Abstract
Allogenic blood is a finite resource, with associated risks. Previous studies show intraoperative cell salvage (ICS) can reduce allogenic transfusion rates in orthopaedic surgery. However, there are concerns regarding efficacy and cost-effectiveness of ICS. This study was carried out to review ICS use in revision hip arthroplasty.
All patients who underwent ICS and re-infusion between 2008 and 2010 in the Southern General Hospital (SGH) were audited. The fall in haemoglobin (Hb), volume of blood re-infused and postoperative allogenic transfusion rates were recorded. This group was compared to a similar SGH cohort who underwent surgery by the same surgeons between 2006 and 2008, and a pre-2005 control group where no ICS was used.
The proportion of patients receiving a postoperative allogenic transfusion fell by 55% in the 2008–2010 ICS cohort compared with the control, and by 40% compared with the previous ICS group. In both instances, there was a statistically significant (p < 0.001) reduction in mean units transfused per patient; in the 2008–2010 ICS cohort, a mean of 0.8 units was used per patient, while 1.4 were used in the 2006–2008 cohort. 3.5 units were used in the control group. There was no statistically significant difference in age or preoperative Hb between the groups, or in length of hospital stay.
In this study, ICS has been shown to be effective in reducing rates and volume of postoperative allogenic transfusion in patients undergoing revision hip surgery at the SGH. However, further work is needed to establish the effect of changing anaesthetic technique on postoperative allogenic transfusion rates.
Keywords: Intraoperative, Cell-salvage, Orthopaedic, Arthroplasty, Blood conservation
1. Introduction
Orthopaedic surgery is often associated with a high volume of blood loss, and hence high rates of postoperative transfusion. Although allogenic (donor) blood is routinely used, it is a finite and increasingly costly resource [1]. The risk of viral infection from allogenic blood is extremely low [2,3]. However, several large studies have shown that allogenic transfusion is associated with increased risk of postoperative bacterial infection [4–6].
Other risks associated with allogenic transfusion include acute transfusion reactions, haemolytic reactions and transfusion-associated acute lung injury. Clinical errors are the most common cause of transfusion related complications, as reported by the Serious Hazards of Transfusion (SHOT) working group [7].
The increasing cost and associated risks of allogenic blood have therefore led to a number of blood saving interventions becoming widely used in orthopaedic surgery. One such intervention has been intraoperative cell-salvage (ICS), a technique whereby blood lost intraoperatively is collected from the operative field, anticoagulated, washed and filtered before being re-infused into the patient either during the procedure, or immediately postoperatively. As half of all units transfused in the UK are used for surgical patients [8], a blood conservation technique like ICS seems well placed to help reduce the national use of allogenic blood. Although ICS can also be successfully carried out in knee arthroplasty, it has been shown to be less effective than ICS in hip arthroplasty [9].
Other measures to reduce allogenic transfusion include the use of tranexamic acid [10], erythropoietin and iron supplementation [11,12]. A recent review by Munoz et al. [13] has shown that both oral and IV pre and perioperative iron reduce the volume of allogenic blood transfusion in orthopaedic and trauma patients. Preoperative autologous donation for patients expected to require >2 units of allogenic blood has also been shown to reduce allogenic blood use [13,14]. It has been suggested that these interventions work best when partnered with cell salvage [15], and indeed cell salvage itself has been shown to offer a safe and cost-effective method of reducing allogeneic blood use [16–18].
Since its introduction in the 1970's, ICS has become routine in a range of surgical specialities. It has been shown to be effective at reducing allogenic transfusions in obstetric [1], vascular [19], cardiac [20], orthopaedic [21] and urological [16] procedures. Indications for ICS include; an anticipated blood loss of >1000 ml; a mean allogenic post-op transfusion of 1 unit or greater; the refusal of transfusion for religious reasons; a low pre-op haemoglobin; risk factors for bleeding or if more than 10% of patients undergoing the operation require a transfusion [16,23].
