Abstract
Premise of the study
Polyploidy plays an important role in race differentiation and eventually speciation. Underlying mechanisms include chromosomal and genomic changes facilitating reproductive isolation and/or stabilization of hybrids. A prerequisite for studying these processes is a sound knowledge on the origin of polyploids. A well-suited group for studying polyploid evolution consists of the three species of Melampodium ser. Leucantha (Asteraceae): M. argophyllum, M. cinereum, and M. leucanthum.
Methods
The origin of polyploids was inferred using network and tree-based phylogenetic analyses of several plastid and nuclear DNA sequences and of fingerprint data (AFLP). Genome evolution was assessed via genome size measurements, karyotype analysis, and in situ hybridization of ribosomal DNA.
Key results
Tetraploid cytotypes of the phylogenetically distinct M. cinereum and M. leucanthum had, compared to the diploid cytotypes, doubled genome sizes and no evidence of gross chromosomal rearrangements. Hexaploid M. argophyllum constituted a separate lineage with limited intermixing with the other species, except in analyses from nuclear ITS. Its genome size was lower than expected if M. cinereum and/or M. leucanthum were involved in its origin, and no chromosomal rearrangements were evident.
Conclusions
Polyploids in M. cinereum and M. leucanthum are of recent autopolyploid origin in line with the lack of significant genomic changes. Hexaploid M. argophyllum also appears to be of autopolyploid origin against the previous hypothesis of an allopolyploid origin involving the other two species, but some gene flow with the other species in early phases of differentiation cannot be excluded.
Keywords: allopolyploidy, Asteraceae, autopolyploidy, genome size, hybridization, Melampodium, polyploid evolution, rDNA
Polyploidy is one of the major processes shaping angiosperm evolution (Wendel, 2000; Hegarty and Hiscock, 2007, 2008; Leitch and Leitch, 2008; Hufton and Panopoulou, 2009). This is evident from early whole-genome duplications in the ancestor of essentially all extant angiosperms (Wendel, 2000; Wolfe, 2001; Adams and Wendel, 2005a, b; De Bodt et al., 2005; Jiao et al., 2011) as well as from more recent poly ploidization events affecting most angiosperms (Masterson, 1994; Leitch and Bennett, 1997; Leitch and Leitch, 2008). Whereas autopolyploids originate from multiplication of the usually identical chromosome sets within a single (sub)species, allopolyploids derive from hybridization and multiplication of the usually differentiated chromosome sets coming from two or more progenitor (sub)species (Comai, 2005; Chen et al., 2007; Otto, 2007). Although the distinction between auto- and allopolyploids is difficult in polyploids exhibiting intermediate chromosome pairing behavior (expected polysomic inheritance in autopolyploids vs. disomic inheritance in allopolyploids; Ramsey and Schemske, 2002; Otto, 2007), both types are unambiguously known to occur frequently in nature (Tate et al., 2005; Soltis et al., 2007), where they often originate recurrently (Soltis et al., 2007; Dixon et al., 2009).
Polyploidy plays an important role in race differentiation and eventually speciation. Polyploidization leads to an instantaneous increase in the amount of genetic material upon which evolution can work, potentially leading to the evolution of new functions in duplicated genes (neofunctionalization; Ohno, 1970; Wolfe, 2001; Liu and Wendel, 2002). Furthermore, polyploids often show accelerated genomic evolution expressed as a higher rate of chromosome structural rearrangements and associated pairing behavior in meiosis (Weiss and Maluszyńska, 2000; Ramsey and Schemske, 2002; Otto, 2007; Schubert and Lysak, 2011), or genome size changes (Bennetzen and Kellogg, 1997; Bennetzen et al., 2005; Leitch and Leitch, 2008; Hawkins et al., 2008), contributing to the isolation of a polyploid from its lower-ploid ancestor(s). Finally, polyploidization contributes to stabilization of genomes after hybridization, a phenomenon common in plants (Anderson and Stebbins, 1954; Grant, 1981; Mallet, 2005, 2007; Rieseberg and Willis, 2007), by restoring regular meiotic pairing and fertility, thus creating crossing barriers with the parental taxa (allopolyploid speciation: Tate et al., 2005; Hegarty and Hiscock, 2007, 2008; Paun et al., 2009).
A prerequisite for studying processes associated with the evolution of polyploid genomes is a sound knowledge of their origin. Molecular sequence data have proven particularly useful for identifying the parental lineages of allopolyploids. Although incongruence between gene trees obtained from plastid DNA (cpDNA; inherited maternally in Asteraceae; Corriveau and Coleman, 1988; Harris and Ingram, 1991), and nuclear DNA (inherited from both parents; Sang et al., 1997; Hughes et al., 2002; Kim et al., 2008) provides evidence for allopolyploidy, this approach is hampered by the often low variation in cpDNA sequences and by concerted evolution of the commonly used nuclear ribosomal internal transcribed spacer (nrITS) region (álvarez and Wendel, 2003). In low-copy nuclear genes, analyses of individual gene copies stemming from the maternal and paternal lineages can be recovered, rendering these genes a powerful and successful tool for inferring the origin of allo polyploids (Ferguson and Sang, 2001; Sang, 2002; Small et al., 2004; Lihová et al., 2006; Fortune et al., 2007; Kim et al., 2008; Shimizu-Inatsugi et al., 2009; Weiss-Schneeweiss et al., 2012).
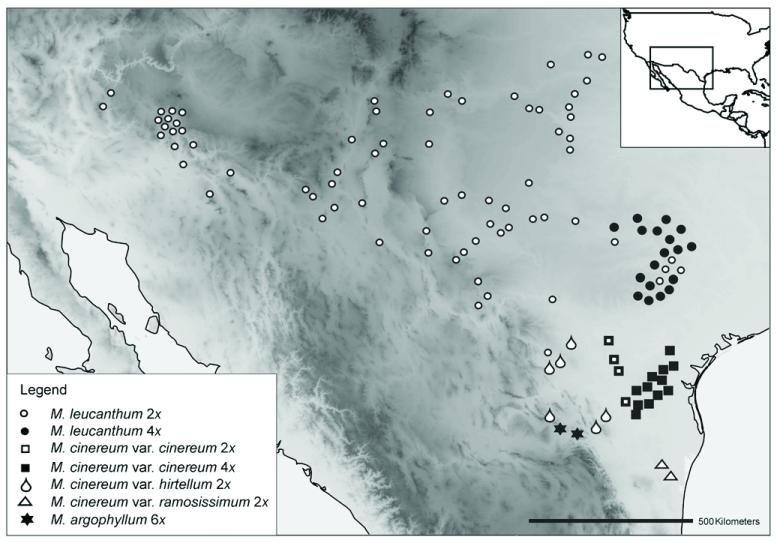
A well-suited group for studying polyploid evolution is the white-rayed complex of Melampodium sect Melampodium ser. Leucantha (Asteraceae; Stuessy, 1972). Characterized by white instead of yellow or orange ray florets, it is a phylogenetically distinct group (Blöch et al., 2009) comprising three xerophytic subshrub species (M. argophyllum, M. cinereum, M. leucanthum) distributed in the arid regions of northern Mexico and the southwestern United States (Fig. 1; Stuessy, 1972; Stuessy et al., 2004). Like the entire sect. Melampodium, species of ser. Leucantha have a basic chromosome number of x = 10 (Weiss-Schneeweiss et al., 2009). The widespread M. cinereum and M. leucanthum comprise both diploid and tetraploid cytotypes (Fig. 1). These originated recurrently via autopolyploidy, as suggested by chromosomal studies and genetic data (Turner and King, 1964; Stuessy et al., 2004; Rebernig et al., 2010a, b). The third species of the complex is the hexaploid M. argophyllum (Stuessy, 1972; Stuessy et al., 2004), restricted to northeastern Mexico (Fig. 1). It shares morphological features with the two other species and has thus been hypothesized to be of allopolyploid origin involving M. cinereum and M. leucanthum (Stuessy et al., 2004). Thus, ser. Leucantha provides an excellent system to compare the type and extent of genomic changes in closely related polyploids with contrasting evolutionary histories.
Fig. 1. Distribution map of the analyzed populations of the white-rayed complex of Melampodium.
The collection area represents the entire distribution of the complex.
Here, we used molecular sequence (plastid and nuclear DNA, including a low-copy gene), fingerprint (AFLPs), and restriction data in conjunction with karyological, and genome size data to investigate the origin and evolution of ser. Leucantha with special emphasis on M. argophyllum. Specifically, we wanted to (1) infer phylogenetic relationships among M. argophyllum, M. cinereum, and M. leucanthum, in particular to test the hypothesis of an allopolyploid origin for M. argophyllum; and (2) compare karyotype structure, genome size, and rDNA loci number and localization in polyploids of presumably different ages and modes of origin (recent auto- vs. ancient allopolyploidy).
Materials And Methods
Study species
Melampodium argophyllum occurs in western Nuevo León and adjacent Coahuila (Mexico) above 1830 m elevation (Stuessy, 1972; Fig. 1), where it is altitudinally separated from the otherwise sympatric M. cinereum var. hirtellum (Stuessy, 1971b). It flowers from February to October (Stuessy, 1972). Melampodium argophyllum shares morphological characters with both M. cinereum (similar leaf shape and head size) and M. leucanthum (similar outer phyllaries; Stuessy, 1971b) and has therefore been described as a variety of M. cinereum (Gray, 1884) as well as of M. leucanthum (Stuessy, 1971b), but is now again considered a distinct species (Stuessy, 1972, following Blake, 1924). The species is known only from the hexaploid level (2n = 6× = 60; Stuessy et al., 2004; Weiss-Schneeweiss et al., 2009).
