Abstract
Background
Mental stress-induced myocardial ischemia is associated with adverse prognosis in coronary artery disease patients. Anger is thought to be a trigger of acute coronary syndromes and is associated with increased cardiovascular risk; however, little direct evidence exists for a link between anger and myocardial ischemia.
Methods
[99mTc]sestamibi single-photon emission tomography was performed at rest, after mental stress (a social stressor with a speech task), and after exercise/pharmacological stress. Summed scores of perfusion abnormalities were obtained by observer-independent software. A summed difference score, the difference between stress and rest scores, was used to quantify myocardial ischemia under both stress conditions. The Spielberger's State-Trait Anger Expression Inventory was used to assess different anger dimensions.
Results
The mean age was 50 years, 50% were female and 60% were non-white. After adjusting for demographic factors, smoking, coronary artery disease severity, depressive and anxiety symptoms, each interquartile range increment in state-anger score was associated with 0.36 units adjusted increase in ischemia as measured by the summed difference score (95% CI: 0.14-0.59); the corresponding association for trait-anger was 0.95 (95% CI: 0.21-1.69). Anger expression scales were not associated ischemia. None of the anger dimensions were related to ischemia during exercise/pharmacological stress.
Conclusion
Anger, both as an emotional state and as a personality trait, is significantly associated with propensity to develop myocardial ischemia during mental stress, but not during exercise/pharmacological stress. Patients with this psychological profile may be at increased risk for silent ischemia induced by emotional stress and this may translate into worse prognosis.
Introduction
Psychological stress has long been suspected to be a risk factor for coronary heart disease (CHD), but its exact role as a trigger of acute ischemia is unclear.1 Mental stress-induced myocardial ischemia is a transient myocardial ischemic response to a standardized mental stress challenge2 which can be induced in approximately one third to one half of patients with CHD.2 Mental stress ischemia is analogous to exercise or pharmacologically-induced myocardial ischemia during standard cardiac testing (here referred together as “physical stress-induced myocardial ischemia”), except that the stressor used is psychological instead of physical.2 Mental stress ischemia has similar prognostic value to physical stress ischemia, but is typically painless, occurs at lower levels of oxygen demand, and is not related to severity of coronary artery disease (CAD)2, 3, or previous revascularization.2, 3 Mental stress, but not physical stress-induced myocardial ischemia, correlates with myocardial ischemia measured in daily life ambulatory monitoring4 and is associated with worse prognosis in subjects with stable CHD, with a twofold increased risk of future cardiac events independent of physical stress-induced ischemia.5
Anger has long been considered a potential precipitant of acute myocardial infarction (MI) and a significant risk factor for CHD. A recent meta-analysis of 44 prospective studies found that anger and hostility were significantly associated with increased CHD risk in both healthy (19% increase) and preexisting CHD populations (24% increase).6 Substantial research also suggests that acute anger is a potential trigger of acute coronary syndromes.1, 7 These previous data suggest that anger could play a role in the development of acute myocardial ischemia. However, direct evidence of a link between anger and myocardial ischemia is scarce.8
To clarify this issue, we examine whether anger as an acute state or as a personality trait, or specific anger expression patterns, are positively associated with the occurrence of mental stress but not physical stress- induced ischemia. Given that emotional distress appears to play a larger role in early-onset CHD than in older age groups,9 we elected to study young and middle-aged (60 years and younger) men and women who survived a recent MI.
Methods
Subjects
Between July-2009 and April-2012, the Myocardial Infarction and Mental Stress Study (MIMS) enrolled 98 patients between the age of 38 and 60 years with a documented history of MI within the previous 6 months (range: 1.3-6 months). Men and women were matched for age (±2 years), type of MI (ST-elevation MI or non-ST-elevation MI) and time since the MI (±2 months). Other inclusion and exclusion criteria and details of sample construction have been described elsewhere.10
Study Design
Subjects underwent three single-photon emission computed tomography (SPECT) imaging studies, one at rest, one with mental stress and one with exercise or pharmacological stress. The two stress scans were obtained in separate days within one week of each other (the order was balanced), and the rest scan was obtained during the first session. All testing was done after an overnight fast, and anti-ischemic medications were held for 24 hours prior to testing. Sociodemographic and psychosocial data were collected at the first visit prior to stress testing. At the end of the study protocol, medical records were abstracted for clinical information. The study protocol was approved by the Emory University Institutional Review Board, and informed consent was obtained from all participants.
