Abstract
The distribution and abundance of Betaproteobacteria and three of its genera: Limnohabitans genus (R-BT065 lineage), Polynucleobacter genus (including two subclusters P. necessarius and P. acidiphobus/difficilis), and Methylophilus genus across the epilimnion of 72 limnologically diverse freshwater habitats was investigated using fluorescence in situ hybridization. Moreover, seasonal development of Betaproteobacteria subgroups along the axis of a longitudinally heterogeneous reservoir was followed. Betaproteobacteria comprised on average 29.1%, Polynucleobacter 11.6%, P. necessarius 10.1%, P. acidiphobus/difficilis 0.5%, Limnohabitans 8.9%, and Methylophilus 0.9% of total bacterioplankton cells in the investigated habitats. P. necessarius and Limnohabitans coexisted in the majority of habitats but showed contrasting abundance patterns along the pH gradient of habitats (pH 3.8–8.5). The observed distribution patterns could be explained by different preferences for substrate sources, i.e. substances of humic origin in acidic waters and algal-derived substances in alkaline waters. However, substrate utilization patterns observed in laboratory experiments indicate no coherent group-specific differences in substrate preferences. Interestingly, similar distribution patterns were revealed for Limnohabitans and P. acidiphobus/difficilis, suggesting similar ecological adaptations of these only distantly related taxa. Our findings further emphasize that at least two major taxa of freshwater Betaproteobacteria represent ecologically diversified groups. Investigations at higher phylogenetic resolution are required for obtaining further insights into the ecology of these important taxa.
Keywords: Polynucleobacter, Limnohabitans, ecological diversification, competition
Introduction
Betaproteobacteria represent one of the key components of freshwater bacterioplankton (Hiorns et al., 1997; Glöckner et al., 2000, Barberan et al., 2010) constituting 23% (three lakes, Glöckner et al., 2000) to 70% of freshwater bacterioplankton (one habitat, Hahn et al., 2005). Freshwater bacteria affiliated with the Betaproteobacteria have formerly been divided into four (Glöckner et al., 2000), six (Zwart et al., 2002) or seven lineages (Newton et al., 2011). However, recent studies (e.g. Hahn et al., 2005; Lindström et al., 2005; Salcher et al., 2008; Jezberová et al., 2010; Šimek et al., 2010a) indicated that in a vast majority of cases, only two of these lineages, i.e. BetI and BetII (Newton et al., 2011), are responsible for the overall abundance of the Betaproteobacteria in freshwater habitats. These two groups are mainly represented by the genus Limnohabitans (Hahn et al., 2010a) and especially by its R-BT065 lineage (Šimek et al., 2001; Kasalický et al., 2010) – a monophyletic cluster within this genus – as well as by the genus Polynucleobacter (Heckmann and Schmidt, 1987; Hahn et al., 2009). So far, much less is known on the other five lineages of Betaproteobacteria, of which only BetIII and BetIV have received some attention (Glöckner et al., 2000, Friedrich et al., 2003, Salcher et al., 2008, Newton et al., 2011).
The genus Limnohabitans constitutes, together with the genus Rhodoferax, the BetI lineage (Newton et al., 2011). So far, Limnohabitans harbors four validly described species (Hahn et al., 2010a; Hahn et al., 2010b; Kasalický et al., 2010).
The genus Polynucleobacter, synonymous with PnecABCD (Wu and Hahn, 2006a), and BetII (Newton et al., 2011), is currently represented by five species, which are more or less equivalent to previously designated subclusters (Hahn, 2003).
A wealth of information is now available on Limnohabitans and Polynucleobacter bacteria, including information on ecophysiology (Hahn et al., 2009; Kasalický et al., 2010), intraspecific ecological differentiation (Jezbera et al., 2011), seasonality (e.g. Crump et al., 2003; Hahn et al., 2005; Wu and Hahn, 2006b), abundance and distribution (e.g. Hahn 2003; Wu et al., 2006; Buck et al., 2009; Jezberová et al., 2010), habitat preferences (Hahn 2003, Hahn et al., 2005, Šimek et al., 2010a), vertical distribution (Wu and Hahn, 2006b; Salcher et al., 2008), niche separation (Šimek et al., 2010b, Jezbera et al., 2011), grazing vulnerability (Šimek et al., 2001, Boenigk et al., 2004, Jezbera et al., 2005), etc. On the other hand, almost all this knowledge has been generated from studies that focused on a single or only a few habitats, or on populations of microorganisms belonging to only one of the lineages, or from results gained from manipulation experiments. What is entirely missing is a comprehensive investigation of the distribution and abundance patterns of the major groups of Betaproteobacteria across a large number of freshwater systems representing a broad ecological spectrum of the habitat types that we offer here.
While several studies have focused on Polynucleobacter and Limnohabitans, considerably less is known on the other groups of freshwater Betaproteobacteria. In this comprehensive study we intended to close this gap by investigating the abundance of four major Betaproteobacteria groups: the genus Limnohabitans, the genus Polynucleobacter and two of its subgroups, and the genus Methylophilus across a large set of 72 systems representing a broad spectrum of habitats, covering for example a pH gradient ranging from 3.8 to 8.5. The major aim of the study was to convincingly document the overall numerical importance and the hypothesized contrasting ecological roles of the two key groups of Betaproteobacteria, i.e. Limnohabitans and Polynucleobacter, not only among Betaproteobacteria alone, but also among the whole bacterioplankton community. In addition, to cover the distribution patterns of the major Betaproteobacteria groups, we decided to use other available genetic probes specific for other betaproteobacterial groups or subgroups of the two mainly studied genera. Last but not least, we intended to explore the fundamental trophic niches of the distinct isolates by following their substrate utilization patterns.
The foremost goals of the presented study thus were (i) to reveal the distribution patterns of the four key subgroups of Betaproteobacteria, (ii) to identify environmental drivers that determine their distribution and abundance, (iii) to identify conditions under which the two major genera, Polynucleobacter and Limnohabitans, co-occur, (iv) and to investigate if they are potentially competing for certain resources. Based on the presented results, we intended to create a generalizing picture on the distribution of Betaproteobacteria across ecologically diverse habitat types serving as a tool for directing future studies on Betaproteobacteria diversity.
Material and Methods
Sampling of habitats
(i) Distribution of taxa across environmental gradients
In total, 72 lentic freshwater habitats (Supplementary Table S1) representing a pH gradient from 3.8 to 8.5 were sampled between 2006 to 2008 (from June to November, respectively). The primary study area was represented by the Salzkammergut Lake District, a mountainous area with numerous lakes and ponds located on the northern slope of the Alps close to the city of Salzburg (Austria). Apart from this area, several other habitats located in Austria and the Czech Republic were sampled. A detailed list of a set of habitats including the ones presented here can be found elsewhere (Jezberová et al., 2010). The selected 72 habitats represented the largest possible variety of lakes, small natural and artificial ponds and puddles differing in a multitude of parameters. Almost all habitats were sampled at a depth of 0.5 meters.