Melampodium cinereum grows in mesquite shrublands in southern Texas and adjacent Mexico (Fig. 1). It differs from M. leucanthum and M. argophyllum by the outer phyllaries being connate only to one fourth their length (Stuessy, 1972). Based on morphological variation of the indumentum of leaves and stems, three varieties, cinereum, hirtellum, and ramosissimum, have been distinguished (Stuessy, 1971b, 1972), but genetic data suggest that var. hirtellum comprises two geographically separated entities (Rebernig et al., 2010b). Flowering time ranges from January to July, and despite some differences between varieties their flowering times overlap for at least 5 months. Melampodium cinereum comprises morphologically weakly and only quantitatively (Stuessy, 1972) distinguishable diploid and tetraploid cytotypes (with occasional tri-, penta-, or hexaploids; Stuessy et al., 2004; R. Obermayer et al., unpublished data), most prominently in var. cinereum (Stuessy et al., 2004; Rebernig et al., 2010b). The tetraploids occur exclusively in southeastern Texas largely to the exclusion of diploids, with no apparent ecological differentiation (Stuessy et al., 2004; Rebernig et al., 2010b).
Melampodium leucanthum grows on calcareous soils in arid habitats from Arizona and New Mexico to central Texas reaching the Edwards Plateau, extending northward to Colorado and Kansas and southward to northern Mexico (Stuessy, 1972; Fig. 1). It can be morphologically distinguished from the other two species by the outer phyllaries being united one half to three fourths their length (Stuessy, 1972). Despite evidence for three separate phylogeographic lineages (Rebernig et al., 2010a), no infraspecific morphological variants of taxonomic value are known. Melampodium leucanthum comprises morphologically indistinguishable diploid and tetraploid cytotypes (Turner and King, 1964; Stuessy, 1971a, b, 1972; Stuessy et al., 2004), with occasional tri, penta-, or hexaploids (Stuessy et al., 2004; Rebernig et al., 2010a). The tetraploids occupy a compact distribution area in eastern Texas around Austin to the (almost complete) exclusion of diploids (Stuessy et al., 2004).
Plant material
Plant material was collected from 22 populations of M. cinereum, 90 populations of M. leucanthum, and two populations of M. argophyllum covering the whole distribution area of the three species (Rebernig et al., 2010a, b; Table 1). AFLP data have been generated for a large data set including all accessions of the white-rayed complex. Plastid and nuclear sequence data comprise a second smaller data set including a selected number of accessions of the species of ser. Leucantha (Table 1).
Table 1. Melampodium species names, localities, voucher numbers, ploidal levels (taken from Weiss-Schneeweiss et al., 2009), and GenBank accession numbers of analyzed taxa.
All vouchers deposited in WU and MEXU; Countries: M, Mexico; USA, United States of America. Collectors: CB, C. Blöch; CR, C. A. Rebernig; EO, E. Ortiz; JV, J. L. Villaseñor; MB, M. Barfuss; TS, T. F. Stuessy.
| Taxon | Accession number | Locality | Ploidal level | ITS | psbA-trnH, ndhF-rpl32, rpl32-trnL | 5S rDNA spacer | PgiCI | PgiCII |
|---|---|---|---|---|---|---|---|---|
| M. argophyllum (A.Gray ex B.L.Rob.) S.F.Blake (6×) | 1 | M, Nuevo León, 2006; TS, JV, EO & CB, 19059. | 6× | FJ697009 |
FJ845873–FJ845875; FJ845867–FJ845869; FJ845879–FJ845881 |
JF277454–JF277466 | – | JF277549 |
| 2 | M, Nuevo León, 2006; TS, JV, EO & CB, 19060. | 6× | FJ697010–FJ697013 |
FJ845876–FJ845878; FJ845870–FJ845872; FJ845882–FJ845884 |
JF277419–JF277453 | JF277491–JF277502 | JF277544–JF277548 | |
| M. cinereum DC. var. cinereum (2×, 4×) | 1 | USA, Texas, Frio Co, 2005; TS & CR, 18688. | 2× | FJ697006 |
FJ456097–FJ456099; FJ456020–FJ456022; FJ456174–FJ456176 |
– | – | – |
| 2 | USA, Texas, Webb Co., 2005; TS & CR, 18690. | 2× | – |
FJ456103–FJ456104; FJ456026–FJ456027; FJ456180–FJ456181 |
JF277413–JF277416 | JF277503–JF277507 | JF277550–JF277556 | |
| 3 | USA, Texas, Zapata Co, 2005; TS & CR, 18694. | 4× | FJ697008 |
FJ456112–FJ456114; FJ456035–FJ456037; FJ456189–FJ456191 |
– | JF277508–JF277511 | JF277533–JF277543 | |
| 4 | USA, Texas, Jim Hogg Co, 2005; TS & CR, 18698. | 4× | FJ697007 |
FJ456118–FJ456120; FJ456041–FJ456043; FJ456195–FJ456197 |
– | – | – | |
| 5 | USA, Texas, Jim Hogg Co., 2005; TS & CR, 18701. | 4× | – |
FJ456127–FJ456129; FJ456050–FJ456052; FJ456204–FJ456206 |
JF277417–JF277418 | – | – | |
| M. cinereum DC. var. hirtellum Stuessy (2×) | 1 | Mexico, Coahuila, 2006; TS, JV, EO & CB, 19054. | 2× | – |
FJ456149–FJ456152; FJ456072–FJ456075; FJ456226–FJ456229 |
JF277404 | – | – |
| 2 | M, Coahuila, 2006; TS, JV, EO & CB, 19057. | 2× | FJ697015 |
FJ456156–FJ456158; FJ456079–FJ456081; FJ456233–FJ456235 |
– | – | – | |
| 3 | M, Nuevo León, 2006; TS, JV, EO & CB, 19061. | 2× | FJ697014 |
FJ456162–FJ456164; FJ456085–FJ456087; FJ456239–FJ456241 |
JF277397–JF277403 | JF277512 | F277557–JF277564 | |
| M. cinereum DC. var. ramosissimum DC. (A.Gray) (2×) | 1 | M, Tamaulipas, 2006; TS, JV & CB, 19063. | 2× | FJ697016 |
FJ456168–FJ456170; FJ456091–FJ456093; |
– | – | – |
| 2 | M, Tamaulipas, 2006; TS, JV & CB, 19064. FJ456245–FJ456247 |
2× | FJ697017 | – | – | – | – | |
| M. leucanthum Torr. & A.Gray (2×, 4×) | 1 | USA, Texas, Medina Co, 2005; TS & CR, 18687. | 4× | FJ697005 |
FJ846101, FJ846102; FJ845899, FJ845900; FJ846303, FJ846304 |
JF277405–JF277412 | JF277483–JF277487 | F277515–JF277519 |
| 2 | USA, Arizona, Graham Co, 2006; CR & MB, 18800. | 2× | FJ697004 |
FJ846189–FJ846191; FJ846987–FJ846989; FJ846391–FJ846393 |
– | – | – | |
| 3 | USA, Arizona, Yavapai Co, 2006; CR & MB, 18808. | 2× | FJ697003 |
FJ846200, FJ846201; FJ846998, FJ846999; FJ846402, FJ846403 |
– | JF277488–JF277490 | F277520–JF277532 |
Achenes were collected from individual plants, where available, and seeds were germinated on wet filter paper for chromosome and genome size analyses. Plant leaf material collected in the field was dried and stored in silica gel until DNA isolation. Herbarium vouchers are deposited in the herbarium of the University of Vienna (WU) with duplicates in MEXU (Tables 1, 2; Appendix 1).
Table 2. Genome size estimation (1C; pg) for selected populations of Melampodium cinereum and M. leucanthum (A) and genome size extrapolation for M. argophyllum (B).