Mental and Physical Stress Procedures
Mental stress was induced by a standardized social stressor using a public speaking task, as previously described.11 Briefly, subjects were asked to imagine a real-life stressful situation, and to make up a realistic story around this scenario. They were given two minutes to plan the story and three minutes to present it in front of a video camera and a small audience wearing white coats. Subjects were told that their speech would be evaluated by the laboratory staff for content, quality and duration. For physical stress, subjects underwent a Bruce protocol by walking on a treadmill, with exercise target set at 85% of maximum predicted heart rate based on the patient's sex and age. For 16 subjects who were unable to reach the heart rate target, we performed a pharmacological stress test with regadenoson (Astellas, Northbrook, IL), an adenosine receptor agonist. Blood pressure and heart rate were monitored during each stress test. Subjective ratings of distress were obtained at baseline and after mental stress with the Subjective Units of Distress Scale12 on a linear scale of 0 to 100 (100=highest level of distress). We also obtained visual analog ratings of nervousness, anxiety, fear and anger with a scale of 0-4, with 4 being extreme.
Myocardial Perfusion Imaging
Subjects underwent [99mTc]-sestamibi SPECT myocardial perfusion imaging at rest, during mental stress and during physical stress on a dedicated ultra-fast solid-state camera (Discovery NM 530c, GE, Milwaukee, WI) without attenuation correction. We used [99mTc]-sestamibi dosages of 10-15 mCi for the rest scan and of 30-45 mCi for the stress scans, according to body weight and with a dose ratio of rest to stress of 1:3. For mental stress, the radioisotope was injected one minute after the onset of the speech, while for exercise stress it was injected at peak exertion following the Bruce protocol. Following standard procedures, stress images were acquired 45-60 minutes after [99mTc]-sestamibi injection.
Myocardial perfusion was quantified by means of the Emory Cardiac Toolbox software, which provides objective (operator-independent) quantitative assessment of perfusion with established validity and reproducibility.13 Briefly, the three-dimensional tracer uptake distribution in the left ventricle was oriented along the short axis and sampled onto a two-dimensional polar map. A summed score, quantifying the extent and severity of perfusion defects across 17 myocardial segments was computed.13 In each region, defect severity was quantified using a 4-point scale from normal (score=0) to absent perfusion (score=4). The regional severity scoring was then summed up across the 17 myocardial segments. Separate scores were obtained for the rest images (summed-rest score, SRS) and the stress images (summed-stress score, SSS). For each stress, a summed-difference score (SDS), quantifying the number and severity of reversible (ischemic) myocardial perfusion defects, was obtained by subtracting the rest-score from the stress-score; a positive SDS would indicate presence of ischemia. We also calculated the percentage of myocardial involvement by dividing the number of myocardial segments with perfusion defects (score >0) by the total number of segments (17). The use of automated image analysis has specific advantages for our study. Quantitative SPECT image analysis is equivalent to visual analysis from expert readers,14, 15 but is more reproducible16, 17 because it eliminates interpreter variability. Thus, it is better suited for protocols with serial SPECT scans such as in our study.