Absolute contraindications are: situations where red cell lysis occurs, such as blood being mixed with sterile water, hydrogen peroxide or alcohol; red cell abnormality, such as sickle cell disease [24]; or procedures with faecal or urine contamination [16,23]. Other more relative, though generally accepted, contraindications include: malignancy; the presence of contaminants too small to be filtered out, for example metal particles from metal on metal hip revisions; and infection. Contamination from fat particles is also cited as a contraindication [16], however fat particles can now be easily eliminated by using a leucocyte depletion filter [22].
Although cell salvage has been shown to be useful in a number of procedures, there is still some debate as to its safety. For example, one of the most commonly cited objections is the theoretical risk of amniotic fluid embolus from blood salvaged during obstetric procedures [25], although evidence for this is weak [26]. There are also debates about its effectiveness and economic viability in some areas of cardiac surgery [27].
The safety and efficacy of ICS in orthopaedic surgery have, however, been well documented. Most studies described the use of ICS in hip and knee arthroplasty patients. Several studies have shown ICS to be effective in primary hip arthroplasty [9,28] A small, case-matched study showed that ICS significantly reduced allogenic transfusion in revision hip arthroplasty [29]. Two large randomised controlled trials (RCTs) and a large retrospective database review have shown similar outcomes in Knee surgery [30–32].
The aim of this study is to assess the impact of ICS on blood transfusion rates in patients undergoing revision hip arthroplasty.
2. Methods
This comparative cohort study was carried out in the Southern General Hospital in Glasgow. The following data was collected from a standardised cell salvage data sheet compiled by theatre staff on the day of operation date of operation; operation details, patient details, cell salvage complications where present, the volume of blood salvaged and re-infused, the total volume of blood lost during the operation and the volume of surgical irrigation and anticoagulant used.
Patient data, such as pre and postoperative haemoglobin (Hb) levels, was obtained from electronic patient records, transfusion data was obtained from both electronic and hard copy databases. Postoperative allogenic transfusions carried out up to and including 10 days postoperatively were recorded, with the day of operation as day zero. Any transfusions after this period were discounted. Length of postoperative stay was recorded, with the day of the operation as day zero. The pre and postoperative Hb levels recorded closest to the operation date were used. Ethical approval was not required.
The Cell Saver 5 salvage machine was used throughout the study period in all patient groups, and salvage was carried out as per manufacturer's (CellSaver) protocols. The same three surgeons carried out all operations throughout all three study periods.
2.1. Transfusion protocol and tranexamic acid
All patients in all cohorts were transfused allogenic packed red cells if their Hb was <80 g/L or if <100 g/L but symptomatic with a background of cardiac disease. All patients in all groups received 1 g tranexamic acid IV at the induction of anaesthesia.
2.2. Inclusion/exclusion criteria
All patients in the Southern General Hospital who underwent a revision hip operation with cell salvage and autologus blood re-infusion were initially included in the study. A small number of patients were excluded due to missing data on the ICS data sheets.
2.3. Statistical analysis
Results were analysed using the statistical software package Prism 4.0 (GraphPad). Graphs were generated by Microsoft Excel (Microsoft 2003). Analysis of variance (ANOVA) non-parametric tests were used to obtain p-values to test significance. The Kruskal–Wallis test was used to determine the significance in variance between all 3 study groups, and Dunn's multiple comparison test was used to compare significance of differences between specific groups. Standard descriptive statistics per group were also calculated, the mean number of units required per patient was calculated from the total post-op units transfused divided by the total number of patients in that revision hip cohort. A p-value of <0.05 was used as the threshold for significance.
3. Results
3.1. Patient recruitment
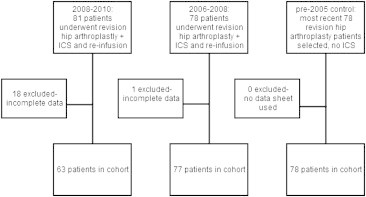
Cell salvage was used in a total of 81 patients who underwent revision hip surgery between 2008 and 2010. Of these, 18 patients were excluded from the study due to unrecorded data such as preoperative Hb.