| Taxon | Pop. no. | Ploidal level | Genome size (1C; pg) | Genome size (1C×; pg) |
|---|---|---|---|---|
| A) M. cinereum and M. leucanthum | ||||
| M. cinereum var. hirtellum | 19055 | 2× | 2.19 | 2.19 |
| M. cinereum var. hirtellum | 19058 | 2× | 2.26 | 2.26 |
| M. cinereum var. hirtellum | 19062 | 2× | 2.17 | 2.17 |
| Mean ± SD | 2.21 ± 0.05 | |||
| M. cinereum var. cinereum | 18689 | 2× | 2.23 | 2.23 |
| M. cinereum var. cinereum | 18690 | 2× | 2.24 | 2.24 |
| M. cinereum var. cinereum | 18692 | 2× | 2.20 | 2.20 |
| Mean ± SD | 2.22 ± 0.02 | |||
| M. cinereum var. cinereum | 18694 | 4× | 4.46 | 2.23 |
| M. cinereum var. cinereum | 18697 | 4× | 4.45 | 2.23 |
| M. cinereum var. cinereum | 18700 | 4× | 4.46 | 2.23 |
| M. cinereum var. cinereum | 18708 | 4× | 4.56 | 2.28 |
| Mean ± SD | 2.24 ± 0.03 | |||
| M. leucanthum | WGS83 MLK | 2× | 2.28 | 2.28 |
| M. leucanthum | 18722 | 2× | 2.31 | 2.31 |
| M. leucanthum | 18727 | 2× | 2.32 | 2.32 |
| M. leucanthum | 18774 | 2× | 2.34 | 2.34 |
| M. leucanthum | 18800 | 2× | 2.35 | 2.35 |
| M. leucanthum | 18808 | 2× | 2.35 | 2.35 |
| M. leucanthum | 20000 | 2× | 2.28 | 2.28 |
| M. leucanthum | 20030 | 2× | 2.31 | 2.31 |
| Mean ± SD | 2.32 ± 0.03 | |||
| M. leucanthum | 18683 | 4× | 4.53 | 2.27 |
| M. leucanthum | 18709 | 4× | 4.61 | 2.31 |
| M. leucanthum | 18712 | 4× | 4.57 | 2.28 |
| M. leucanthum | 18720 | 4× | 4.27 | 2.14 |
| M. leucanthum | 18779 | 4× | 4.66 | 2.33 |
| M. leucanthum | 18780 | 4× | 4.46 | 2.23 |
| Mean ± SD | 2.26 ± 0.07 | |||
| Taxon | Pop. no. | Ploidal level | Rel. fluor. | 1C/1C× (pg) |
|---|---|---|---|---|
| B) M. argophyllum | ||||
| M. argophyllum | 19059 | 6× | 4.4 ± 0.17 | 5.68/1.89 |
| M. argophyllum | 19060 | 6× | 4.4 ± 0.01 | 5.75/1.92 |
| Mean ± SD | 5.72 ± 0.05/1.9 ± 0.02 |
Notes: Extrapolated values are given for 1C/1C×. Relative fluorescence (Rel. fluor.) values are from flow cytometry measurements of 35 individuals of silica-gel dried leaf material. Pop. no. = population number.
Laboratory methods
Since most populations of M. cinereum and M. leucanthum form genetically cohesive groups (Rebernig et al., 2010a, b), we used one randomly selected individual per population of these two species for the final AFLP analyses. Additionally, eight individuals of M. argophyllum from the two available populations (Table 1) were added to previously published AFLP data sets (Rebernig et al., 2010a, b). Total genomic DNA was extracted, and AFLPs were generated and scored as described in Rebernig et al. (2010a, b), employing five primer combinations (fluorescent dyes in parentheses): Eco RI-ACA/Mse I-CAT (FAM), Eco RI-ACG/Mse I-CAA (VIC), Eco RI-ACC/MseI-CAG (NED), Eco RI-ACT/MseI-CAC (FAM), Eco RI-AGG/Mse I-CAA (VIC).
Sequences for three noncoding plastid regions (psbA-trnH, rpl32-trnL, ndhF-rpl32) were generated as described in Rebernig et al. (2010a, b). All sequences are deposited in GenBank (accession numbers in Table 1).
Amplification, cloning, and sequencing of the nuclear ITS regions followed the protocols described in Blöch et al. (2009). The complete 5S rDNA region including the nontranscribed spacer was amplified as described in Stuessy et al. (2011). The region between exons 11 and 16 of the low-copy nuclear gene PgiC (two paralogues in Melampodium; Stuessy et al., 2011) was amplified using newly developed specific primers (Stuessy et al., 2011).
The PCR products of the 5S rDNA spacer and the partial PgiC gene paralogues were cloned into pGEM-T-easy vector and transformed into JM109 competent cells (Promega, Vienna, Austria) according to the manufacturer’s instructions. Inserts of 6–18 positive clones for the 5S rDNA spacer region (on average six clones per diploid genome) and 10–20 clones per diploid genome for PgiC were amplified using colony PCR with universal M13 primers, whereby recombinant colonies were added directly into the PCR reaction and inserts amplified using reagents and conditions described in Park et al. (2007). Colony PCR products were purified using E. coli Exonuclease I and Calf Intestine Alkaline Phosphate (CIAP; MBI-Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instructions. The purified DNA fragments were directly sequenced using dye terminator chemistry following the manufacturer’s protocol (Applied Biosystems, Foster City, California, USA). The cycle sequencing reactions were performed using M13 universal primers, either in one or in both directions. Sequencing reactions were run on a 3130×l Genetic Analyzer automated sequencer (Applied Biosystems).
Data analyses
AFLP data were analyzed using the neighbor-net method implemented in the program SplitsTree 4.8 (Huson and Bryant, 2006) based on Nei-Li distances (Nei and Li, 1979) calculated with the program FAMD 1.108 (Schlüter and Harris, 2006). Bootstrap analysis based on 2000 replicates was conducted in the program TREECON 1.3 (van de Peer and de Wachter, 1994).
Sequences were assembled using the program SeqMan II (DNAstar, Madison, USA), aligned manually or with the program Muscle 3.6 (Edgar, 2004) using default settings and further improved by visual inspection using the program BioEdit 7.0.9.0 (Hall, 1999). Prior to all analyses of the cpDNA data, inversions in the plastid sequence data detected by visual inspection (trnH-psbA and ndhF-rpl32) were reverted (Rebernig et al., 2010a, b). In the 5S and the PgiCII data sets, clearly unalignable regions (combinations of mononucleotide repeats and microsatellites) in the 5′-end of the 5S rDNA spacer region (11–27 nucleotides) and the 3′-end of the PgiCII sequences (16–32 nucleotides) were excluded from further analyses. Likewise, a single recombinant PgiCII sequence was removed as well. Each data set was analyzed separately without outgroups, because the phylogenetic distance between ser. Leucantha and its presumptive sister group is relatively large and the introduction of distant lineages might have negative effects on phylogenetic inference (especially due to long-branch attraction) within ser. Leucantha. Maximum likelihood analyses were conducted using the program RAxML 7.2.2 (Stamatakis, 2006) employing the rapid bootstrap analysis plus maximum likelihood tree search (option f-a) with 1000 bootstrap replicates and a GTR+τ model (option-m GTRGAMMA). As phylogenetic trees are not suited for displaying reticulate patterns, the data were also analyzed using parsimony split networks (Bandelt and Dress, 1993) in the program SplitsTree 4.8 (Huson and Bryant, 2006) employing 1000 bootstrap replicates to assess support for splits. For this analysis, indels longer than 1 bp were recoded to be counted as single mutational steps only, and these indels were subsequently treated as a fifth character state.
Amplification and restriction digest of ITS regions
The ITS sequence analyses supported the hypothesis of a hybrid origin of M. argophyllum (see Results). All three species of the white-rayed complex, therefore, including multiple individuals of M. cinereum (also those sharing plastid haplotypes with M. leucanthum; Rebernig et al., 2010b), were investigated in more detail via restriction patterns of ITS. Restriction patterns in already available ITS sequences (Blöch et al., 2009) were identified using Sequence Manipulation Suite (Stothard, 2000). The primers used for amplification were designed based on the ITS region sequence data set from Blöch et al. (2009) and were anchored in 18S and 26S rDNA for the entire ITS and in 5.8S and 26S rDNA for ITS2, respectively. These regions were amplified using 1× Reddy Mix PCR Master Mix (including 2.5 mmol/L MgCl 2; ABgene, Epsom, UK), 0.5 μmol/L each of forward and reverse primer, 0.8 μg/μL dimethyl sulfoxide (DMSO) and ca. 50 ng of DNA template in a T-CY thermocycler (Creacon Technologies, Emmen, Netherlands) under the following conditions: 3 min at 90°C; 35 cycles of 30 s at 94°C, 30 s at 52°C, and 45 s at 72°C; followed by 10 min at 72°C. The entire ITS region was digested with Bst UI (Bsh 12361; MBI-Fermentas) at 37°C for 16 h according to the manufacturer’s protocol. The ITS2 region was digested with the FastDigest Hae III restriction enzyme (MBI-Fermentas) at 37°C for 10 min, following the manufacturer’s recommendation. Resulting DNA fragments were separated electrophoretically (60 min at 80 V) on a 2% agarose gel.
Ploidal level and genome size estimation
Measurements of DNA ploidal levels of all individuals used in the molecular analyses were conducted as described in Rebernig et al. (2010a, b). Additionally, absolute genome size was measured for three populations of diploid M. cinereum var. hirtellum, seven populations of M. cinereum var. cinereum (three diploid and four tetraploid populations), and 14 populations of M. leucanthum (eight diploid and six tetraploid populations), totaling 24 populations (Table 2). Approximately 10 mm 2 leaf tissue of Glycine max ‘Idefix’ (1C = 1.28 pg, Dolezel et al., 1998) and two seedlings of Melampodium were chopped together in 500 μL cold Otto I isolation buffer (Otto et al., 1981). After another 500 μL Otto I buffer was added, the nuclei solution was filtered through a 30 μL nylon mesh. Then 50 μL of RNase A (3 mg/mL; MBI-Fermentas) was added to each sample, which was incubated at 37°C for 30 min to remove RNA. The samples were incubated for ca. 90 min in Otto II buffer (Otto et al., 1981) containing propidium iodide (0.1 mmol/L; Sigma, Vienna, Austria). Analysis was conducted with a Partec CyFlow ML (Partec, Münster, Germany) equipped with a green laser beam, and each sample was measured four times to check for value accuracy. Due to insufficient number of viable achenes of M. argophyllum, its genome size was calculated from the relative fluorescence intensity values obtained from measurements of silica-gel dried leaf material (Rebernig et al., 2010a, b) using the 24 samples of M. cinereum and M. leucanthum with known genome sizes as calibration points (linear regression through the origin).