Measurement of Anger and Other Covariates
Anger was assessed using the Spielberger's State-Trait Anger Expression Inventory (STAXI-2), a 57 item questionnaire which measures the following anger dimensions: 1) state-anger (intensity of anger at a particular time), 2) trait-anger (disposition to experience angry feelings as a trait), and 3) anger expression, including anger-out (anger expressed toward others or the environment), anger-in (suppression of anger), and anger-control, the latter consisting of two subscales: anger-control (out), the ability to limit expression of anger, and anger-control (in), the ability to calm down.18, 19 Larger scores for each dimension indicate more severity of anger, except for the anger-control subscales (higher score indicating better anger control). All scales have good internal consistency (alphas ranging from 0.70-0.87) and validity.19
Sociodemographic factors and medical history were assessed using standardized questionnaires. Angiographic data were obtained from the coronary angiogram performed in conjunction with the index-MI. CAD severity was quantified using the Gensini semi-quantitative angiographic scoring system,20 which takes into account the degree of luminal narrowing along with a multiplier for specific coronary tree locations. If a patient underwent revascularization, the percentage of coronary obstruction used in the scoring reflected the post-revascularization results. Depressive symptoms were assessed with the Beck Depression Inventory-II (BDI-II).21 We also administered the State-Trait Anxiety Inventory (STAI) to measure state and trait anxiety22 and the Seattle Angina Questionnaire to assess angina symptoms.23
Statistical Analysis
Multiple linear regression models were used to assess the association between summed scores of myocardial perfusion with mental/physical stress and the six anger subscales, adjusting for possible confounding factors. The SDS for ischemia quantification was our main outcome variable of interest. Since the SDS for both mental and physical stress was skewed, while the SSS for both conditions was normally distributed, we used the SSS scores as dependent variables while adjusting for the rest score (SRS). Because of the mathematical relationship between these scores, the coefficient from a model with SSS as dependent variable, adjusted for SRS, is identical to that from a model where the dependent variable is the SDS. This strategy allowed us to obtain non-biased standard errors and p values.
In cumulative hierarchical models, we adjusted for a set of factors that were considered a priori either possible confounding factors or mediators of the relationships under study. Because of the relatively small sample size, we were careful to develop parsimonious models. Adjustment factors included sociodemographic and lifestyle characteristics (age, sex, race, and current cigarette smoking), CAD severity (Gensini score), depressive symptoms (BDI-II score) and trait-anxiety. To allow comparison of effects across different anger subscales with unequal score range, we used the interquartile range (IQR) as scaling factor, i.e., the distance between the 25th and 75th percentiles. We also assessed the interaction of sex and age (≤50 years and >50 years) for each anger subscale in the final models. We performed thorough regression diagnostics to rule out collinearity and outliers;24 these analyses showed that our models were appropriate and no influential data points were present. Additionally, we repeated the analysis using non-parametric generalized additive modeling,25 which yielded similar results.
Results
Study Sample
The mean and median age was 50 years, half of patients were women, and 60% were non-white (Table 1). Almost half of the patients had an ST-elevation MI (45%), and after the index MI 75% underwent percutaneous coronary interventions and 11% coronary artery bypass surgery. Psychosocial factors were common, with almost one third of patients (32%) reporting income below poverty, and 37% having significant depressive symptoms (BDI-II score >13).
Table 1.
Characteristics of the study population.
| Variables | Characteristics (n=98) |
|---|---|
| Demographics | |
| Age in years, mean (SD) | 50 (6) |
| Age ≤ 50 years (%) | 49 (50%) |
| Female (%) | 49 (50%) |
| Non-white (%) | 59 (60%) |
| Income below poverty line (%)* | 31 (32%) |
| Current smokers (%) | 28 (29%) |
|
| |
| Medical history and CHD risk factors | |
| ST- elevation MI (%) | 44 (45%) |
| Time since MI (months), mean (SD) | 4.8 (1.3) |
| Hypertension (%)† | 67 (69%) |
| Hyperlipidemia (%)† | 71 (73%) |
| Diabetes (%)† | 20 (21%) |
| BMI (kg/m2), mean (SD)† | 31 (6) |
| Obese (BMI ≥30 kg/m2) (%)† | 45 (46%) |
| Gensini score, mean (SD) | 17 (32) |
| > 0 (%) | 65 (66%) |
| At least 1 angina episode in past month (%) | 41 (42%) |
|
| |
| Treatment history and current medications | |
| Percutaneous Coronary Intervention (%) | 73 (75%) |
| Coronary Artery Bypass Graft (%) | 11 (11%) |
| Aspirin (%)† | 85 (88%) |
| Beta blockers (%)† | 85 (88%) |
| ACE inhibitors (%)† | 53 (55%) |
| Statins (%)† | 85 (88%) |
| Anti-depressants (%)† | 13 (13%) |
|
| |
| Psychological factors | |
| BDI-II score, mean (SD) | 11 (9) |
| > 13 | 36 (37%) |
| Anxiety-state, mean (SD)† | 38 (11) |
| Anxiety-trait, mean (SD)† | 39 (11) |
SD: standard deviation; BMI: Body Mass Index; BDI: Beck Depression Inventory;
2 observations missing;
1 observation missing.