78 patients underwent revision hip surgery between 2006 and 2008, one of whom was excluded due to missing data. A control group of 78 revision hip patients who had no cell salvage was obtained from records dating from pre-2005, where cell salvage was not used.
Patient recruitment is summarised in the flow diagram below:
3.2. Comparison of cohorts
Patients who underwent revision hip arthroplasty in the new (2008–2010) and previous (2006–2008) cell-salvage group characteristics were compared with the pre-2005 control group. All 3 groups were found to be comparable in terms of age and preoperative Hb (p > 0.05) (Table 1). There was no significant difference between male and female patients in any parameter (p > 0.05).
Table 1.
Characteristics of the 2010–2008 and 2006–2008 cohorts, who had ICS, and the pre-2005 control where no ICS was carried out.
| All hip revisions | 2008–2010: ICS | Significance of difference: 2008–2010 vs 2006–2008 | 2006–2008: ICS | Significance of difference: 2006–2008 vs non-ICS control | Non-ICS (pre-2005) control | Significance of difference: 2008–2010 vs non-ICS control |
|---|---|---|---|---|---|---|
| No. of patients | 63 | 77 | 78 | |||
| Average age (yrs) | 66.3 (CI 62.9–69.7) | p > 0.05 | 65.5 (CI 62.9–68.0) | p > 0.05 | 68.2 (CI 65.3–71.1) | p > 0.05 |
| Average pre-op Hb (g/L) | 127.5 | 128.8 | 127.4 | |||
| Average reduction in Hb postoperatively (g/L) | 28.1 (CI 24–32.2) | p > 0.05 | 32.6 (CI 28.5–36.8) | p < 0.001 | 86.0 (CI 82.5–89.6) | p < 0.001 |
| No. of patients – allogenic transfusion | 8 13% (8/63) | 41 53% (41/77) | 54 68% (54/78) | |||
| Mean units required per patient | 0.4 (CI 0.1–0.8) | p < 0.001 | 1.8 (CI 1.0–2.7) | p < 0.05 | 3.5 (CI 2.6–4.5) | p < 0.001 |
| Mean length of post-op hospital stay (days) | 10.9 (CI 8.1–13.7) | p > 0.05 | 12.6 (CI 10.0–15.2) | p > 0.05 | 11.4 (CI 9.5–13.2) | p > 0.05 |
The proportion of patients who received at least one allogenic postoperative transfusion fell by 55% in the 2008–2010 ICS cohort compared with the non-ICS group (68–13%). The number of patients transfused in the 2008–2010 ICS cohort was also reduced by 40% (52–12%) compared with the previous ICS study group. In both instances, this was accompanied by a statistically significant reduction in the mean number of units transfused per patient (of the total number of patients in that cohort, not just transfused patients); 0.4 units were transfused in the new ICS cohort, compared with 3.5 (p < 0.001) in the control and 1.8 (p < 0.001) in the previous ICS group (Table 1).
Along with a reduction in both the number of patients transfused and mean units used, a difference in the average postoperative Hb reduction was also noted between the three groups. A significant difference in the postoperative drop in Hb was identified between the 2008–2010 cell salvage and control group (28.1 g/L vs 86.0 g/L, p < 0.001) however no significant difference was identified between the 2006–2008 and 2008–2010 ICS groups (32.6 vs 28.1, p > 0.05).
Although the average length of postoperative hospital stay seemed to be ∼1 day longer in the 2006–2008 cohort than the 2008–2010 ICS group, this difference did not reach statistical significance (Table 1).