Cytogenetic analyses
Chromosome numbers, karyotypes, and ideograms were established for selected accessions of all cytotypes and species of the white-rayed complex (Weiss-Schneeweiss et al., 2009). Fluorescent in situ hybridization (FISH) using 35S rDNA and 5S rDNA as probes followed protocols of Weiss-Schneeweiss et al. (2006, 2008). In M. argophyllum, FISH could not be performed due to the insufficient number of viable achenes.
Results
AFLP and sequence analysis
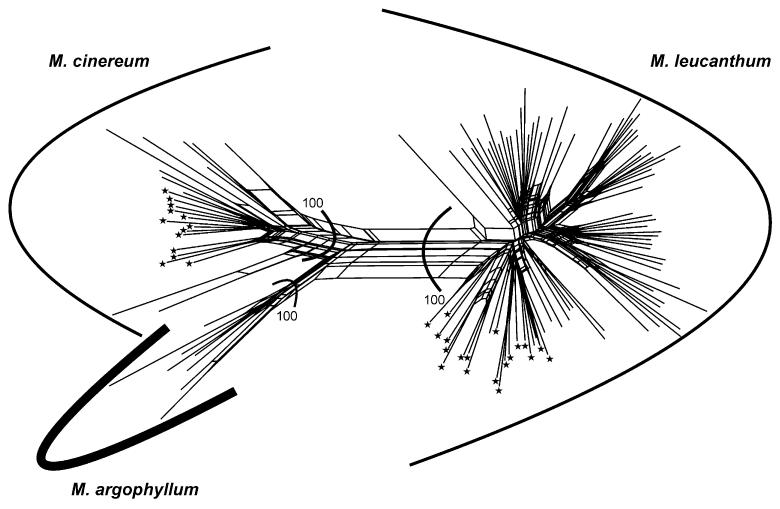
The five AFLP primer combinations chosen for the analysis generated 715 unambiguously scorable fragments in the size range from 100 to 500 bp: 167 with Eco RI-ACA/Mse I-CAT (FAM), 135 with Eco RI-ACG/Mse I-CAA (VIC), 123 with Eco RI-ACC/Mse I-CAG (NED), 160 with Eco RI-ACT/Mse I-CAC (FAM), and 130 with Eco RI-AGG/Mse I-CAA (VIC). All 113 individuals analyzed had a unique AFLP profile. The error rate (Bonin et al., 2004), based on comparisons among replicated individuals, amounted to 4%. The neighbor-net analysis of the AFLP data revealed a network of three well-supported (bootstrap support [BS] 100%) monophyletic groups corresponding to the three species (Fig. 2). The major split separated M. leucanthum from M. cinereum and M. argophyllum.
Fig. 2. Neighbor-net for the AFLP data using Nei-Li distances.
Numbers indicate bootstrap values from a neighbor joining analysis. Asterisks indicate tetraploid cytotypes.
Relationships among the three species of ser. Leucantha were complicated and not always concordant among sequenced regions (sequence characteristics in Table 3). In all sequenced regions, tetraploid accessions of M. cinereum and M. leucanthum grouped with conspecific diploid accessions. Plastid sequence data revealed three well-supported groups (Fig. 3A, B). With the exception of some individuals of M. cinereum sharing haplotypes with M. leucanthum (this pattern is insensitive to sample size: data not shown; Rebernig et al., 2010b), these groups correspond to the three species.
Table 3. Sequence characteristics of DNA regions studied in Melampodium ser. Leucantha.
| Character | Plastid regions | 5S rDNA spacer | ITS | PgiCI | PgiCII |
|---|---|---|---|---|---|
| Alignment length unedited / edited | 2233 / 2233 | 538 / 499 | 791 / 791 | 702 / 702 | 741 / 679 |
| Number of variable characters | 65 | 283 | 34 | 111 | 153 |
| Number of parsimony-informative characters | 59 | 163 | 19 | 67 | 97 |
| Maximum likelihood (ln) | −3383.37 | −3950.33 | −1385.19 | −1782.19 | −2133.99 |
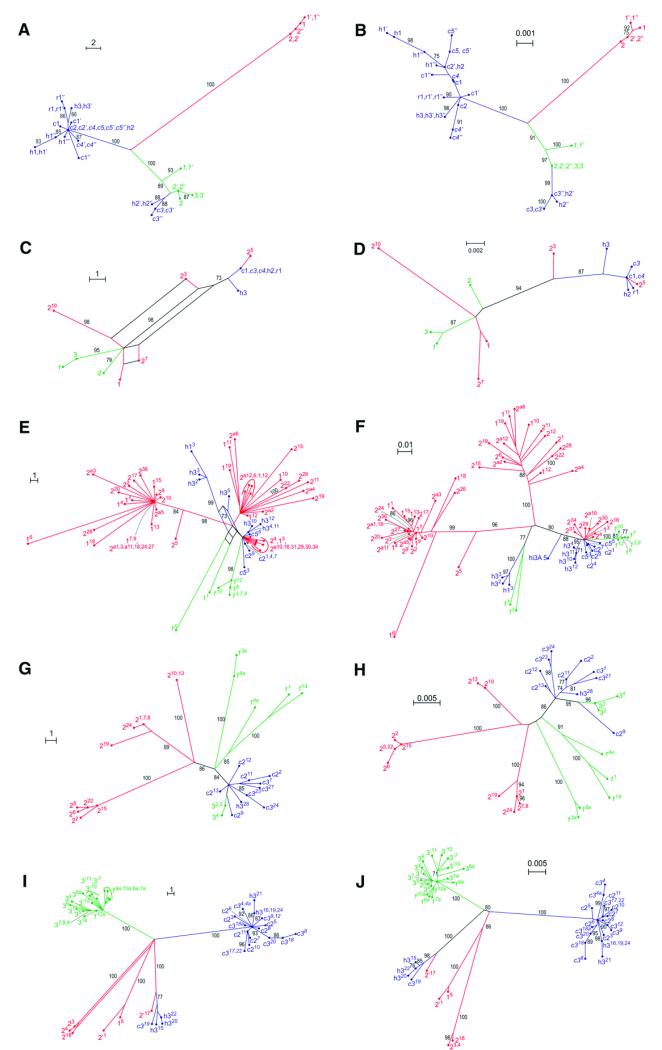
Fig. 3. Genealogical relationships of nuclear and plastid DNA sequences of Melampodium cinereum(blue; the three varieties cinereum, hirtellum, and ramosissimum indicated by their initials), M. leucanthum (green) and M. argophyllum (red) inferred via parsimony splits networks (A, C, E, G, I) and maximum likelihood trees (B, D, F, H, J).
(A, B) Plastid data, (C, D) nuclear ITS, (E, F) 5S rDNA nontranscribed spacer, (G, H) PgiC copy I, (I, J) PgiC copy II. Numbers at splits or branches are bootstrap support values of 70 or higher. Population numbers as in Table 1, different individuals from the same population indicated by apostrophes. In (E-J), different clones are designated with superscript numbers.
Analyses of nuclear ITS sequences (Fig. 3C, D) inferred two groups (BS 94–98) corresponding to M. cinereum and M. leucanthum, each additionally containing sequences of M. argophyllum. None of the three species was found to be monophyletic from analyses of 5S rDNA spacer sequences (Fig. 3E, F). Instead, the majority of clones isolated from M. cinereum and M. leucanthum fell together with some clones of M. argophyllum in a group (supported only in the maximum likelihood analysis), whereas the phylogenetic position of the remaining sequences from M. cinereum and M. leucanthum is unclear due to homoplasy (reticulations in the parsimony splits network: Fig. 3E) or poor resolution and insufficient support (maximum likelihood: Fig. 3F). The remaining sequences from M. argophyllum were distributed into two moderately to well-supported groups (Fig. 3E, F).
Relationships inferred from PgiC sequences suggested three groups with different degrees of cohesiveness and support (Fig. 3G-J). These largely corresponded to the three species with the exception of a few clones of M. leucanthum grouping with M. cinereum in PgiCI (Fig. 3G, H) and a single distinct clone of M. cinereum grouping with M. argophyllum in PgiCII (Fig. 3I, J). In both paralogues, sequences isolated from M. argophyllum fell into three distinct groups (Fig. 3G-J), although in PgiCII these were not found in all individuals studied. In M. leucanthum, lineage heterogeneity was found only in PgiCI (Fig. 3G, H).