Myocardial perfusion could not be quantified in 5 subjects due to poor image quality. For mental stress, the mean and standard deviation for the SDS for ischemia quantification was 2.31±2.69 (range, 0-13). For physical stress, the mean SDS was 2.74±3.24 (range, 0-13). Based on a pre-defined cut-off point of SDS ≥3 for mental stress and ≥4 for exercise/pharmacological stress,26 36 patients (39%) had mental stress ischemia, 32 (34%) had physical stress ischemia and 18 (20%) had both mental and physical stress ischemia. Seven (9%) subjects had angina during exercise, and 18 (22%) had ST-segment depression with exercise stress. Descriptive characteristics of anger subscales are shown in Table 2.
Table 2.
Characteristics of anger subscales.
| Anger subscales | No of Items | Scale score mean (SD) | Scale score range | Scale item mean (SD)* |
|---|---|---|---|---|
| Anger-state | 15 | 18.0 (7.5) | 15-60 | 1.20 (0.50) |
| Anger-trait | 10 | 15.8 (4.9) | 10-36 | 1.58 (0.49) |
| Anger expression | ||||
| Anger-out | 8 | 13.9 (3.5) | 8-27 | 1.73 (0.44) |
| Anger-in | 8 | 15.8 (4.3) | 8-28 | 1.98 (0.54) |
| Anger-control (out) | 8 | 24.1 (5.3) | 14-32 | 3.01 (0.66) |
| Anger-control (in) | 8 | 23.1 (5.2) | 10-32 | 2.89 (0.65) |
SD: standard deviation
Scale items ranged between 1 and 4 on a likert scale from 1= Almost never, to 4= Almost always
Correlates of Anger Subscales
Age was inversely associated with state and trait-anger, indicating that the older the age, the less severe the anger scores (online appendix supplementary table 1). There were no significant differences based on other demographic factors. Current smokers, hypertensive subjects and subjects reporting at least one angina episode per month tended to have higher anger-out scores, but the levels of other anger dimensions were similar. All anger subscales were positively associated with higher depressive and anxiety symptoms.
Changes in Hemodynamic Measures and Subjective Distress with Mental Stress
Heart rate, systolic and diastolic blood pressure and rate-pressure product (heart rate times systolic blood pressure) significantly increased with mental stress (online appendix supplementary table 2) and with exercise/pharmacological stress. None of these changes were significantly associated with anger dimensions (data not shown). Changes in subjective ratings of distress, nervousness, fearfulness and anxiety with mental stress were also not related to anger, but the change in subjective ratings of anger was weakly but significantly associated with trait-anger (regression-coefficient: 0.05, 95% CI: 0.01-0.09). None of the above subjective ratings were found to be associated with ischemia during mental stress.
Mental Stress-Induced Myocardial Ischemia and Anger
State-anger, trait-anger, and anger expression-out were all significantly associated with the SDS with mental stress in unadjusted analysis, denoting more ischemia (Table 3). After adjustment for age, sex, race, smoking status, Gensini score, and depression and anxiety symptoms, both state and trait-anger remained significantly associated with the SDS with mental stress. Each incremental IQR increase in state-anger score (corresponding to 3 score points) was associated with 0.36 units adjusted increase in SDS (95% CI: 0.14-0.59); the corresponding association for trait-anger was 0.95 (95% CI: 0.21-1.69) per IQR increase (corresponding to 6 points). These associations translated into 2.1% increased myocardium ischemic involvement with each IQR progressively higher state-anger score (95% CI: 1.0%-3.2%), and 5.4% increased myocardial ischemic involvement with each IQR higher trait-anger score (95% CI: 1.8%-8.9%). For both state and trait-anger, the SDS was higher for higher levels of scale item means (Figure 1). For state-anger, being on average moderately angry or very angry was associated with about 4-times higher summed-difference score compared to the two lower categories. For trait-anger, there was a gradual increase in SDS going from 1 (almost never angry), to 4 (almost always angry). Anger-out was no longer significantly associated with the SDS after adjustment for depression and anxiety symptoms. No association was found for the other anger dimensions. No significant sex or age interactions were found.