4. Discussion
Autologous blood collection techniques, such as ICS, have been the source of debate over the last 30 years since their introduction. While the benefits of blood salvage in Orthopaedic surgery have been well described [9,21,28–32], there remain concern that it should be used more discriminatingly. These concerns may stem from a number of studies that have shown ICS to either be ineffective or costly, across a number of other surgical specialities [2,25,33]. For example, one study by Guerra et al., published in 1995, found that ICS was not cost effective, as often only a small volume of blood was salvaged and so did not decrease the need for allogenic blood [34]. Further to this, a study reviewing the use of ICS in paediatric orthopaedic surgery [35] found that ICS was only cost effective in a small number of cases with a relatively low blood loss. It is important therefore to continue to document the efficacy of ICS.
Interestingly, ICS cannot be used in revision hip surgery if infection or adverse reaction to metal debris (ARMD) is the indication, or for revision of metal on metal hip replacements [23,24]. However, even excluding cases with the above indications, revision hip arthroplasty is a common enough operation to yield a relatively large patient group for this study who did undergo ICS. In this study both the 2008–2010 and 2006–2008 cohort showed a decrease in the proportion of patients transfused and the mean transfusion requirement per patient, compared to a historical control group. The results of this study would therefore support the use of ICS in revision hip arthroplasty.
A reduction in allogenic transfusion requirement was also demonstrated between the 2006–2008 and 2008–2010 ICS cohorts. This could be due to increased competency and experience of staff in using a complex system, which could have lead to a higher percentage of blood being salvaged and reinfused in the later cohort. The two ICS groups showed less of a reduction in Hb post-op compared with the control, which was highly significant. The small difference in average Hb reduction between the ICS groups of 4.5 g/L failed to reach statistical significance. However, the percentage of patients with postoperative Hb's of <80 g/L and <100 g/L (the transfusion thresholds) were less in the 2008–2010 cohort than the 2006–2008 group (8% (5/63) and 51% (32/63) respectively in 2008–2010, compared with 17% (13/77) and 60% (46/77) in the earlier ICS group). This may account for the decreased allogenic transfusion rate in the 2008–2010 cohort, despite the difference in average Hb reduction between the two groups being non-significant.
Patient demographics between the cohorts were very similar in terms of age, preoperative Hb levels and geographical area. Although multi-centre studies are useful, studying patient groups treated in the same hospital reduce variability in practices, equipment (e.g. the ICS machine) and electronic recording systems. Similarly, studying patients who were operated on by the same surgeons over time should reduce variations in operative technique. Although technique and cell salvage use may vary between surgeons, this is in keeping with a realistic departmental ICS model – ICS would be adopted by departments as opposed to individual surgeons.
As cohorts were not run in parallel, potential confounders included changes in anaesthetic technique, surgical technique and transfusion policy over time. Transfusion policies did not change between the two ICS cohorts, although there were some minor changes in transfusion policy pre-2006. However, transfusion thresholds were not altered outside the range of 70 g/L–90 g/L, with most elderly patients being transfused at a threshold of 90 g/L, as set out in the SIGN 54 clinical guideline [36]. Although this guideline has now been withdrawn from SIGN, it was the guideline on which transfusion policy was based at the time of data collection.
There were no reported changes in surgical technique since before 2005, but there have been changes in anaesthetic technique, such as the increased use of spinal anaesthesia. It has been argued that this reduces blood loss, however current research is divided as to whether or not anaesthetic technique has a significant effect on intraoperative blood loss [37–39]. It is therefore unclear how much of an effect changes in anaesthetic technique have had on the large reduction in postoperative allogenic transfusion rates between the control and ICS groups.
There are several different types of revision hip operations, which in turn could induce different volumes of blood loss. Anecdotally, the vast majority of the revision hip arthroplasties in this study are single stage, elective revisions. However, data was not recorded on some of the cell salvage forms as to which type of revision was performed- single stage, two stage, emergency or elective. Operation details such as which type of acetabular component was used were also not available. This study therefore does not distinguish between different types of revision hip surgery, which could be a potential confounding factor.