Restriction endonuclease digestion of ITS and ITS2
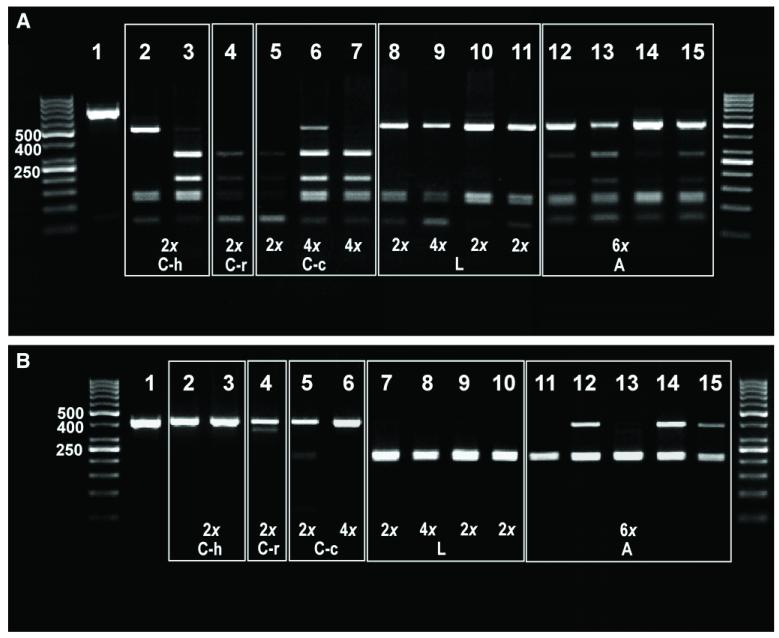
PCR amplifications of both the entire ITS region and of ITS2 alone always resulted in single bands of ca. 700 bp and ca. 350 bp, respectively (Fig. 4). Digestion of the entire ITS PCR product was always complete (Fig. 4A). In both cytotypes of M. cinereum var. cinereum (lanes 5–7), in eastern diploid var. hirtellum (lane 3; Rebernig et al., 2010b), and in diploid var. ramosissimum (lane 4) the digestion resulted in several bands, two of which were specific for M. cinereum (ca. 300 bp and ca. 200 bp). The other bands (ca. 500 bp, 150 bp and 100 bp; not all present in each population) were shared with M. leucanthum, where they are the only ones found (lanes 8–11). All analyzed individuals of western M. cinereum var. hirtellum (Rebernig et al., 2010b) had the same digestion pattern as M. leucanthum irrespective of whether they possessed a cinereum or a leucanthum cpDNA haplotype (lane 2, data not shown). All seven tetraploid individuals of M. cinereum var. cinereum, on the other hand, revealed the digestion pattern typical for M. cinereum irre spec tive of their cpDNA haplotype (lanes 6 and 7 and data not shown). All 10 individuals of M. argophyllum analyzed had almost identical banding patterns (lanes 12–15 and data not shown), sharing all bands specific for both M. cinereum and M. leucanthum .
Fig. 4. Restriction patterns for ITS from Melampodium.
(A) The entire ITS digested with Bst UI: 1, undigested ITS amplification product; 2, M. cinereum var. hirtellum 19057C, 2×; 3, M. cinereum var. hirtellum 19061B, 2×; 4, M. cinereum var. ramosissimum 19065H, 2×; 5, M. cinereum var. cinereum 18688H, 2×; 6, M. cinereum var. cinereum 18697F, 4×; 7, M. cinereum var. cinereum 18694J, 4×; 8, M. leucanthum 18814B, 2×; 9, M. leucanthum 18785G, 4×; 10, M. leucanthum 20033C, 2×; 11, M. leucanthum 19056B, 2×; 12, M. argophyllum 19059E, 6×; 13, M. argophyllum 19060A, 6×; 14, M. argophyllum 19060CI, 6×; 15, M. argophyllum 19060E, 6×. (B) ITS2 only digested with Hae III: 1, undigested ITS2 amplification product; 2, M. cinereum var. hirtellum 19057B, 2×; 3, M. cinereum var. hirtellum 19061B, 2×; 4, M. cinereum var. ramosissimum 19065H, 2×; 5, M. cinereum var. c inereum 18688H, 2×; 6, M. cinereum var. cinereum 18694N, 4×; 7, M. leucanthum 18814B, 2×; 8, M. leucanthum 18785G, 4×; 9, M. leucanthum 20033C, 2×; 10, M. leucanthum 19056B, 2×; 11, M. argophyllum 19059A, 6×; 12, M. argophyllum 19059E, 6×; 13, M. argophyllum 19059I, 6×; 14, M. argophyllum 19060A, 6×; 15, M. argophyllum 19060C, 6×. Abbreviations: C-h, M. cinereum var. hirtellum; C-r, M. cinereum var. ramosissimum; C-c, M. cinereum var. cinereum; L, M. leucanthum; A, M. argophyllum. Unlabelled lanes contain markers (DNA ladder).
The ITS2 region (ca. 350 bp) of M. cinereum (Fig. 4B; lanes 2–6) remained essentially undigested, whereas that of M. leucanthum was completely digested resulting in a single band due to the overlap of the two restriction products each of ca. 175 bp in length (lanes 7–11). Contrary to the results of the whole ITS digestion, the individuals of western M. cinereum var. hirtellum sharing haplotypes with M. leucanthum did not share the ITS2 restriction pattern with M. leucanthum (Fig. 4B). In most accessions of M. argophyllum, undigested and digested bands were observed, albeit often of different strength, a few individuals possessing only the M. leucanthum-specific band (lanes 11–15).
Ploidal level and genome size estimation
In contrast to M. cinereum and M. leucanthum, which comprise two main ploidal levels each (DNA-diploids and DNA-tetraploids: Rebernig et al., 2010a, b), M. argophyllum was uniformly hexaploid (36 individuals; mean value given in Table 2). Flow cytometry measurements of genome size in newly germinated seedlings from selected populations showed no significant differences between varieties of M. cinereum (t = 0.559, P = 0.61; Table 2). Whereas diploid M. cinereum had a significantly smaller genome size than diploid M. leucanthum (2.22 pg/1C vs. 2.32 pg/1C, respectively; t = −0.103, P < 0.001), these differences disappeared at the tetraploid level (4.48 pg/1C vs. 4.52 pg/1C, respectively; t = −0.034, P = 0.66; Table 2). Monoploid genome sizes (C× values: Greilhuber et al., 2005) were not (M. cinereum) or only marginally significantly different (M. leucanthum) between cytotypes (t = −0.028, P = 0.21 and t = 0.058, P = 0.05, respectively). Due to the lack of fresh material, the genome size of M. argophyllum had to be calculated from the relative fluorescence values obtained from silica-gel dried leaf material using the regression Eq. 1C = 1.2785 × relative fluorescence (R 2 = 1). The thus-obtained mean genome size value for M. argophyllum (5.72 pg/1C; 1.9 pg/1C×; Table 2) is 14–16% lower than the expected additive value from an allopolyploid origin (span of 1C = 6.66 to 1C = 6.80 pg, depending on the assumed combination of parental diploid and tetraploid genomes) and 14–18% lower than the expected value from an autopolyploid origin of either species (based on mean genome sizes of current diploids).
Cytogenetic analyses
Melampodium cinereum and M. leucanthum encompassed both diploid (2n = 2× = 20) and tetraploid (2n = 4× = 40) cytotypes (Fig. 5A-D). In tetraploids, aneuploid variation was observed in both species (2n = 41 and 42; Fig. 5C, D). As the size of the extra chromosomes in these aneuploid individuals was similar to that of the smallest chromosomes of the regular A-complement, it is not possible to determine whether the aneuploid numbers can be attributed to the presence of B-chromosomes or accessory A-chromosomes. Melampodium argophyllum was exclusively and regularly hexaploid (2n = 6× = 60; Fig. 5E).
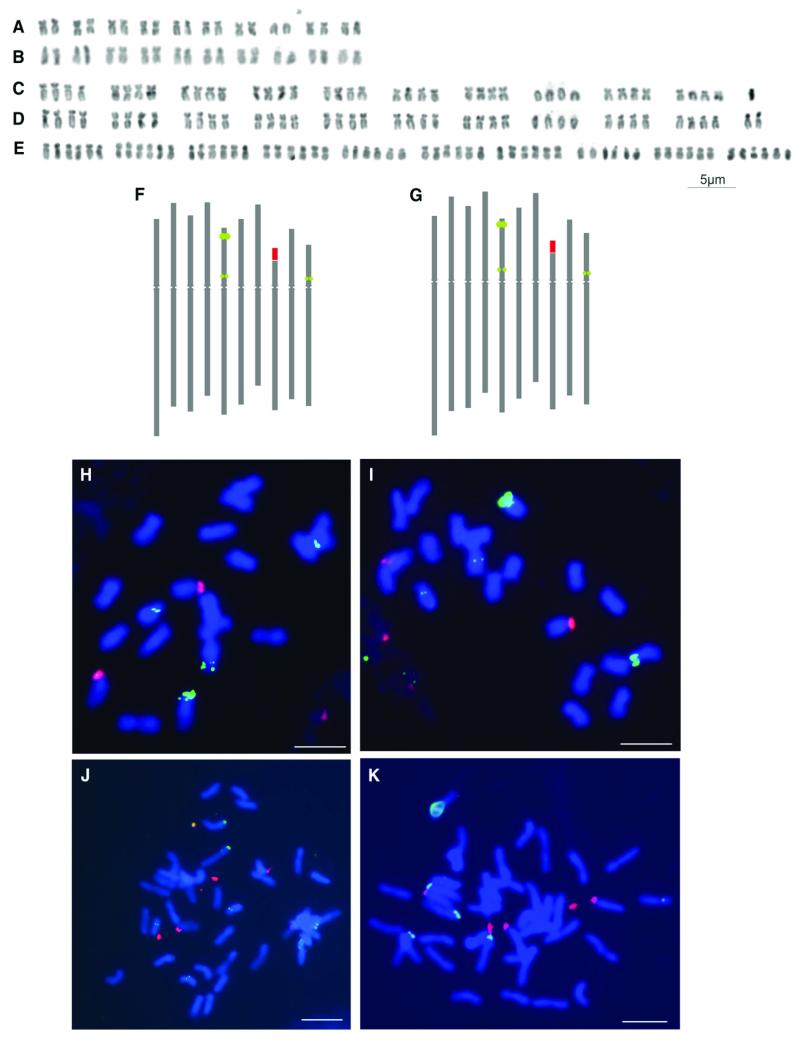
Fig. 5. Chromosomal analyses of the white-rayed species of Melampodium.