Table 3.
Association between anger subscale scores and myocardial ischemia severity, as quantified by the SDS, during mental stress.
| Anger subscales | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Δ (95% CI) | P | Δ (95% CI) | P | Δ (95% CI) | P | |
| Anger-state | 0.40 (0.20-0.60) | <.001 | 0.41 (0.20-0.62) | <.001 | 0.36 (0.14-0.59) | 0.002 |
| Anger-trait | 1.02 (0.39-1.65) | 0.002 | 1.12 (0.46-1.78) | 0.001 | 0.95 (0.21-1.69) | 0.01 |
| Anger Expression | ||||||
| Anger-out | 0.68 (0.08-1.29) | 0.03 | 0.68 (0.06-1.31) | 0.03 | 0.52 (-0.15-1.18) | 0.13 |
| Anger-in | 0.67 (-0.09-1.42) | 0.08 | 0.68 (-0.10-1.46) | 0.09 | 0.31 (-0.62-1.23) | 0.52 |
| Anger-control (out) | -0.69 (-1.51-0.13) | 0.10 | -0.72 (-1.54-0.10) | 0.08 | -0.52 (-1.39-0.35) | 0.24 |
| Anger-control (in) | -0.27 (-1.22-0.67) | 0.57 | -0.33 (-1.27-0.61) | 0.49 | 0.01 (-0.99-1.02) | 0.98 |
Δ: Unit change in SDS per increase in IQR of corresponding anger subscale
Model 1: Unadjusted model
Model 2: Adjusted for age, gender, race, smoking status, and Gensini score.
Model 3: Adjusted for all the covariates in model 2 + BDI-II score and trait-anxiety.
Figure 1. Mental stress summed-difference scores according to state and trait anger levels.
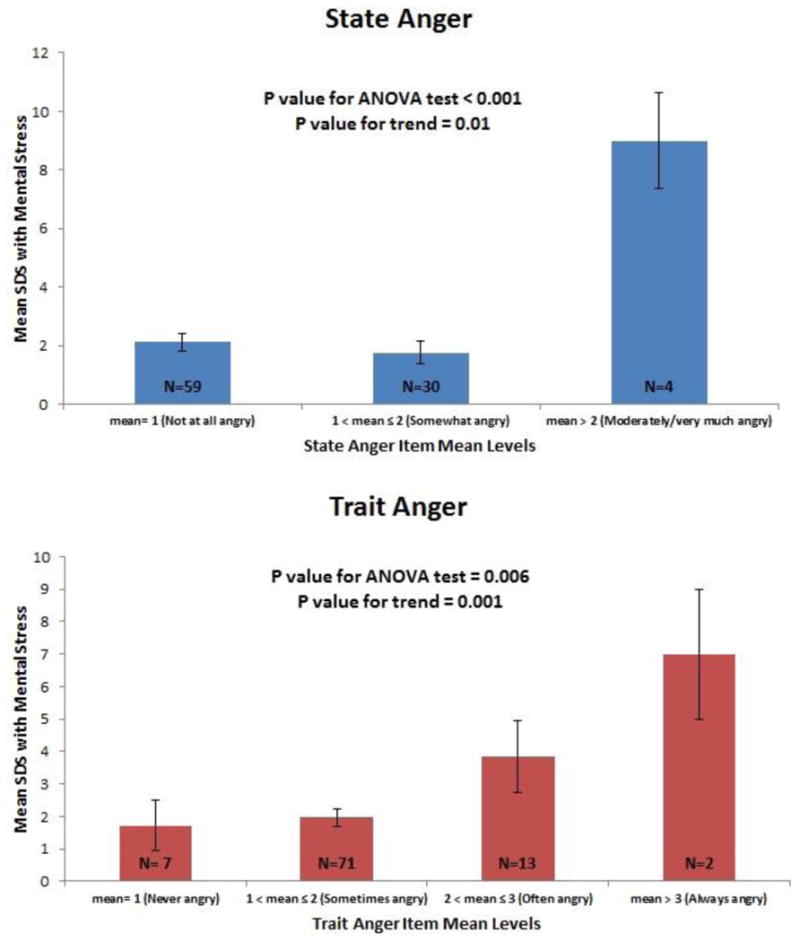
Shown are mental-stress mean SDS and standard errors according to state and trait anger score item mean levels. Numbers on bars indicate number of patients. For state-anger, categories 3 and 4 were collapsed due to limited sample size.