Although a contemporary, larger non-ICS cohort of patients would be useful in answering this important question, there was a small cohort of 10 patients who had revision hip surgery between June and July 2012, where ICS was not available for technical reasons. This cohort was not included in the ‘Results’ section as it was underpowered, and so results and statistics should be viewed with caution. However, there are some interesting points to take from it nonetheless. The group seemed to be comparable with the 3 larger groups in the study, as no significant difference was calculated between average age or preoperative Hb (p > 0.05), although the group did contain a higher proportion of women:men than the other cohorts (8 females:2 males). Despite the smaller drop in post-operative haemoglobin in the 2012 cohort (demonstrated in Fig. 1), ninety percent of patients received a postoperative allogenic transfusion, compared with 13% of the patients from the 2008–2010 ICS group. This is demonstrated in Fig. 2. The average number of allogenic units transfused per transfused patient in the 2012 cohort was 2.55, similar to 2.9 units in the 2008–2010 group (Fig. 3).
Fig. 1.
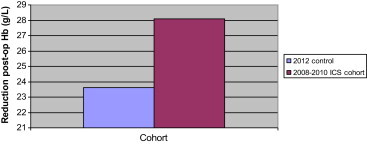
Comparison between 2012 control cohort and 2008–2010 ICS cohort: postoperative Hb reduction.
Fig. 2.
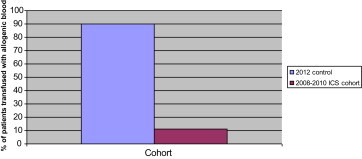
Comparison between 2012 control cohort and 2008–2010 ICS cohort: percentage of patients transfused with allogenic blood.
Fig. 3.
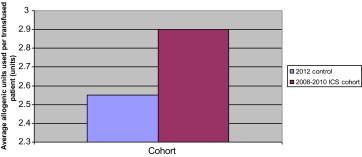
Comparison between 2012 control cohort and 2008–2010 ICS cohort: average number allogenic units used per transfused patient.
Enhanced recovery and a drive to reduce length of stay in hospital have been a recent goal in patient management. ICS does not appear to directly contribute to this but may do so on an individual patient basis. Other modalities of care appear more directly relevant to reducing length of stay.
5. Conclusions
Allogenic blood is a finite resource with associated risks. ICS has been shown to be effective in reducing rates and volume of postoperative allogenic transfusion in patients undergoing revision hip arthroplasty in this single centre, comparative cohort study. ICS has also been used with increasing efficiency over time.
Funding sources
No funding was obtained for this study.
Conflict of interest statement
None to declare.
Acknowledgements
Theatre staff and ICS technicians in the Southern General Hospital.
References
- 1.Catling S. Blood conservation techniques in obstetrics: a UK perspective. Int J Obstet Anesth. 2007;16(3):241–249. doi: 10.1016/j.ijoa.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber G.B., Busch M.P., Kleinman S.H., Korelitz J.J. The risk of transfusion transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 3.Tosti M.E., Solinas S., Prati D., Salvaneschi L., Manta M., Francesconi M. An estimate of the current risk of transmitting blood-borne infections through blood transfusion in Italy. Br J Haematol. 2002;117:215–219. doi: 10.1046/j.1365-2141.2002.03334.x. [DOI] [PubMed] [Google Scholar]
- 4.Carson J.L., Altman D.G., Duff A., Noveck H., Weinstein M.P., Sonnenberg F.A. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694–700. doi: 10.1046/j.1537-2995.1999.39070694.x. [DOI] [PubMed] [Google Scholar]
- 5.Bierbaum B.E., Callaghan J.J., Galante J.O., Rubash H.E., Tooms R.E., Welch R.B. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Rosencher N., Kerkkamp H.E., Macheras G., Munuera L.M., Menichella G., Barton D.M., OSTHEO Investigation Orthopedic Surgery Transfusion Haemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 7.SHOT annual report 2011. Available from: http://www.shotuk.org/wp-content/uploads/2012/07/SHOT-Summary_FinalWebVersion2012_06_261.pdf. cited 06 Dec 2012.