Karyotypes (A–E) and idiograms (F, G) of M. cinereum var. cinereum (A, F: 2×, 18688; C: 4×, 18703), M. leucanthum (B, G: 2×, 19056; D: 4× 18683), and M. argophyllum (E: 6×; 19059). (H–K) In situ chromosomal localization of 5S rDNA (green signals) and 35S rDNA loci (red signals) in diploid (18690; H) and tetraploid (18700; J) M. cinereum var. cinereum, as well as diploid (19056; I) and tetraploid (18683; K) M. leucanthum. Scale bar (H–K) = 5 μm.
The karyotypes of the diploid cytotypes of M. cinereum and M. leucanthum were very similar in morphology and structure, and nearly all chromosome pairs were distinguishable based on chromosome structure alone (Fig. 5A, B). Likewise, the tetraploid karyotypes of both species were very similar to one another and to those of the diploid cytotypes and did not show any apparent chromosomal rearrangements (Fig. 5A-D). However, sometimes the four homologues of each chromosome were differentiated into two pairs, rather than forming a group of four identical chromosomes, particularly in M. cinereum (Fig. 5C).
Molecular cytogenetic localization of 5S rDNA and 35S rDNA using FISH in diploid cytotypes revealed the presence of one locus of 35S rDNA and three loci of 5S rDNA (Fig. 5F-I). The single 35S rDNA locus (NOR) was found at a subterminal position in the short arm of chromosome 8. Two of the 5S rDNA loci were localized in the short arm of chromosome 5, a larger one in the subtelomeric region (locus 1) and a weaker one in the pericentromeric region (locus 2), while the third weak 5S rDNA locus (locus 3) was found in a pericentromeric position in the smallest submetacentric chromosome 10 (Fig. 5F-I).
Tetraploid cytotypes of both M. cinereum and M. leucanthum possessed twice as many loci with the same localization as the diploids (Fig. 5J, K). Although the 35S rDNA locus was present in all four chromosomes, it showed differentiation into two larger and two smaller signals, suggesting ongoing differentiation of the two homologous chromosome pairs. All four signals mark active loci, which in the interphase are decondensed and attached to the nucleolus/nucleoli (data not shown). The major 5S rDNA locus (locus 1) was clearly identifiable in all tetraploid individuals analyzed. The minor loci were also mostly present, but occasionally with one or two signals not clearly discernable in chromosomal spreads, particularly of locus 2 due to its close proximity to large locus 1 (Fig. 5J, K). As depending on the degree of chromosome condensation loci 1 and 2 were sometimes indistinguishable also in diploid plants (data not shown), there is no evidence for substantial rDNA loci loss in tetraploid genomes.
Discussion
Phylogenetic relationships and origin of M. argophyllum
Melampodium cinereum and M. leucanthum constitute well-separated and distinct gene pools (Fig. 2), in agreement with their morphological, geographical, and ecological separation (Stuessy, 1971b). Although currently no hybrids between the two species are known, their past evolution appears to have been shaped by multiple hybridization events and gene flow. Specifically, western M. cinereum var. hirtellum has experienced introgression from M. leucanthum as indicated by leucanthum-type plastid haplotypes and ITS sequences in at least some of the var. hirtellum populations (Figs. 3, 4). Whereas conversion of the 35S rDNA cistron is biased toward the maternal parent, even in the presence of extensive back-crossing (Lim et al., 2000), in western M. cinereum var. hirtellum ITS at least partially homogenized toward M. leucanthum irrespective of its parentage, a pattern observed also in other allopolyploid Melampodium species (Weiss-Schneeweiss et al., 2012). Repeated introgression of M. leucanthum into western var. hirtellum may explain its genetic distinctness from the morphologically indistinguishable eastern var. hirtellum (Rebernig et al., 2010b), but evidently this gene flow did not blur the border between M. cinereum and M. leucanthum (in line with a genic view of speciation; Wu, 2001). A second zone of introgression includes a few tetraploid M. cinereum var. cinereum populations in the east of the species’ distribution, where distinct leucanthum-type haplotypes are present (Fig. 3A, B; Rebernig et al., 2010b). In these populations, the 35S rDNA cistron has not been affected, which may be related to an altogether lower amount of gene flow and strong backcrossing, or biased and parent-specific ribotype conversion (Arnold et al., 1988; Lim et al., 2008).
The situation for M. argophyllum is less clear. Plastid and, to a lesser extent, 5S rDNA and PgiC sequence data suggest M. argophyllum as a distinct third lineage. This agrees with a hypothesis put forward by Stuessy (1971b) based on morphological analyses that M. argophyllum resembles the progenitor of the whole complex. This renders M. argophyllum an altitudinally and ecologically isolated relict taxon of putative autopolyploid origin, possibly having arisen from the ancestral taxon of the whole complex. If so, the presence of three distinct groups in 5S rDNA and PgiC sequences might reflect independent evolution of the three paraloguous loci that resulted from autopolyploidization likely involving diploid and autotetraploid parental cytotypes. Furthermore, sequences grouping with those from M. cinereum (Fig. 3) were either remnants of ancient polymorphisms or the results of introgression into M. argophyllum. Such introgressions may have occurred prior to polyploidization, because there is no evidence for current hybridization between M. argophyllum and any of the other two taxa. At present, this hexaploid species grows in a higher elevation rocky habitat and does not co-occur with M. cinereum or M. leucanthum, but there is still much to learn about M. argophyllum. It is confined to isolated mountains in northern Mexico that are difficult to access; therefore, the species has not yet been extensively sampled for cytological or genetic variation among existing populations.
Alternatively, M. argophyllum has been hypothesized to have originated via allopolyploidy from crosses between M. cinereum and M. leucanthum (Stuessy et al., 2004). A certain morphological intermediacy is reflected in previous classifications, which treated M. argophyllum as a variety either under M. cinereum (Gray, 1884) or M. leucanthum (Stuessy, 1969). Evidence for this hypothesis comes from nrITS sequence data (Figs. 3C, 3D, 4). As outlined already, M. cinereum and M. leucanthum apparently did hybridize repeatedly in their history. In general, formation and establishment of viable and fertile hybrids between different species on the diploid level is rather rare (Stebbins, 1958; Baack and Rieseberg, 2007; Mallet, 2005, 2007) due to genomic instability or hybrid sterility (Hegarty et al., 2005, 2006). Polyploidization following early hybridization may have resulted in genome stabilization (Hegarty and Hiscock, 2008) and restoration of fertility (Rieseberg, 2001; Salmon et al., 2005), resulting in allopolyploid speciation as known in several other cases in sect. Melampodium (Stuessy et al., 2011; Weiss-Schneeweiss et al., 2012).
Despite the use of several molecular markers, therefore, the mode of origin of M. argophyllum remains uncertain. Paradoxically, the sequence marker, nuclear ITS, considered most likely to lose signal for reticulation due to homogenization (álvarez and Wendel, 2003; Poczai and Hyvönen, 2009) is the only one showing strong evidence for an allopolyploid origin, whereas signal from the other markers is consistent with an autopolyploid origin with some gene flow. Evidence for an autopolyploid origin is also evident with the two PgiC paralogues, although their interpretation is complicated by intraindividual variation (due to, for instance, allelic variation, intraspecific gene duplications, or simply cloning artifacts; Brysting et al., 2011) and by partial failure to recover the expected number of copies in some individuals. Possibly the origin of M. argophyllum predates (full) separation of M. cinereum and M. leucanthum, when gene flow between paternal and descendant lineages still did occur. Not mutually exclusive, the origin of M. argophyllum may involve both auto- and allopolyploidization, but further data will be necessary to test these hypotheses.
Genome evolution
Members of ser. Leucantha have significantly larger genomes than those of the presumptive sister group ser. Cupulata (ca. 2.2-fold increase; Weiss-Schneeweiss et al., 2012) or any other species group within section Melampodium (H. Weiss-Schneeweiss et al., unpublished data). This may be connected to different life histories of this group (perennial and xerophilous life form) because annuals tend to have lower nuclear DNA content than perennials (Bennett, 1972). Previous studies have found positive correlations between the duration of the vegetative period and genome sizes in various angiosperms (Bennett, 1987; Turpeinen et al., 1999). Additionally, the shift toward arid environments in ser. Leucantha may have triggered additional genome size increase (Knight and Ackerly, 2002; Knight et al., 2005), possibly via transposable element mobility induced by environmental stress (Fontdevila, 2005; Vitte and Panaud, 2005). Although correlations of water stress and genome size are usually negative, in some plant groups they have been shown to be positive (Knight and Ackerly, 2002).
Autopolyploidization in M. cinereum and M. leucanthum did not significantly affect genome size, which is within the range of the expected additive values (Table 2). This pattern agrees with that seen in young autopolyploids of other plant groups (e.g., Ozkan et al., 2006; Eilam et al., 2009). In contrast to nascent autopolyploids, established autopolyploids (i.e., those which usually are cytologically already diploidized) were often reported to show substantial genome reorganization compared with their diploid relatives (Eilam et al., 2010; Parisod et al., 2010). In contrast to the young autopolyploids of M. cinereum and M. leucanthum, M. argophyllum has a lower genome size than would be expected irrespective of the mode of origin (autopolyploidy vs. allopolyploidy). This is congruent with the likely older age of this polyploid and thus agrees with the general trend of genome downsizing in polyploids (Leitch and Bennett, 2004; Weiss-Schneeweiss et al., 2006; Lysak et al., 2009). Alternatively, it may reflect smaller genome size of its ancestor(s), which remain unknown at this point, as is conceivable given the evidence for genome size increase in the evolution of the entire ser. Leucantha from its relatives.