Using a similar analytical strategy, we found that none of the anger dimensions were significantly associated with the summed-difference score during exercise or pharmacological stress (online appendix supplementary table 3).
To rule out the possibility that SPECT imaging artifacts may have influenced our results, we performed a sensitivity analysis after excluding 9 subjects with significant artifacts identified through a systematic review of all SPECT scans by an experienced cardiologist. Such exclusion did not change the association between anger subscales and both mental and physical stress SDS (online appendix supplementary table 4).
Discussion
In a sample of young and middle-aged survivors of acute MI, we found that patients scoring higher in either state or trait-anger were more likely to develop myocardial ischemia due to emotional stress than those with lower anger scores. The association was robust and clinically significant; each incremental IQR for both state and trait anger led to about 2% and 5% increase in ischemic myocardium, respectively. In addition, the association was specific to mental stress, since it was not seen for exercise or pharmacologically-induced ischemia. Furthermore, it was independent of traditional coronary risk factors and CAD severity, and held true even after adjustment for other indicators of psychosocial distress (depression and anxiety symptoms). These results suggest that patients with higher levels of anger, either as a transitory emotional response or as a personality trait, are at increased risk of silent ischemia induced by emotional stress. This psychological profile may help identify patients at risk for mental stress-induced adverse outcomes.
To the best of our knowledge, ours is the first study to systematically examine the association between various anger dimensions and mental stress-induced ischemia measured by myocardial perfusion imaging. A previous study of 180 patients with stable CHD and a positive exercise stress test27 reported no association between anger expression and mental stress-induced ischemia assessed by radionuclide ventriculography and electrocardiography, but state and trait-anger were not measured. This study did find a relationship between anger/irritability ratings in response to the speech stressor and myocardial ischemia. On the other hand, in a study of 30 CHD patients with a positive exercise or pharmacological stress test, Burg et al28 found that higher trait-anger and lower anger-control predicted the development of mental stress ischemia measured by radionuclide ventriculography. Anger-in and anger-out were not associated with mental stress ischemia, while state-anger was not measured. Additional studies have provided evidence that anger experimentally induced in the laboratory can provoke myocardial ischemia, both in patients and in animal models.29, 30 Thus, our results extend these important previous findings by examining a comprehensive set of anger dimensions, by using myocardial perfusion imaging, which is currently the gold standard for myocardial ischemia assessment, and by including a well-characterized sample of MI patients enriched with younger individuals, women and minorities, a diverse group with a substantial level of psychosocial burden.
Acute anger has been consistently implicated as an important precipitant for angina and/or MI.1, 7 A recent meta-analysis found that both anger and hostility were significantly associated with increased risk of CHD events. Higher trait-anger was associated with 98% increased risk of CHD events in subjects with pre-existing CHD.6 However, whether anger is an independent risk factor for CHD, or rather an epiphenomenon of unmeasured risk factors, or even a prodrome of CHD itself, is still debated.8 Our findings of a relationship between anger, both as a state and as a trait, and mental stress-induced ischemia, are consistent with the notion that anger is indeed linked to the risk of CHD. Our data also provide a novel mechanistic pathway through which young and middle-aged survivors of MI might be at risk of recurrent CHD events.