- 8.Regan F., Taylor C. Blood transfusion medicine. BMJ. 2002;325(7356):143–147. doi: 10.1136/bmj.325.7356.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason L., Fitzgerald C., Powell-Tuck J., Rice R. Intraoperative cell salvage versus postoperative autologous blood transfusion in hip arthroplasty: a retrospective service evaluation. Ann R Coll Surg Engl. 2011;93(5):398–400. doi: 10.1308/003588411X579801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irisson E., Hemon Y., Pauly Y., Parratte S., Argenson J.N., Kerbaul F. Tranexamic acid reduces blood loss and financial cost in primary total hip and knee replacement surgery. Orthop Traumatol Surg Res. September 2012;98(5):477–483. doi: 10.1016/j.otsr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Orthopedic Perioperative Erythropoietin Study Group (Epo study group) Effectiveness of perioperative recombinant human erythropoietin in elective hip replacement. Lancet. 2003;341(8855):1227–1232. [PubMed] [Google Scholar]
- 12.Eschbach J.W. Iron requirements in erythropoietin therapy. Best Pract Res Clin Haematol. 2005;18(2):347–361. doi: 10.1016/j.beha.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Munoz M., Garcia-Erce J.A., Cuenca J., Bisbe E., Naveira E., Spanish Anaemia Working Group (AWGE) On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10(1):8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obama K., Maruyama Y., Osame M. The correlation between erythropoiesis and thrombopoiesis as an index for preoperative autologous blood donation. Transfus Sci. 1999;21(3):207–210. doi: 10.1016/s0955-3886(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz M., García-Erce J.A., Villar I., Thomas D. Blood conservation strategies in major orthopaedic surgery: efficacy, safety and European regulations. Vox Sang. 2009;96:1–13. doi: 10.1111/j.1423-0410.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 16.Esper S.A., Waters J.H. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus. 2011;9(2):139–147. doi: 10.2450/2011.0081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies L., Brown T.J., Haynes S., Payne K., Elliott R.A., McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;(44):1–210. doi: 10.3310/hta10440. iii–iv, ix–x. [DOI] [PubMed] [Google Scholar]
- 18.Carless P.A., Henry D.A., Moxey A.J., O'connell D.L., Brown T., Fergusson D.A. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;14(4):CD001888. doi: 10.1002/14651858.CD001888.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spark J.I., Chetter I.C., Kester R.C., Scott D.J. Allogeneic versus autologous blood during abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 1997;14(6):482–486. doi: 10.1016/s1078-5884(97)80128-1. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich W, Thuermel K, Heyde S, Busley R, Berger K. Autologous blood donation in cardiac surgery: reduction of allogeneic blood transfusion and cost-effectiveness. J Cardiothorac Vasc Anesth 19(5):589–96. [DOI] [PubMed]
- 21.Rosenblatt M.A. Strategies for minimizing the use of allogeneic blood during orthopedic surgery. Mt Sinai J Med. 2002;69(1–2):83–87. [PubMed] [Google Scholar]
- 22.Munoz M., Slappendel R., Thomas D. Transfusion of post-operative shed blood: laboratory characteristics and clinical utility. Eur Spine J. 2004;13(Suppl. 1):S107–S113. doi: 10.1007/s00586-004-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Health. Better blood transfusion: better use of blood 2010. Available from: http://www.transfusionguidelines.org.uk/?publication=bbt§ion=22&pageid=1473. cited 08 Dec 2012.