(ii) Temporal and spatial distribution of taxa in a single habitat
A seasonal distribution study was performed on the canyon-shaped meso-eutrophic Římov reservoir located near the city of České Budějovice (Czech Republic). The reservoir was sampled along its longitudinal axis at three sites (assigned as DAM, MIDDLE and RIVER) in three-week intervals between March and November 2005, as described previously (Šimek et al., 2008).
Determination of physicochemical parameters of the water samples
Temperature, pH, oxygen concentration and conductivity were measured on site. In the habitat survey, dissolved organic carbon (DOC) and concentrations of humic substances were estimated photospectroscopically, as described previously (Supplementary Table S1 and Jezberová et al., 2010). In the seasonal study of the Římov reservoir, DOC, dissolved reactive phosphorus (DRP), particulate reactive phosphorus (PRP), total phosphorus (TP), particulate phosphorus (PP), total primary production (PPtot), primary production in 1-2 μl filtrate (PP1-2μl) and chlorophyll a (ChlA) were measured as described in Šimek et al. (2008).
Determination of bacterial abundance
Subsamples were fixed with formaldehyde (2% final concentration), stained with DAPI, (Sigma), filtered onto black 0.2 μm-pore-size Nuclepore filters (Osmonic Inc., Livermore, USA) and enumerated using an epifluorescence microscope under UV excitation at a magnification of 1250×. At least 500 bacterial cells were enumerated.
Absolute and relative abundance of selected bacterial taxa
The abundances of Betaproteobacteria and of major Betaproteobacteria subgroups – the genus Limnohabitans, the PnecC subcluster (P. necessarius) and the PnecB subcluster (P acidiphobus and P. difficilis) and the genus Methylophilus – were determined by Catalyzed Reporter Deposition Fluorescence in situ Hybridization (CARD-FISH) following the protocols of Pernthaler et al. (2002) and Sekar et al. (2003). The CARD-FISH probes deployed and their specificity are listed in Tab. 1.
Table 1.
Oligonucleotide CARD-FISH probes used in this study, their specificity and reference papers.
| Probe | Specificity | Reference |
|---|---|---|
| BET42a | Betaproteobacteria | Manz et al. (1992) |
| R-BT065 | R-BT lineage within the Limnohabitans genus | Šimek et al. (2001) |
| PnecABCD-445 | Polynucleobacter genus (= PnecABCD) | Hahn et al. (2005) |
| PnecC-16S-445 | Polynucleobacter necessarius (= PnecC) | Hahn et al. (2005) |
| PnecB-23S-166 | P. acidiphobus & P. difficilis (= PnecB ) | Wu & Hahn (2006) |
| Met1217 | Methylophilus genus | Friedrich et al. (2003) |
Incorporation of radioactively labeled substrates assessed by MAR-FISH
To track bacterial cells with active biomass synthesis and the potential production of storage matter, duplicate samples and formaldehyde-fixed blanks of 5 ml were incubated with either L-[3H]-leucine (Leu, final concentration 20 nmol l−1, specific activity 6.4 TBq mmol−1, MP Biomedicals) or D-[3H]-glucose (Glc, final concentration 20 nmol l−1, specific activity 2.22 TBq mmol−1, MP Biomedicals). Samples were incubated for 2 h at in situ temperature in the dark, preserved in formaldehyde (final concentration 2%) and filtered onto 0.2 μm polycarbonate filters, as described previously (Horňák et al., 2006). Filters were rinsed with Milli-Q water, air-dried, and kept frozen (−20°C) until further processing. After the CARD-FISH procedure, the filters were transferred onto slides coated with autoradiography emulsion (NTB, Kodak). After 24 to 48 h of exposure in the dark, the cells were stained with DAPI (final concentration 1 μg ml−1). The relative abundances of hybridized cells were enumerated by epifluorescence microscopy. At least 500 DAPI-stained cells were counted per sample. MAR-FISH experiments were performed with water samples from Loipersbacher Pond 1 and 2 (acidic ponds, Hahn et al., 2005), Lake Mondsee (alkaline, deep lake, Wu and Hahn, 2006a), and the Římov Reservoir (circum-neutral canyon-shaped reservoir, this study).
Substrate assimilation analyses
Previously published as well as unpublished data on substrate utilization by Limnohabitans and Polynucleobacter strains (Hahn et al., 2009, Kasalický et al., 2010, Hahn et al.,2010a+b+c;Hahn et al.,2011a+b; Hahn et al., in press; Kasalický unpubl. data; Hahn unpubl. data) were compiled and analyzed. All the established data were obtained by following the same protocol. Briefly, growth of bacterial isolates based on utilizing specific substrates was determined by comparison of the optical density measured at 575 nm (OD575) in liquid one-tenth-strength NSY medium (0.3 g/l, Hahn, 2003) with and without 0.5 g of the test substrate. Differences in OD575 of 10, 10–50 and 50% and more compared with growth on the medium lacking the test substance after 10 days of growth were scored as no utilization (−), weak utilization (w) and good utilization (+), respectively.
Statistical analysis
The program CANOCO (TerBraak and Šmilauer, 1998) was used for the multivariable analysis. Redundancy analyses (RDA) were performed, and the results were visualized by CanoDraw for Windows (TerBraak and Šmilauer, 1998).
Results
Occurrence of Betaproteobacteria groups along the pH gradient of investigated habitats
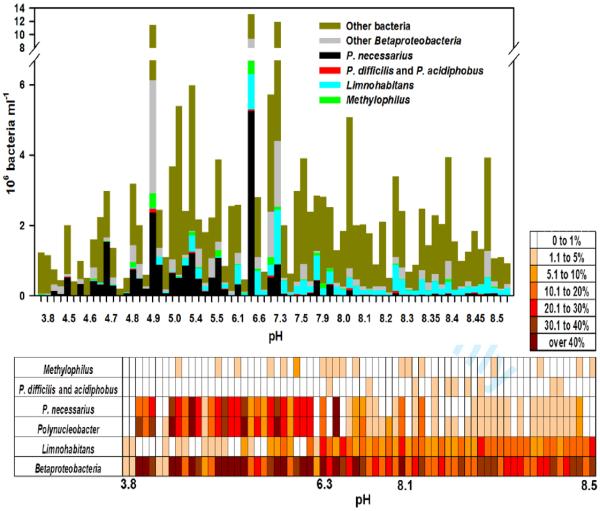
Total bacterial numbers differed strongly between the sampled habitats (Fig. 1, upper panel). Betaproteobacteria formed almost one third (29.1%) of all heterotrophic bacteria when averaged across all 72 habitats. Differences in Betaproteobacteria numbers were more pronounced in acidic habitats (pH 3.8-7.4), ranging there from 1.5 – 72% of all bacteria. In the alkaline pH range from 7.4 to 8.5, Betaproteobacteria numbers were more stable, ranging from 11.3 to 44.8% (Fig. 1). On average, Betaproteobacteria contributed less to total bacterial numbers in alkaline habitats than in acidic ones (Fig. 1, lower panel), which can be attributed to the dominant role of PnecC bacteria in the acidic habitats (Fig. 1). In acidic habitats, Betaproteobacteria contributed, on average, 34.3% to the total bacteria, in contrast to alkaline habitats where they constituted 23.9% of all bacteria, on average.
