawada R., Obayashi K., Togi K. Effects of amlodipine and cilnidipine on cardiac sympathetic nervous system and neurohormonal status in essential hypertension. Hypertension. 1999;33:1447–1452. doi: 10.1161/01.hyp.33.6.1447. [DOI] [PubMed] [Google Scholar]
- 25.Vesalainen R., Kantola I., Airaksinen K.E.J., Tahvanainen K.O., Kaila T. Vagal cardiac activity in essential hypertension: the effects of metoprolol and ramipril. Am J Hypertens. 1998;11:649–658. doi: 10.1016/s0895-7061(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 26.Bilge A.K., Atilgan D., Tükek T., Özcan M., Özben B., Koylan N. Effects of amlodipine and fosinopril on heart rate variability and left ventricular mass in mild-to-moderate essential hypertension. Int J Clin Pract. 2005;59:306–310. doi: 10.1111/j.1742-1241.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- 27.Blumenfeld J.D., Sealey J.E., Mann S.J., Bragat A., Marion R., Pecker M.S. Β-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens. 1999;12:451–459. doi: 10.1016/s0895-7061(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 28.Bradley H.A., Wiysonge C.S., Volmink J.A., Mayosi B.M., Opie L.H. How strong is the evidence for use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens. 2006;24 doi: 10.1097/01.hjh.0000249685.58370.28. [DOI] [PubMed] [Google Scholar]
- 29.Aksamit T.R., Floras J.S., Victor R.G., Aylward P.E. Paroxysmal hypertension due to sinoaortic baroreceptor denervation in humans. Hypertension. 1987;9:309–314. doi: 10.1161/01.hyp.9.3.309. [DOI] [PubMed] [Google Scholar]
- 30.Mahfoud F., Cremers B., Janker J., Link B., Vonend O., Ukena C. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- 31.Ukena C., Mahfoud F., Kindermann I., Barth C., Lenski M., Kindermann M. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2011;58:1176–1182. doi: 10.1016/j.jacc.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Symplicity HTN-1 Invetsigators Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 33.Symplicity HTN-2 Investigators Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): a randomised controlled trial. The Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 34.Felber Dietrich D., Schwartz J., Schindler C., Gaspoz J., Barthélémy J., Tschopp J. Effects of passive smoking on heart rate variability, heart rate and blood pressure: an observational study. Int J Epidemiol. 2007;36:834–840. doi: 10.1093/ije/dym031. [DOI] [PubMed] [Google Scholar]
- 35.Ohira T., Tanigawa T., Tabata M., Imano H., Kitamura A., Kiyama M. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension. 2009;53:13–19. doi: 10.1161/HYPERTENSIONAHA.108.114835. [DOI] [PubMed] [Google Scholar]
- 36.Malpas S.C., Whiteside E.A., Maling T.J. Heart rate variability and cardiac autonomic function in men with chronic alcohol dependence. Br Heart J. 1991;65:84–88. doi: 10.1136/hrt.65.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund-Johnson P. Newer thinking on the hemodynamics of hypertenion. Curr Opin Cardiol. 1994;9 doi: 10.1097/00001573-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Task Force of the European Society of Cardiology the North American Society of Pacing Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]