The karyotype in ser. Leucantha, as assessed by chromosome morphology as well as by number and localization of 5S and 35S ribosomal RNA genes (no data available for M. argophyllum), is very stable both across species and across ploidal levels. This supports the relatively recent origin of polyploids in M. cinereum and M. leucanthum as suggested previously (Stuessy et al., 2004, Rebernig et al., 2010a, b). Only in tetraploid M. cinereum, and to a lesser extent in hexaploid M. argophyllum, some of the homologous chromosomes can form distinguishable pairs/groups (Fig. 5) as seen in other groups (e.g., Spartina, Aïnouche et al., 2004; Gossypium, Adams and Wendel, 2004). This suggests ongoing diploidization of the tetraploids, likely acting as one of the processes that restore fertility and allow further evolution of the genomes (Eilam et al., 2009).
APPENDIX 1
Sampling location coordinates, voucher (collector) number, number of analyzed individuals (N), and ploidal level of populations of species of the white rayed complex analyzed with AFLPs.
| Voucher | Location | N | Ploidal level |
|---|---|---|---|
| Melampodium leucanthum | |||
| 18676 | N 30.194 W 30.1938 | 1 | 2× |
| 18677 | N 30.216 W 98.238 | 1 | 2× |
| 18679 | N 30.227 W 98.382 | 1 | 2× |
| 18681 | N 30.280 W 98.906 | 1 | 2× |
| 18682 | N 30.170 W 98.382 | 1 | 2× |
| 18683 | N 29.891 W 98.407 | 1 | 4×* |
| 18686 | N 29.616 W 98.756 | 1 | 4× |
| 18687 | N 29.529 W 98.848 | 1 | 4× |
| 18709 | N 30.616 W 97.860 | 1 | 4× |
| 18710 | N 30.694 W 97.981 | 1 | 4× |
| 18711 | N 30.696 W 98.253 | 1 | 4× |
| 18712 | N 30.671 W 98.255 | 1 | 4× |
| 18714 | N 30.387 W 98.366 | 1 | 4× |
| 18720 | N 29.718 W 101.364 | 1 | 4×* |
| 18725 | N 30.789 W 104.033 | 1 | 2× |
| 18726 | N 31.005 W 104.825 | 1 | 2× |
| 18727 | N 30.999 W 103.755 | 1 | 2×* |
| 18729 | N 31.352 W 103.579 | 1 | 2× |
| 18730 | N 31.640 W 102.634 | 1 | 2× |
| 18731 | N 31.698 W 102.572 | 1 | 2× |
| 18732 | N 31.934 W 101.865 | 1 | 2× |
| 18733 | N 31.871 W 101.646 | 1 | 2× |
| 18734 | N 31.900 W 100.716 | 1 | 2× |
| 18735 | N 32.964 W 102.011 | 1 | 2× |
| 18737 | N 32.288 W 102.611 | 1 | 2× |
| 18738 | N 31.852 W 103.114 | 1 | 2× |
| 18753 | N 35.326 W 102.369 | 1 | 2× |
| 18756 | N 35.003 W 101.918 | 1 | 2× |
| 18757 | N 34.986 W 101.717 | 1 | 2× |
| 18758 | N 36.221 W 101.333 | 1 | 2× |
| 18760 | N 36.449 W 100.372 | 1 | 2× |
| 18761 | N 36.427 W 99.883 | 1 | 2× |
| 18762 | N 35.845 W 100.396 | 1 | 2× |
| 18764 | N 35.432 W 100.770 | 1 | 2× |
| 18768 | N 33.860 W 100.851 | 1 | 2× |
| 18769 | N 34.219 W 100.888 | 1 | 2× |
| 18770 | N 34.380 W 101.110 | 1 | 2× |
| 18771 | N 34.788 W 100.898 | 1 | 2× |
| 18772 | N 35.009 W 100.895 | 1 | 2× |
| 18774 | N 31.226 W 99.757 | 1 | 2× |
| 18776 | N 31.781 W 99.779 | 1 | 4× |
| 18778 | N 31.761 W 98.899 | 1 | 4× |
| 18779 | N 31.689 W 98.813 | 1 | 4× |
| 18781 | N 31.358 W 98.129 | 1 | 4× |
| 18782 | N 31.170 W 98.183 | 1 | 4× |
| 18783 | N 30.928 W 98.001 | 1 | 4× |
| 18785 | N 31.064 W 97.571 | 1 | 4× |
| 18786 | N 31.575 W 97.834 | 1 | 4× |
| 18787 | N 31.793 W 98.227 | 1 | 4× |
| 18800 | N 33.238 W 110.253 | 1 | 2× |
| 18801 | N 34.002 W 111.313 | 1 | 2× |
| 18802 | N 33.480 W 111.443 | 1 | 2× |
| 18805 | N 33.962 W 111.863 | 1 | 2× |
| 18807 | N 34.349 W 112.153 | 1 | 2× |
| 18808 | N 34.605 W 111.857 | 1 | 2× |
| 18809 | N 34.617 W 111.843 | 1 | 2× |
| 18810 | N 34.650 W 111.760 | 1 | 2× |
| 18811 | N 34.639 W 111.807 | 1 | 2× |
| 18812 | N 34.712 W 111.880 | 1 | 2× |
| 18813 | N 34.730 W 111.968 | 1 | 2× |
| 18814 | N 34.755 W 112.091 | 1 | 2× |
| 18816 | N 24.747 W 112.105 | 1 | 2× |
| 18817 | N 34.825 W 111.778 | 1 | 2× |
| 18819 | N 34.756 W 111.765 | 1 | 2× |
| 18820 | N 35.121 W 113.666 | 1 | 2× |
| 18822 | N 32.602 W 110.744 | 1 | 2× |
| 19056 | N 28.228 W 101.056 | 1 | 2×* |
| 20000 | N 32.263 W 107.232 | 1 | 2× |
| 20002 | N 31.953 W 107.675 | 1 | 2× |
| 20003 | N 32.640 W 107.954 | 1 | 2× |
| 20005 | N 32.786 W 108.139 | 1 | 2× |
| 20007 | N 32.953 W 107.489 | 1 | 2× |
| 20010 | N 33.275 W 107.281 | 1 | 2× |
| 20011 | N 34.146 W 106.907 | 1 | 2× |
| 20014 | N 33.793 W 106.274 | 1 | 2× |
| 20016 | N 34.009 W 105.941 | 1 | 2× |
| 20017 | N 34.945 W 106.191 | 1 | 2× |
| 20021 | N 35.288 W 106.216 | 1 | 2× |
| 20029 | N 35.230 W 103.767 | 1 | 2× |
| 20030 | N 35.395 W 104.179 | 1 | 2× |
| 20031 | N 34.897 W 104.718 | 1 | 2× |
| 20032 | N 34.036 W 104.747 | 1 | 2× |
| 20033 | N 32.490 W 104.348 | 1 | 2× |
| 20034 | N 32.529 W 103.801 | 1 | 2× |
| 20035 | N 32.506 W 103.126 | 1 | 2× |
| 20038 | N 30.239 W 103.380 | 1 | 2× |
| 20039 | N 29.784 W 103.177 | 1 | 2× |
| 20040 | N 29.516 W 103.403 | 1 | 2× |
| 20043 | N 31.003 W 104.824 | 1 | 2× |
| 20044 | N 31.610 W 104.857 | 1 | 2× |
| 20045 | N 31.315 W 106.078 | 1 | 2× |
| 20046 | N 32.397 W 106.614 | 1 | 2× |
| M. argophyllum | |||
| 19059 | N 26.249 W 100.895 | 6 | 6×* |
| 19060 | N 26.232 W 100.677 | 2 | 6×* |
| M. cinereum var. cinereum | |||
| 18689 | N 28.608 W 99.841 | 1 | 2×* |
| 18690 | N 28.035 W 99.529 | 1 | 2× |
| 18691 | N 27.781 W 99.451 | 1 | 2× |
| 18693 | N 26.890 W 99.262 | 1 | 2× |
| 18694 | N 26.697 W 99.109 | 1 | 4× |
| 18697 | N 26.823 W 98.855 | 1 | 4× |
| 18698 | N 26.941 W 98.619 | 1 | 4× |
| 18699 | N 27.195 W 98.622 | 1 | 4× |
| 18700 | N 27.264 W 98.486 | 1 | 4×* |
| 18701 | N 27.278 W 98.734 | 1 | 4× |
| 18702 | N 27.232 W 98.934 | 1 | 4× |
| 18704 | N 27.405 W 98.576 | 1 | 4× |
| 18705 | N 27.513 W 98.481 | 1 | 4× |
| 18706 | N 27.733 W 98.263 | 1 | 4× |
| 18707 | N 27.870 W 98.088 | 1 | 4× |
| 18708 | N 28.242 W 98.106 | 1 | 4× |
| M. cinereum var. hirtellum | |||
| 19054 | N 27.945 W 101.257 | 1 | 2× |
| 19055 | N 27.929 W 101.343 | 1 | 2× |
| 19057 | N 28.193 W 101.072 | 1 | 2× |
| 19058 | N 26.509 W 101.352 | 1 | 2× |
| 19061 | N 26.366 W 100.122 | 1 | 2× |
| 19062 | N 26.375 W 100.025 | 1 | 2×* |
| M. cinereum var. ramosissimum | |||
| 19063 | N 25.181 W 98.199 | 1 | 2×* |
| 19065 | N 24.816 W 98.185 | 1 | 2× |
Notes: Populations counted chromosomally (other determined only by flow cytometry).