Fig. 1.
Upper panel, distribution of subgroups of Betaproteobacteria along the pH gradient (from 3.8 to 8.5) of 72 different habitats in absolute cell numbers. Lower panel, heat map of the relative (%) proportions of distinct Betaproteobacteria groups along the gradient of increasing pH (from 3.8 to 8.5) of the investigated 72 habitats. Relative proportion classes used are 0-1%, 1.1-5%, 5.1-10%, 10.1-20%, 20.1-30%, 30.1-40%, and over 40%.
Limnohabitans bacteria contributed on average to about 8.9% of bacterioplankton cells and showed a clear trend of increasing abundance with increasing pH. However, they were also present, though in smaller quantities, in habitats of lower or very low pH (close to 3.8). In the alkaline range of the pH gradient, at approximately pH 7.5 and higher, Limnohabitans bacteria were clearly the dominant group among Betaproteobacteria (Fig. 1).
The entire genus Polynucleobacter contributed on average to 11.6% of bacteria in the investigated samples. The vast majority of the detected Polynucleobacter cells were affiliated with the species P. necessarius (Fig. 1). This species alone accounted for approximately 10% of all bacteria. The B-lineage of Polynucleobacter (i.e. PnecB consisting of P. acidiphobus and P. difficilis) comprised about 0.5% of bacteria detected across all studied habitats. A clear trend in the distribution of these two taxa was observed. The B-lineage was more abundant exclusively in the alkaline part of the sampled habitat range, being most abundant in the large prealpine lakes of the Salzkammergut area (Fig. 1 and 2). P. necessarius showed a completely opposite trend, displaying a clear preference for low-pH habitats, where its abundance ranged between 5 to 60% of all detected bacteria. This species was also detectable, however, in alkaline habitats, though in much lower percentages. The three species P. necessarius, P. difficilis and P. acidiphobus constituted together almost 90% of all Polynucleobacter bacteria.
Fig. 2.
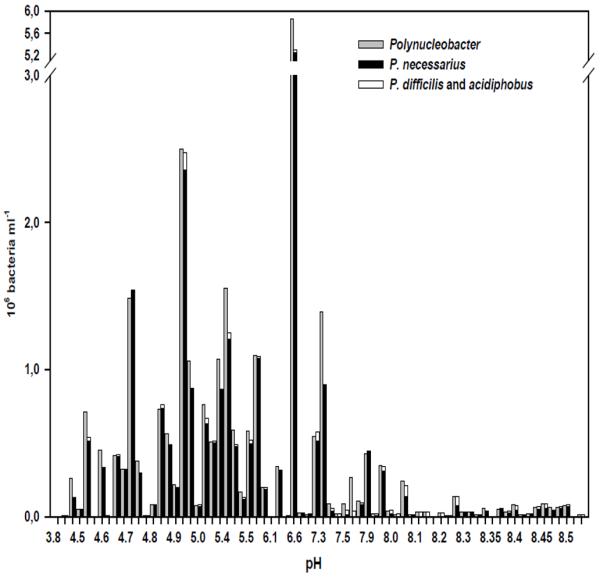
Abundance of the B-lineage of the genus Polynucleobacter (P. difficilis and P. acidiphobus), P. necessarius and the entire Polynucleobacter genus as detected by respective FISH probes along the pH gradient of the 72 investigated habitats.
In contrast to Limnohabitans and Polynucleobacter, bacteria belonging to the genus Methylophilus were never highly abundant, reaching on average only 0.9% of all bacteria and displaying no clear trend across the investigated habitats (Fig. 1).
Importantly, by using the CARD-FISH probes for Polynucleobacter, Limnohabitans and Methylophilus, we were able to cover on average almost three quarters (72.3%) of all Betaproteobacteria across the wide range of sampled habitats.
Comparison of substrate assimilation patterns
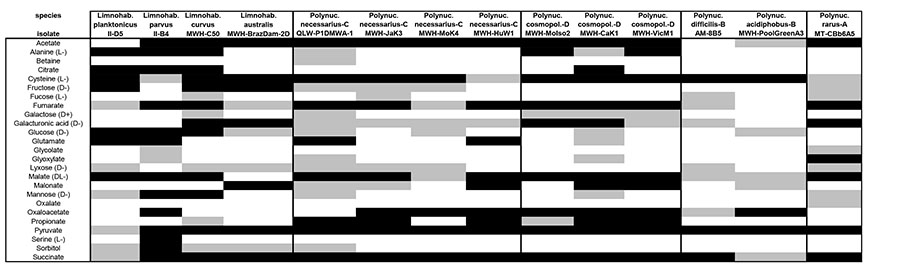
No clear pattern in substrate utilization that would clearly separate Limnohabitans from Polynucleobacter bacteria was observed (Tab. 2). Moreover, large differences in patterns even among strains of the same genus were observed for both genera. Pronounced differences in utilization patterns for strains of the same species (e.g. P. necessarius) were also obvious.
Table 2.
Substrate utilization by Polynucleobacter and Limnohabitans isolates. Adopted from Kasalický et al., 2010, Hahn et al., 2010a,bc, Hahn et al., 2011a,b, Hahn et al., in press, Kasalický unpublished data , Hahn unpublished data. White, no assimilation; grey, weak assimilation; black, 3 good assimilation (for details see Methods section). Note that L. curvus and L. australis are not targeted by the R-BT065 FISH probe.
|
Even when using a large array of substances, we were unable to find a sole substrate utilized by all members of one genus and not utilized at all by all members of the other genus (Tab. 2). The only distinct traceable trends were higher preferences of Limnohabitans for monosaccharides (namely fructose, glucose, mannose etc.) and certain amino acids (L-alanine) contrasting with no or very weak utilization of these substances by P. acidiphobus, P. difficilis, and P. necessarius. Another important result was a positive and strong preference of Limnohabitans and P. necessarius for acetate as opposed to no utilization of acetate by P. acidiphobus and P. difficilis (PnecB lineage).