Our study has several strengths. We included a well-defined population of young and middle-aged post-MI patients, who are likely to have enhanced vulnerability to psychological stressors, with balanced representation of women and minorities. In addition, we assessed ischemia using state-of-the-art myocardial perfusion imaging and a quantitative, reader independent ischemia scoring system.2, 31
The main limitation of our study is the relatively small sample size that may have caused wide confidence intervals. Small sample size may also have precluded conclusively proving the association (or lack thereof) between anger expression subscales and mental stress-induced ischemia. Nonetheless, the directionality of the associations consistently holds true with the underlying assumption, with anger-out and anger-in being directly related, and anger-control indirectly related, with mental stress-induced myocardial ischemia. Most of our MI patients received revascularization procedures, reflecting current treatment standards, which may have affected the detection of ischemia. However, mental stress-induced myocardial ischemia is known to be unrelated to severity of coronary obstruction or previous revascularization, and can occur in the setting of a negative exercise- or adenosine-stress test.3 We are unable to explain why mental stress-induced myocardial ischemia shows a robust relationship only with state/trait anger, and not with anger expression, but our results are consistent with a recent meta-analysis,6 where an association with CHD was found for trait anger but not for anger-expression. Another limitation is the lack of CHD outcome data. Therefore, our results need to be replicated in larger studies with prospective follow up to assess if mental stress-induced ischemia explains the relationship between anger and increased risk of cardiac events.
Conclusion
Among young and middle-aged survivors of an MI, anger, both as a psychological state and as a personality trait, is associated with a greater propensity to develop myocardial ischemia with emotional stress. Our results suggest that MI patients with this psychological profile are at increased risk of silent ischemia induced by emotional stress, which in turn may increase their risk for adverse outcomes. Incorporation of psychological evaluations for anger assessment, especially among young post-MI patients, may help identify patients at risk for mental stress-induced adverse outcomes. New treatments should be evaluated that specifically target anger to reduce the risk of future cardiac events.
Acknowledgments
This work was supported by the National Institutes of Health (R21-HL093665, R21-HL093665-01A1S1, R01-HL109413, 2R01-HL068630, 2K24-HL077506, K24-MH076955, R01-MH056120, R01-HL088726, and P01-HL 101398).
Footnotes
Conflicts of Interest: No authors report conflict of interest.
The authors are solely responsible for the design and conduct of this study, including all study analyses, the drafting-editing of the manuscript, and its final content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steptoe A, Brydon L. Emotional triggering of cardiac events. Neurosci Biobehav Rev. 2009;33(2):63–70. doi: 10.1016/j.neubiorev.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24(8):690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 3.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2(5):e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, et al. Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: The psychophysiologic investigations of myocardial ischemia (PIMI) Study. J Am Coll Cardiol. 1999;33(6):1477–1484. doi: 10.1016/s0735-1097(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 5.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, et al. Meta-Analysis of Mental Stress-Induced Myocardial Ischemia and Subsequent Cardiac Events in Patients With Coronary Artery Disease. Am J Cardiol. 2014 doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53(11):936–46. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Gabbay FH, Krantz DS, Kop WJ, Hedges SM, Klein J, Gottdiener JS, et al. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: physical and mental activities, anger and smoking. J Am Coll Cardiol. 1996;27(3):585–92. doi: 10.1016/0735-1097(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 8.Edmondson D, Newman JD, Whang W, Davidson KW. Emotional triggers in myocardial infarction: do they matter? Eur Heart J. 2013;34(4):300–6. doi: 10.1093/eurheartj/ehs398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ketterer MW, Fitzgerald F, Thayer B, Moraga R, Mahr G, Keteyian SJ, et al. Psychosocial and traditional risk factors in early ischaemic heart disease: cross-sectional correlates. J Cardiovasc Risk. 2000;7(6):409–13. doi: 10.1177/204748730000700603. [DOI] [PubMed] [Google Scholar]