- 24.Waters J.H. Indications and contraindications of cell salvage. Transfusion. 2004;44:40S–44S. doi: 10.1111/j.0041-1132.2004.04176.x. [DOI] [PubMed] [Google Scholar]
- 25.Fong J., Gurewitsch E., Kang H.J., Kump L., Mack P.F., Birbach D. An analysis of transfusion practice and the role of intraoperative red blood cell salvage during cesarean delivery. Anesth Analg. 2007;104:666–672. doi: 10.1213/01.ane.0000253232.45403.e5. [DOI] [PubMed] [Google Scholar]
- 26.Ashworth A., Klein A.A. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105(4):401–416. doi: 10.1093/bja/aeq244. [DOI] [PubMed] [Google Scholar]
- 27.Attaran S., McIlroy D., Fabri B.M., Pullan M.D. The use of cell salvage in routine cardiac surgery is ineffective and not cost-effective and should be reserved for selected cases. Interact Cardiovasc Thorac Surg. 2011;12(5):824–826. doi: 10.1510/icvts.2010.249136. [DOI] [PubMed] [Google Scholar]
- 28.Kubota Combined preoperative autologous blood donation and intra-operative cell salvage for hip surgery. J Orthop Surg (Hong Kong) 2009;17(3):288–290. doi: 10.1177/230949900901700308. [DOI] [PubMed] [Google Scholar]
- 29.Bridgens J.P., Evans C.R., Dobson P.M.S., Hamer A.J. Intraoperative red blood-cell salvage in revision hip surgery. J Bone Joint Surg (American volume) 2007;89:270–275. doi: 10.2106/JBJS.F.00492. [DOI] [PubMed] [Google Scholar]
- 30.Shenolikar A., Wareham K., Newington D., Thomas D., Highes J., Downes M. Cell salvage auto transfusion in total knee replacement surgery. Transfus Med. 1997;7:277–280. doi: 10.1046/j.1365-3148.1997.d01-43.x. [DOI] [PubMed] [Google Scholar]
- 31.Sinclair K.C., Clarke H.D., Noble B.N. Blood management in total knee arthoplasty: a comparison of techniques. Orthopedics. 2009;32:19. doi: 10.3928/01477447-20090101-21. [DOI] [PubMed] [Google Scholar]
- 32.Thomas D., Wareham K., Cohen D., Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2000;86:669–673. doi: 10.1093/bja/86.5.669. [DOI] [PubMed] [Google Scholar]
- 33.Waters J.H., Dyga R.M., Waters J.F., Yazer M.H. The volume of returned red blood cells in a large blood salvage program: where does it all go? Transfusion. 2011;51(10):2126–2132. doi: 10.1111/j.1537-2995.2011.03111.x. [DOI] [PubMed] [Google Scholar]
- 34.Guerra J.J., Cuckler J.M. Cost effectiveness of intraoperative autotransfusion in total hip arthroplasty surgery. Curr Orthop Pract. 1995;315 [PubMed] [Google Scholar]
- 35.Simpson M.B., Georgopoulos G., Eilert R.E. Intraoperative blood salvage in children and young adults undergoing spinal surgery with predeposited autologous blood: efficacy and cost effectiveness. J Paediat Orthop. 1993;13(6) doi: 10.1097/01241398-199311000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Scottish Intercollegiate Guidelines Network (SIGN) Oct 2001. SIGN 54: perioperatove blood transfusion for elective surgery, a national clinical guideline. [Google Scholar]
- 37.Urwin S.C., Parker M.J., Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomised control trials. Br J Anaesth. 2000;84(4):450–455. doi: 10.1093/oxfordjournals.bja.a013468. [DOI] [PubMed] [Google Scholar]
- 38.Valentin N., Lomholt B., Jensen J.S., Heigaard N., Kreiner S. Spinal or general anaesthesia for surgery of the fracture hip. Br J Anaesth. 1986;58(3):284–291. doi: 10.1093/bja/58.3.284. [DOI] [PubMed] [Google Scholar]
- 39.Niemi T.T., Pitkanen M., Syrjaia M., Rosenberg P.H. Comparison of hypotensive epidural anaesthesia and spinal anaesthesia on blood loss and coagulation during and after total hip arthroplasty. Acta Anaesthesiol Scand. 2000;44(4):457–464. doi: 10.1034/j.1399-6576.2000.440418.x. [DOI] [PubMed] [Google Scholar]