Activity measurements by MAR-FISH
Uptake of two radioactively labeled substances (leucine and glucose) by Polynucleobacter and Limnohabitans bacteria (targeted by the R-BT065 probe) were investigated in four habitats representing three limnologically contrasting habitat types. Uptake of both substrates by the two bacterial groups was observed in all four habitats (Supplementary Table S2). In acidic Loiperbacher ponds 1 and 2 (habitats #11 and 12 in Supplementary Table S2), as well as in large, alkaline Lake Mondsee (habitat #51 in Supplementary Table S2), Polynucleobacter displayed a markedly lower affinity for leucine than did Limnohabitans bacteria. On the other hand, glucose was similarly assimilated by both groups. In the meso-eutrophic, circum-neutral Římov reservoir (Supplementary Table S2), similar trends were observed. On average, 92% of Limnohabitans bacteria incorporated leucine as opposed to approximately 56% of Polynucleobacter bacteria actively incorporating leucine. A similar pattern was observed for the utilization of glucose, where approximately 81% of Limnohabitans and only 45% of Polynucleobacter bacteria incorporated this substrate.
Factors influencing the distribution of the major Betaproteobacteria groups across 72 habitats and during one season in the Římov reservoir
In 2005, temporal and spatial development of the B-lineage of Polynucleobacter (P. difficilis and P. acidiphobus), P. necessarius, and Limnohabitans bacteria were followed at the DAM, MIDDLE and RIVER stations of the Římov reservoir (Fig. 3). The B-lineage and P. necessarius showed clearly contrasting trends. In the DAM area, which is primarily supplied by autochthonous primary production, the B-lineage markedly dominated over P. necessarius during most of the season, as opposed to the allochthonously loaded RIVER station, where P. necessarius formed the major part of the whole Polynucleobacter assemblage. In the MIDDLE station both groups alternated. Note that on the 23rd of August, when sampling was not feasible due to a flood event, the high P. necessarius numbers, normally typical of the RIVER station, were projected as far as to the MIDDLE station. Redundancy analyses (RDA) were performed for the set of 72 distinct habitats (Fig. 4A) as well as for the seasonal data from the Římov reservoir (Fig. 4B). Both RDA analyses confirmed contrasting roles of the B-lineage and P. necessarius. The B-lineage (P. difficilis and P. acidiphobus) was positively related to changing ChlA concentrations, primary production, extracellular primary production (EPP) and temperature, and was more abundant in the “lake” part of the Římov reservoir (Fig. 4B). In contrast, P. necessarius bacteria showed a positive correlation with dissolved reactive phosphorus and humic substances, being more abundant in the inflow (“river” part) of the reservoir. Limnohabitans bacteria did not show any trend within the Římov reservoir, while their distribution patterns were clearly indicated in the highly heterogeneous set of 72 habitats (Fig. 4A). In this analysis, the B-lineage of Polynucleobacter (PnecB) and Limnohabitans preferred habitats with higher pH, higher conductivity and lower amounts of humic substances, whereas P. necessarius was more abundant in habitats at higher altitude. Interestingly, P. necessarius, compared to PnecB bacteria and Limnohabitans, displayed exactly opposite relationships to the investigated environmental parameters. Methylophilus bacteria showed no clear distribution trend across the 72 investigated habitats (data not shown).
Fig. 3.
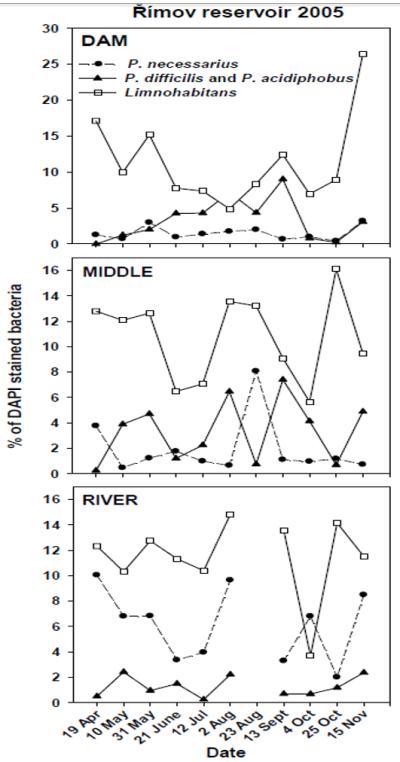
Distribution of relative proportions of the major Betaproteobacteria subgroups as detected by FISH at the DAM, MIDDLE and RIVER stations located along a longitudinal transect of the canyon-shaped Římov reservoir in the period from mid of March until mid of November 2005.
Fig. 4.
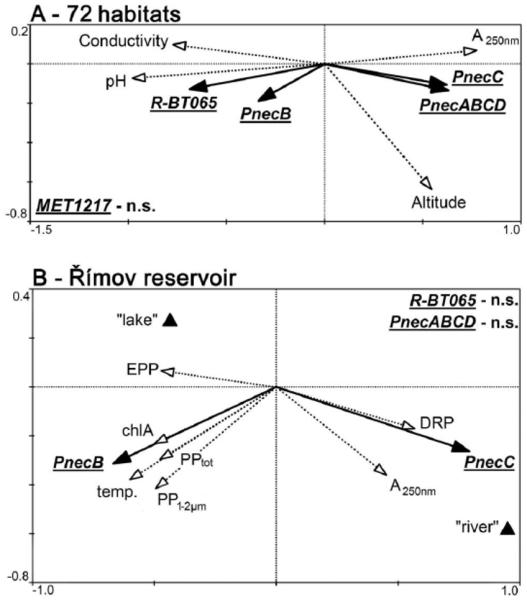
Panel A – 72 different habitats, Panel B – the Římov reservoir, a seasonal study. Redundancy analysis (RDA) showing only the significant parameters responsible for the distribution of the entire Polynucleobacter genus (labeled as PnecABCD); P. necessarius, (PnecC); the B-lineage of Polynucleobacter, (PnecB, representing P. difficilis and P. acidiphobus); Limnohabitans, (R-BT065) bacteria; and Methylophilus genus (MET1217). PPtot, total primary production; A250nm, absorption at 250 nm (DOC proxy); DRP, dissolved reactive phosphorus; PP1-2um, primary production in the 1-2 μm size fraction; chlA, chlorophyll a; temp., temperature; EPP, extracellular primary production; “lake” triangle represents pooled data for the station DAM and MIDDLE; “river” triangle represents data for the RIVER stations (see Methods section); n.s., not significant.