- 10.Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014 doi: 10.1097/PSY.0000000000000045. epub March 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann PG, M R, Becker LC, Bertolet B, Bonsall R, Chaitman B, Cohen JD, Forman S, Goldberg AD, Freedland K, Ketterer MW, Krantz DS, Pepine CJ, Raczynski J, Stone PH, Taylor H, Knatterud GL, Sheps DS. The Psychophysiological Investigations of Myocardial Ischemia (PIMI) study: objective, methods, and variability of measures. Psychosom Med. 1998 doi: 10.1097/00006842-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Wolpe J. The practice of behavior therapy. New York: New York Pergamon Press; 1969. [Google Scholar]
- 13.Garcia EV, DePuey EG, DePasquale EE. Quantitative planar and tomographic thallium-201 myocardial perfusion imaging. Cardiovasc Intervent Radiol. 1987;10(6):374–83. doi: 10.1007/BF02577348. [DOI] [PubMed] [Google Scholar]
- 14.Esteves FP, Galt JR, Folks RD, Verdes L, Garcia EV. Diagnostic performance of low-dose rest/stress Tc-99m tetrofosmin myocardial perfusion SPECT using the 530c CZT camera: quantitative vs visual analysis. J Nucl Cardiol. 2014;21(1):158–65. doi: 10.1007/s12350-013-9827-7. [DOI] [PubMed] [Google Scholar]
- 15.Arsanjani R, Xu Y, Hayes SW, Fish M, Lemley M, Jr, Gerlach J, et al. Comparison of fully automated computer analysis and visual scoring for detection of coronary artery disease from myocardial perfusion SPECT in a large population. J Nucl Med. 2013;54(2):221–8. doi: 10.2967/jnumed.112.108969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udelson JE. Lessons from the development of new adenosine A2A receptor agonists. JACC Cardiovasc Imaging. 2008;1(3):317–20. doi: 10.1016/j.jcmg.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Mahmarian JJ, Cerqueira MD, Iskandrian AE, Bateman TM, Thomas GS, Hendel RC, et al. Regadenoson induces comparable left ventricular perfusion defects as adenosine: a quantitative analysis from the ADVANCE MPI 2 trial. JACC Cardiovasc Imaging. 2009;2(8):959–68. doi: 10.1016/j.jcmg.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger CD. Professional manual for the State-Trait Anger Expression Inventory. Research Ed. Tampa, Fla: University of South Florida; 1988. [Google Scholar]
- 19.al'Absi M, Bongard S. Neuroendocrine and behavioral mechanisms mediating the relationship between anger expression and cardiovascular risk: assessment considerations and improvements. J Behav Med. 2006;29(6):573–91. doi: 10.1007/s10865-006-9077-0. [DOI] [PubMed] [Google Scholar]
- 20.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. BDI-II. Beck Depression Inventory: Second Edition. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety (STAI) manual. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 23.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbaum D, Kupper L, Nizam A, Rosenberg E. Applied regression analysis and other multivariable methods. 5. Boston: Cengage Learning; 2013. [Google Scholar]
- 25.Hastie TJ, Tibshirani RJ. Generalized additive models. 1. Boca Raton: CRC Press; 1990. [Google Scholar]
- 26.Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, et al. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 2006;47(5):987–91. doi: 10.1016/j.jacc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 27.Ketterer MW, Freedland KE, Krantz DS, Kaufmann P, Forman S, Greene A, et al. Psychological Correlates of Mental Stress-induced Ischemia in the Laboratory: The Psychophysiological Investigation of Myocardial Ischemia (PIMI) Study. J Health Psychol. 2000;5(1):75–85. doi: 10.1177/135910530000500112. [DOI] [PubMed] [Google Scholar]
- 28.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22(2):440–8. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- 29.Ironson G, Taylor CB, Boltwood M, Bartzokis T, Dennis C, Chesney M, et al. Effects of anger on left ventricular ejection fraction in coronary artery disease. Am J Cardiol. 1992;70(3):281–5. doi: 10.1016/0002-9149(92)90605-x. [DOI] [PubMed] [Google Scholar]
- 30.Verrier RL, Hagestad EL, Lown B. Delayed myocardial ischemia induced by anger. Circulation. 1987;75(1):249–54. doi: 10.1161/01.cir.75.1.249. [DOI] [PubMed] [Google Scholar]
- 31.Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM, Sheps DS. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. J Nucl Cardiol. 2003;10(1):56–62. doi: 10.1067/mnc.2003.26. [DOI] [PubMed] [Google Scholar]