Discussion
Polynucleobacter and Limnohabitans bacteria as omnipresent components of freshwater systems
In the 72 freshwater habitats investigated, Polynucleobacter and Limnohabitans bacteria together formed the vast majority of Betaproteobacteria (on average more than 70%). However, it is important to note that the FISH probe used for the detection of Limnohabitans bacteria does not target the entire genus but only an assumed core lineage within this diverse taxon (Table 1; Kasalický et al., 2010). Other lineages within the genus Limnohabitans, not detected by the probe, also represent bacteria frequently inhabiting the pelagic zones of freshwater systems (Zwart et al., 2002; Lindström et al., 2005; Hahn et al., 2010a,b). For instance, the type species of L. curvus and some closely related strains have been isolated from Lake Mondsee, which was included in the set of habitats investigated here. Due to the lack of complete coverage of the genus by the probe, underestimation of the contributions of the entire genus Limnohabitans to total bacterioplankton is quite likely. However, due to the lack of suitable FISH probes, it is currently impossible to estimate how significant this underestimation is.
In this study we used three independent FISH probes to cover the B and C lineages of Polynucleobacter as well as the entire genus Polynucleobacter. Based on previous studies, where the numerical abundance of A and D lineages was found to be negligible (Wu and Hahn, 2006a), we decided not to deploy probes targeting these two lineages. This decision is well supported by the fact that probes specific for the B and C lineages detected on average 91.3% of all Polynucleobacter bacteria enumerated by using the genus-specific probe.
In contrast to the other two genera studied, the genus Methylophilus constituted only a small part of the bacterial communities, as well as of the whole Betaproteobacteria assemblages in the investigated habitats. However, it is important to note that we have mainly investigated epilimnetic samples that were more or less oxygen-saturated. We did not sample deeper, oxygen more depleted water layers that were reported to be richer in Methylophilus bacteria (Salcher et al., 2008). Potentially, this sampling strategy could have resulted in an underestimation of the importance of this bacterial taxon. Just recently (Hutalle et al., 2010), it has been demonstrated that bacteria such as Methylophilus can be enriched on phenol or humic matter additions that might to a certain extent link these microbes to direct degradation of humic substances and probably not so much to the utilization of methane produced in anaerobic zones. The study of Hutalle et al., can not however be generalized, since only few isolates and clones were analyzed. If this assumption would be true one would expect a tight relation of Methylophilus spp. numbers to concentration of humic matter which was not so far confirmed.
We have omitted a detailed analysis of the environmental drivers controlling distribution and abundance of Methylophilus bacteria because the cell numbers determined by FISH were frequently close to the detection limit, resulting in low accuracy of the determined data. Aside from this, these data indicate also a rather negligible role of these bacteria in the overall carbon flow of the systems studied.
We believe that the observed distribution pattern of major groups of Betaproteobacteria can be generalized for the majority of freshwater habitats worldwide.
There are inevitably other factors that may influence the distribution of a certain bacterial taxon, to name just a few: water retention time, dispersal and biotic interactions. Considering the amount of habitats sampled, none of these was unfortunately in the scope of our study. Nonetheless, especially the biotic factors came just recently into attention (Eiler et al., 2011) showing that certain network modeling can predict that biotic factors (including the presence of specific organisms, be it bacteria or higher organisms) can shape network structures. Subsequently it can influence the presence or absence of key species including microorganisms.
(ii) Contrasting distribution patterns of bacteria along the pH gradient
We observed the coexistence of Polynucleobacter and Limnohabitans bacteria in the majority of investigated lakes, but the two groups showed also rather contrasting abundance patterns regarding pH of the habitats (Fig. 1). Limnohabitans occurred, on average, with higher relative and absolute abundances in circum-neutral and alkaline habitats, while the opposite trend was observed for the genus Polynucleobacter. However, the two Polynucleobacter subgroups considered in our study display both differences in total abundance as well as opposite abundance patterns along the pH gradient (Fig. 1, lower panel). Newton and coworkers (2011) also demonstrated a negative correlation of P. necessarius (PnecC subcluster) occurrence and a positive correlation of the Polynucleobacter B-lineage (PnecB subcluster) with lake pH. Interestingly, in this meta-analysis, the R-BT065 lineage of Limnohabitans was split in two subclusters (Lhab-A1 and Lhab-A2) for which opposite - i.e. positive and negative – pH correlations were revealed (Newton et al., 2011).
Several previous investigations revealed pH as one of the key drivers, or even as the strongest driver influencing bacterioplankton composition (Lindström et al., 2005; Yanarell and Triplett, 2005), or unveiled significant distribution differences of related taxa based on lake pH (Schauer et al., 2005; Newton et al., 2007). Despite the well-documented role of pH as the factor strongly shaping bacterioplankton composition (Lindström et al., 2005), it is not known if pH acts as a direct factor linked to pH adaptation of the respective bacteria or as an indirect factor influencing other growth conditions. In the case of Polynucleobacter and Limnohabitans bacteria, the distribution trends observed cannot simply be explained by differences in pH adaptation. The MAR-FISH investigations indicated that populations of both taxa, actively incorporating selected substrates, were present in all four investigated habitats, which represented a pH range of more than three units (pH 4.7 to 8.3, Supplementary Table S2). Other investigations performing similar MAR-FISH experiments on other habitats previously indicated that metabolically active bacteria affiliated with the genus Polynucleobacter, and especially with the species P. necessarius, are present in habitats strongly differing in pH (Buck et al., 2009; Alonso et al., 2009; Salcher et al., 2010). Additionally, recent findings suggested differences in pH preferences across subgroups of P. necessarius (Jezbera et al., 2011).
An alternative explanation for the opposite distribution trends observed could be utilization of different major substrate sources. Previous analyses indicated utilization of different substrate pools for Limnohabitans (R-BT065 lineage) and P. necessarius. Šimek et al. (2008) proposed that Limnohabitans bacteria mainly rely on algal-derived substrates, i.e. utilize direct or indirect products of autochthonous primary production. Moreover, it has been documented that L. planktonicus and L. parvus grow well even in diluted exudates produced by axenic cultures of typical planktonic algae (Šimek et al., 2011). In contrast, bacteria affiliated with the species P. necessarius are believed to utilize mainly photooxidation products of humic substances (Watanabe et al., 2009; Jezberová et al., 2010, Hahn et al., submitted) – i.e., they utilize allochthonous organic matter that is, at least partially, of terrestrial origin. In a previous analysis by Jezberová et al., (2010), which investigated a similar set of habitats, it was revealed that pH of habitats and concentrations of humic substances were negatively correlated. On the other hand, the majority of circum-neutral and alkaline habitats studied in the current study represent non-humic lakes with low allochthonous DOC input, which should favor bacteria utilizing autochthonous production.
A previous investigation indicated that not all free-living Polynucleobacter rely on humic substances. Wu and Hahn (2006b) proposed that Polynucleobacter bacteria of the B-lineage mainly utilize substrates derived from algal primary production and are frequently found in stock cultures of various algae as accompanying bacteria (Šimek et al., 2011). Thus, utilization of similar substrate pools was suggested for Limnohabitans (R-BT065 lineage) and P. acidiphobus/P. difficilis. Interestingly, both PnecB and Limnohabitans groups share similar distribution patterns along the investigated pH gradient.
Both PnecB and Limnohabitans appear in alkaline and circum-neutral habitats in higher relative abundances and in lower proportions in some of the acidic habitats (Fig. 1). Detection of the Polynucleobacter B-lineage in acidic habitats is in disagreement with the previously reported lack of detection in acidic waters (Wu and Hahn, 2006b). These contradicting observations may have resulted from differences in the sensitivity of the FISH methods deployed (CARD-FISH versus FISH).
Previously, there have been numerous studies documenting the effect of protozoan grazing on bacterial community composition. The effect of grazing on Limnohabitans and Polynucleobacter bacteria specifically can be found for instance in Boenigk et al., 2004, Jezbera et al., 2005 and Šimek et al., 2007. It was documented that bacteria belonging to the Limnohabitans genus (partially represented by the R-BT065 cluster) are highly susceptible to grazing and are selectively grazed upon by protists. The manuscript by Hahn et al. (submitted) provides a thorough discussion on the effect of grazing on a specific lineage within the Polynucleobacter cluster.
Generally, the above mentioned studies on grazing on Limnohabitans and Polynucleobacter bacteria seem to suggest that the natural predation mortality of Limnohabitans bacteria is higher than that of Polynucleobacter bacteria; however, large intra-genus differences in predation vulnerability can be expected for both taxa.
(iii) Environmental gradient analysis versus single habitat analysis
Opposite distribution patterns of P. necessarius and Limnohabitans (R-BT065 lineage) along broad pH gradients (Fig. 1) were also observed in separate previous investigations (Jezberová et al., 2010; Šimek et al., 2010a). By contrast, our presented seasonal study of the Římov reservoir revealed no significant differences in the relation of the two taxa to parameters related to primary production or phytoplankton biomass. Since Limnohabitans growth is expected to depend on a substrate pool derived from algal production (Šimek et al., 2011), the two taxa could be expected to reveal different relationships to these variables. However, the pH and conductivity values in the Římov reservoir remained virtually unchanged during the season, showing only small diurnal fluctuations (data not shown). This hints that other variables may be responsible for the segregation of these two groups, apart from self-correlating ones such as pH and conductivity. Interestingly, no direct significant relationship of Limnohabitans bacteria to ChlA was found, strongly contrasting with its positive relationship to certain algal groups, e.g. cryptophytes (Šimek et al., 2008). Jones and co-workers (2009), however, recently suggested a proxy, i.e. the ratio of water color to chlorophyll A (the CtCH ratio), which indicates the dominance of allochthonous versus autochthonous organic carbon sources available for bacteria. This proxy showed significant correlation with the relative proportions of R-BT065 bacteria in total Betaproteobacteria (Šimek et al., 2010a), which further corroborates the finding on enhanced proportions of the Limnohabitans bacteria in non-humic habitats where autochthonous organic carbon sources dominate.
On the other hand, the B-lineage of Polynucleobacter shows a positive correlation with the above-mentioned variables related to primary production and phytoplankton biomass (Fig. 4), which agrees with previous findings (Wu and Hahn, 2006b).
(iv) Lack of taxon-specific trends in substrate utilization patterns
The spectrum of substrates used in substrate utilization tests included several types of substances (e.g. acetate, pyruvate and other short-chain fatty acids) reported as typical products of photooxidation of humic substances (Moran and Zepp, 1997), as well as substances reported as algal exudates (e.g. carbohydrates, Giroldo et al., 2007). If P. necessarius and Limnohabitans tend to utilize different substrate pools, one could expect different preferences in the assimilation tests. Obviously, the performed tests indicated no taxon-specific trends in substrate preferences, although one has to take into account that these tests were performed at significantly higher substrate concentrations than usually inherent in the water environment. The predictive power of the utilization tests performed should thus not be overemphasized. It may be that the investigated taxa differ in substrate affinity, but not in substrate uptake potential, i.e., the ability to absorb available substrate. Only the latter was tested by the substrate utilization tests.
We are aware of the fact that by using relatively high substrate concentrations for testing of the utilization in the lab, we might have overlooked differences between strains in substrate affinity, which could play an important role in niche partitioning. On the other hand, the revealed small genome sizes of Polynucleobacter bacteria (Hahn et al., submitted; Vannini et al., 2007) suggest that these bacteria can only encode a rather small number of substrate utilization pathways.
(v) Potential limitations of ecological analyses caused by ecological diversification of taxa
Several contradictions revealed above indicate that the current pictures of the ecology of P. necessarius and Limnohabitans (R-BT065) are too simple. Several recent findings suggest intra-taxon ecological diversification resulting in differently adapted ecotypes for both groups (Šimek et al., 2010b; Jezbera et al., 2011). It seems that – at least for future ecological investigations – a taxonomy with a higher phylogenetic resolution as well as molecular tools enabling detection and quantification of these taxa will be required. We demonstrated that the two taxa P. necessarius and Limnohabitans represent together the majority of Betaproteobacteria in a broad range of habitat types. However, it seems that the postulated within-taxon diversity currently limits further insights into the ecological function of these bacterial groups.
Supplementary Material
Acknowledgements
This study was mainly supported by GAČR projects P504/10/0566 awarded to JJ and the Austrian Science Fund Project P19853 granted to MWH, and partially also by GAČR 206/08/0015 project awarded to KŠ. Anton Baer, a native speaker, is greatly acknowledged for English proofreading.
References
- Alonso C, Zeder M, Piccini C, Conde D, Pernthaler J. Ecophysiological differences of betaproteobacterial populations in two hydrochemically distinct compartments of a subtropical lagoon. Environ Microbiol. 2009;11:867–876. doi: 10.1111/j.1462-2920.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Barberan A, Casamayor EO. Global phylogenetic community structure and beta-diversity patterns in surface bacterioplankton metacommunities. Aquat Microb Ecol. 2010;1:1–10. [Google Scholar]
- Boenigk J, Stadler P, Wiedlroither A, Hahn MW. Strain-specific differences in the grazing sensitivities of closely related ultramicrobacteria affiliated with the Polynucleobacter cluster. Appl Environ Microbiol. 2004;70:5787–5793. doi: 10.1128/AEM.70.10.5787-5793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck U, Grossart HP, Amann R, Pernthaler J. Substrate incorporation patterns of bacterioplankton populations in stratified and mixed waters of a humic lake. Environ Microbiol. 2009;11:1854–1865. doi: 10.1111/j.1462-2920.2009.01910.x. [DOI] [PubMed] [Google Scholar]
- Burkert U, Warnecke F, Babenzien D, Zwirnmann E, Pernthaler J. Members of a readily enriched b-proteobacterial clade are common in surface waters of a humic lake. Appl Environ Microbiol. 2003;69:6550–6559. doi: 10.1128/AEM.69.11.6550-6559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump BC, Kling GW, Bahr M, Hobbie JE. Bacterioplankton community shifts in an Arctic lake correlate with seasonal changes in organic matter source. Appl Environ Microbiol. 2003;69:2253–2268. doi: 10.1128/AEM.69.4.2253-2268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler A, Heinrich F, Bertilsson S. Coherent dynamics and association networks among lake bacterioplankton taxa. ISME J. 2011;1:1–13. doi: 10.1038/ismej.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U, Van Langenhove H, Altendorf K, Lipski A. Microbial community and physicochemical analysis of an industrial waste gas biofilter and design of 16S rRNA-targeting oligonucleotide probes. Environ Microbiol. 2003;3:183–201. doi: 10.1046/j.1462-2920.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- Giroldo D, Ortolano PIC, Vieira AAH. Bacteria-algae association in batch cultures of phytoplankton from a tropical reservoir: the significance of algal carbohydrates. Freshwater Biol. 2007;7:1281–1289. [Google Scholar]
- Glöckner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J, Pernthaler A, Amann R. Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of Actinobacteria. Appl Environ Microbiol. 2000;66:5053–5065. doi: 10.1128/aem.66.11.5053-5065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW. Isolation of strains belonging to the cosmopolitan Polynucleobacter necessarius cluster from freshwater habitats located in three climatic zones. Appl Environ Microbiol. 2003;69:5248–5254. doi: 10.1128/AEM.69.9.5248-5254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Lang E, Brandt U, Wu QL, Scheuerl T. Emended description of the genus Polynucleobacter and the species P. necessarius and proposal of two subspecies, P. necessarius subspecies necessarius subsp. nov. and P. necessarius subsp. asymbioticus subsp. nov. Int J Syst Evol Microbiol. 2009;59:2002–2009. doi: 10.1099/ijs.0.005801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Pöckl M. Ecotypes of planktonic Actinobacteria with identical 16S rRNA genes adapted to thermal niches in temperate, subtropical, and tropical freshwater habitats. Appl Environ Microbiol. 2005;71:766–773. doi: 10.1128/AEM.71.2.766-773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Pöckl M, Wu QL. Low intraspecific diversity in a Polynucleobacter subcluster population numerically dominating bacterioplankton of a freshwater pond. Appl Environ Microbiol. 2005;71:4539–4547. doi: 10.1128/AEM.71.8.4539-4547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Kasalický V, Jezbera J, Brandt U, Jezberová J, Šimek K. Limnohabitans curvus gen. nov., sp. nov., a planktonic bacterium isolated from a freshwater lake. Int J Syst Evol Microbiol. 2010a;6:1358–1365. doi: 10.1099/ijs.0.013292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Kasalický V, Jezbera J, Brandt U, Šimek K. Limnohabitans australis sp. nov., isolated from a freshwater pond, and emended description of the genus Limnohabitans. Int J Syst Evol Microbiol. 2010b;60:2946–2950. doi: 10.1099/ijs.0.022384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Lang E, Brandt U, Sprőer C. Polynucleobacter acidiphobus sp. nov., a representative of an abundant group of planktonic freshwater bacteria. Int J Syst Evol Microbiol. 2011a;61:788–794. doi: 10.1099/ijs.0.023929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Lang E, Tarao M, Brandt U. Polynucleobacter rarus sp. nov., a free-living planktonic bacterium isolated from an acidic lake. Int J Syst Evol Microbiol. 2011b;61:781–787. doi: 10.1099/ijs.0.017350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Minasyan A, Lang E, Koll U, Sprőer C. Polynucleobacter difficilis sp. nov., a planktonic freshwater bacterium affiliated with subcluster B1 of the genus Polynucleobacter. Int J Syst Evol Microbiol. doi: 10.1099/ijs.0.031393-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Scheuerl T, Jezberová J, Koll U, Jezbera J, Šimek K, Vannini C, Petroni G, Wu QL. The passive yet successful way of planktonic life: genomic and experimental analysis of the ecology of a planktonic Polynucleobacter population. Submitted to PLoS One. doi: 10.1371/journal.pone.0032772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann K, Schmidt HJ. Polynucleobacter necessarius gen. nov., sp. nov., an obligately endosymbiotic bacterium living in the cytoplasm of Euplotes. Int J Syst Bacteriol. 1987;37:456–457. [Google Scholar]
- Hiorns WD, Methé EA, Nierzwickibauer SA, Zehr JP. Bacterial diversity in Adirondack mountain lakes as revealed by 16S rRNA gene sequences. Appl Environ Microbiol. 1997;63:2957–2960. doi: 10.1128/aem.63.7.2957-2960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horňák K, Jezbera J, Nedoma J, Gasol JM, Šimek K. Bacterial leucine incorporation under different levels of resource availability and bacterivory in a freshwater reservoir. Aquat Microb Ecol. 2006;45:277–289. [Google Scholar]
- Hutalle-Schmelzer KML, Zwirnmann E, Krueger A, Grossart HP. Enrichment and cultivation of pelagic bacteria from a humic lake using phenol and humic matter additions. FEMS Microb Ecol. 2010;1:58–73. doi: 10.1111/j.1574-6941.2009.00831.x. [DOI] [PubMed] [Google Scholar]
- Jezbera J, Horňák K, Šimek K. Food selection by bacterivorous protists: insight from the analysis of the food vacuole content by means of fluorescence in situ hybridization. FEMS Microb Ecol. 2005;52:351–363. doi: 10.1016/j.femsec.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Jezbera J, Jezberová J, Brandt U, Hahn MW. Ubiquity of Polynucleobacter necessarius subspecies asymbioticus results from ecological diversification. Environ Microbiol. 2011;13:922–931. doi: 10.1111/j.1462-2920.2010.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezberová J, Jezbera J, Brandt U, Lindström ES, Langenheder S, Hahn MW. Ubiquity of Polynucleobacter necessarius subsp asymbioticus in lentic freshwater habitats of a heterogenous 2000 km2 area. Environ Microbiol. 2010;12:658–669. doi: 10.1111/j.1462-2920.2009.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Newton RJ, McMahon KD. Evidence for structuring of bacterial community composition by organic carbon source in temperate lakes. Environ Microbiol. 2009;11:2463–2472. doi: 10.1111/j.1462-2920.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- Kasalický V, Jezbera J, Šimek K, Hahn MW. Limnohabitans planktonicus sp. nov. and Limnohabitans parvus sp. nov., planktonic Betaproteobacteria isolated from a freshwater reservoir, and emended description of the genus Limnohabitans. Int J Syst Evol Microbiol. 2010;60:2710–2714. doi: 10.1099/ijs.0.018952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström ES, Kamst-Van Agterveld MP, Zwart G. Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Appl Environ Microbiol. 2005;71:8201–8206. doi: 10.1128/AEM.71.12.8201-8206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- Newton RJ, Jones SE, Helmus MR, McMahon KD. Phylogenetic ecology of the freshwater Actinobacteria acI lineage. Appl Environ Microbiol. 2007;73:7169–7176. doi: 10.1128/AEM.00794-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S. A guide to the natural history of freshwater lake bacteria. Microb Mol Biol Rev. 2011;75:14–49. doi: 10.1128/MMBR.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MA, Zepp RG. Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol Oceanogr. 1997;42:1307–1316. [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol. 2002;6:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcher MM, Pernthaler J, Zeder M, Psenner R, Posch T. Spatio-temporal niche separation of planktonic Betaproteobacteria in an oligo-mesotrophic lake. Environ Microbiol. 2008;10:2074–2086. doi: 10.1111/j.1462-2920.2008.01628.x. [DOI] [PubMed] [Google Scholar]
- Salcher MM, Pernthaler J, Posch T. Spatiotemporal distribution and activity patterns of bacteria from three phylogenetic groups in an oligomesotrophic lake. Limnol Oceanogr. 2010;2:846–856. [Google Scholar]
- Schauer M, Kamenik C, Hahn MW. Ecological differentiation within a cosmopolitan group of planktonic freshwater bacteria (SOL cluster, Saprospiraceae, Bacteroidetes) Appl Environ Microbiol. 2005;71:5900–5907. doi: 10.1128/AEM.71.10.5900-5907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar R, Pernthaler A, Pernthaler J, Warnecke F, Posch T, Amann R. An improved protocol for quantification of freshwater Actinobacteria by fluorescence in situ hybridization. Appl Environ Microbiol. 2003;5:2928–2935. doi: 10.1128/AEM.69.5.2928-2935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimek K, Pernthaler J, Weinbauer MG, Horňák K, Dolan JR, Nedoma J, Mašín M, Amann R. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl Environ Microbiol. 2001;6:2723–2733. doi: 10.1128/AEM.67.6.2723-2733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimek K, Weinbauer MG, Horňák K, Jezbera J, Nedoma J, Dolan JR. Grazer and virus-induced mortality of bacterioplankton accelerates development of Flectobacillus populations in a freshwater community. Environ Microbiol. 2007;3:789–800. doi: 10.1111/j.1462-2920.2006.01201.x. [DOI] [PubMed] [Google Scholar]
- Šimek K, Horňák K, Jezbera J, Nedoma J, Znachor P, Hejzlar J, Sed’a J. Spatio-temporal patterns of bacterioplankton production and community composition related to phytoplankton composition and protistan bacterivory in a dam reservoir. Aquat Microb Ecol. 2008;3:249–262. [Google Scholar]
- Šimek K, Kasalický V, Jezbera J, Jezberová J, Hejzlar J, Hahn MW. Broad habitat range of the phylogenetically narrow R-BT065 cluster representing a core group of the betaproteobacterial genus Limnohabitans. Appl Environ Microbiol. 2010a;76:631–639. doi: 10.1128/AEM.02203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimek K, Kasalický V, Horňák K, Hahn MW, Weinbauer MG. Assessing niche separation among coexisting Limnohabitans strains through interactions with a competitor, viruses, and a bacterivore. Appl Environ Microbiol. 2010b;11:3762–3762. doi: 10.1128/AEM.02517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimek K, Kasalický V, Zapomělová E, Horňák K. Algal-derived substrates select for distinct betaproteobacterial lineages and contribute to niche separation in Limnohabitans strains. Appl Environ Microbiol. 2011;77:7307–7315. doi: 10.1128/AEM.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBraak CJF, Smilauer P. CANOCO for Windows Version 4.02.Wageningen. Centre for Biometry Wageningen, CPRO-DLO; the Netherlands: 1998. [Google Scholar]
- Vannini C, Pöckl M, Petroni G, Wu QL, Lang E, Stackebrandt E, Schrallhammer M, Richardson PM, Hahn MW. Endosymbiosis in statu nascendi: Close phylogenetic relationship between obligately endosymbiotic and obligately free-living Polynucleobacter strains (Betaproteobacteria) Environ Microbiol. 2007;9:347–359. doi: 10.1111/j.1462-2920.2006.01144.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Komatsu N, Ishii Y, Negishi M. Effective isolation of bacterioplankton genus Polynucleobacter from freshwater environments grown on photochemically degraded dissolved organic matter. FEMS Microb Ecol. 2009;67:57–68. doi: 10.1111/j.1574-6941.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- Wu QL, Hahn MW. Differences in structure and dynamics of Polynucleobacter communities in a temperate and a subtropical lake revealed at three phylogenetic levels. FEMS Microb Ecol. 2006a;57:67–79. doi: 10.1111/j.1574-6941.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- Wu QL, Hahn MW. High predictability of the seasonal dynamics of a species-like Polynucleobacter population in a freshwater lake. Environ Microbiol. 2006b;8:1660–1666. doi: 10.1111/j.1462-2920.2006.01049.x. [DOI] [PubMed] [Google Scholar]
- Wu QL, Schauer M, Kamst-van Agterveld MP, Zwart G, Hahn MW. Bacterioplankton community composition along a salinity gradient of sixteen high mountain lakes located on the Tibetan Plateau, China. Appl Environ Microbiol. 2006;72:5478–5485. doi: 10.1128/AEM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannarell AC, Triplett EW. Geographic and environmental sources of variation in lake bacterial community composition. Appl Environ Microbiol. 2005;1:227–239. doi: 10.1128/AEM.71.1.227-239.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart G, Crump BC, Kamst-van Agterveld MP, Hagen F, Han SK. Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat Microb Ecol. 2002;28:141–155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.