Footnotes
The authors thank M. Lenko (University of Vienna, Austria), E. Ortiz (UNAM, México), M. Reed and H. Wilson (Texas A&M University, USA), and D. Bailey and P. Alexander (New Mexico State University, USA) for help with collections of material. The authors thank G. Kohl (University of Vienna, Austria) for technical assistance. The study was financially supported by Austrian Science Fund (FWF) grants P18201-B03 (to T.F.S.) and Hertha-Firnberg postdoctoral fellowship T-218 (to H.W.-S.), and by Austrian Academy of Sciences Kiös P2007-12 (to T.F.S.).
Literature Cited
- Adams KL, Wendel JF. Exploring the genomic mysteries of polyploidy in cotton. Biological Journal of the Linnean Society. 2004;82:573–581. [Google Scholar]
- Adams KL, Wendel JF. Novel patterns of gene expression in polyploid plants. Trends in Genetics. 2005a;21:539–543. doi: 10.1016/j.tig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Adams KL, Wendel JF. Polyploidy and genome evolution in plants. Current Opinion in Plant Biology. 2005b;8:135–141. doi: 10.1016/j.pbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Aïnouche ML, Baumel A, Salmon A. Spartina anglica C. E. Hubbard: A natural model system for analysing early evolutionary changes that affect allopolyploid genomes. Biological Journal of the Linnean Society. 2004;82:475–484. [Google Scholar]
- álvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Molecular Phylogenetics and Evolution. 2003;29:417–434. doi: 10.1016/s1055-7903(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Anderson E, Stebbins GL. Hybridization as an evolutionary stimulus. Evolution. 1954;8:378–388. [Google Scholar]
- Arnold ML, Contreras N, Shaw DD. Biased gene conversion and asymmetrical introgression between subspecies. Chromosoma. 1988;96:368–371. [Google Scholar]
- Baack EJ, Rieseberg LH. A genomic view of introgression and hybrid speciation. Current Opinion in Genetics and Development. 2007;17:513–518. doi: 10.1016/j.gde.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelt H-J, Dress AWM. A relational approach to split decomposition. In: Opitz O, Lausen B, Klar R, editors. Information and classification. Springer; Berlin, Germany: 1993. pp. 123–131. [Google Scholar]
- Bennett MD. Nuclear DNA content and minimum generation time in herbaceous plants. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1972;181:109–135. doi: 10.1098/rspb.1972.0042. [DOI] [PubMed] [Google Scholar]
- Bennett MD. Variation in genomic form in plants and its ecological implications. The New Phytologist. 1987;106:177–200. [Google Scholar]
- Bennetzen JL, Kellogg EA. Do plants have a one-way ticket to genomic obesity? The Plant Cell. 1997;9:1509–1514. doi: 10.1105/tpc.9.9.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen JL, MA J, Devos KM. Mechanisms of recent genome size variation in flowering plants. Annals of Botany. 2005;95:127–132. doi: 10.1093/aob/mci008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake SF. New American Asteraceae. Contributions from the United States National Herbarium. 1924;22:587–661. [Google Scholar]
- Blöch C, Weiss-Schneeweiss H, Schneeweiss GM, Barfuss MHJ, Rebernig CA, Villaseñor JL, Stuessy TF. Molecular phylogenetic analyses of nuclear and plastid DNA sequences support dysploid and polyploid chromosome number changes and reticulate evolution in the diversification of Melampodium (Millerieae, Asteraceae) Molecular Phylogenetics and Evolution. 2009;53:220–233. doi: 10.1016/j.ympev.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin A,E, Bellemain PB, Eidesen F, Pompanon C, Rochmann B, Taberlet P. How to track and assess genotyping errors in population genetics studies. Molecular Ecology. 2004;13:3261–3273. doi: 10.1111/j.1365-294X.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- Brysting AK, Mathiesen C, Marcussen T. Challenges in polyploid phylogenetic reconstruction: A case story from the arcticalpine. Cerastium alpinum complex. Taxon. 2011;60:333–347. [Google Scholar]
- Chen JZ, HA M, Soltis DE. Polyploidy: Genome obesity and its consequences. The New Phytologist. 2007;174:717–720. doi: 10.1111/j.1469-8137.2007.02084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L. The advantages and disadvantages of being polyploid. Nature Reviews Genetics. 2005;6:836–846. doi: 10.1038/nrg1711. [DOI] [PubMed] [Google Scholar]
- Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. American Journal of Botany. 1988;75:1443–1458. [Google Scholar]
- De Bodt S, Maere S, Van De Peer Y. Genome duplication and the origin of angiosperms. Trends in Ecology and Evolution. 2005;20:591–597. doi: 10.1016/j.tree.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Dixon CJ, Schönswetter P, Suda J, Wiedermann MM, Schneeweiss GM. Reciprocal Pleistocene origin and postglacial range formation of an allopolyploid and its sympatric ancestors (Androsace adfi nis group, Primulaceae) Molecular Phylogenetics and Evolution. 2009;50:74–83. doi: 10.1016/j.ympev.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Dolezel J, Greilhuber J, Lucretti S, Meister A, Lysák MA, Nardi L, Obermayer R. Plant genome size estimation by flow cytometry: Inter-laboratory comparison. Annals of Botany. 1998;82(Supplement A):17–26. [Google Scholar]
- Edgar RC. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam T, Anikster Y, Millet E, Manisterski J, Eldman MF. Genome size in natural and synthetic autopolyploids and in a natural segmental allopolyploid of several Triticeae species. Genome. 2009;52:275–285. doi: 10.1139/G09-004. [DOI] [PubMed] [Google Scholar]
- Eilam T, Anikster Y, Millet E, Manisterski J, Feldman M. Genome size in diploids, allopolyploids, and autopolyploids of Mediterranean Triticeae. Journal of Botany. 2010:341380. doi: 10.1155/2010/341380. [Google Scholar]
- Ferguson D, Sang T. Speciation through homoploid hybridization between allotetraploids in peonies (Paeonia) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3915–3919. doi: 10.1073/pnas.061288698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontdevila A. Hybrid genome evolution by transposition. Cytogenetic and Genome Research. 2005;110:49–55. doi: 10.1159/000084937. [DOI] [PubMed] [Google Scholar]
- Fortune PM, Schierenbeck KA, Ainouche AK, Jacquemin J, Wendel JF, Ainouche ML. Evolutionary dynamics of Waxy and the origin of hexaploid Spartina species (Poaceae) Molecular Phylogenetics and Evolution. 2007;43:1040–1055. doi: 10.1016/j.ympev.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Grant V. Plant speciation. Columbia University Press; New York, New York, USA: 1981. [Google Scholar]
- Gray A. Synoptical flora of North America. Ivison, Blakeman, Taylor, and Co.; New York, New York, USA: 1884. [Google Scholar]
- Greilhuber J, Dolezel J, Lysák MA, Bennett MD. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Harris SA, Ingram R. Chloroplast DNA and biosystematics: The effect of intraspecific diversity and plastid transmission. Taxon. 1991;40:393–412. [Google Scholar]
- Hawkins JS, Grover CE, Wendel JF. Repeated big bangs and the expanding universe: Directionality in plant genome size evolution. Plant Science. 2008;174:557–562. [Google Scholar]
- Hegarty M, Barker GL, Wilson ID, Abbott RJ, Edwards KJ, Hiscock SJ. Transcriptome shock after interspecific hybridization in Senecio is ameliorated by genome duplication. Current Biology. 2006;16:1652–1659. doi: 10.1016/j.cub.2006.06.071. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Jones JM, Wilson ID, Barker GL, Coghill JA, Sanchez-Baracaldo P, Liu G, et al. Development of anonymous cDNA microarrays to study changes in the Senecio floral transcriptome during hybrid speciation. Molecular Ecology. 2005;14:2493–2510. doi: 10.1111/j.1365-294x.2005.02608.x. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Hiscock SJ. Polyploidy: Doubling up for evolutionary success. Current Biology. 2007;17:R927–R929. doi: 10.1016/j.cub.2007.08.060. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Current Biology. 2008;18:R435–R444. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Hufton AL, Panopoulou G. Polyploidy and genome restructuring: A variety of outcomes. Current Opinion in Genetics and Development. 2009;19:600–606. doi: 10.1016/j.gde.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Bailey CD, Harris SA. Divergent and reticulate species relationships in Leucaena (Fabaceae) inferred from multiple data sources: Insight into polyploid origins and nrDNA polymorphism. American Journal of Botany. 2002;89:1057–1073. doi: 10.3732/ajb.89.7.1057. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- Kim ST, Sultan SE, Donoghue MJ. Allopolyploid speciation in Persicaria (Polygonaceae): Insights from a low-copy nuclear region. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12370–12375. doi: 10.1073/pnas.0805141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CA, Ackerly DD. Genome size variation across environmental gradients in the California flora. Ecology Letters. 2002;5:66–76. [Google Scholar]
- Knight CA, Molinari NA, Petrov DA. The large genome constraint hypothesis: Evolution, ecology and phenotype. Annals of Botany. 2005;95:177–190. doi: 10.1093/aob/mci011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch AR, Leitch IJ. Genome plasticity and diversity of polyploid plants. Science. 2008;320:481–483. doi: 10.1126/science.1153585. [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Bennett MD. Polyploidy in angiosperms. Trends in Plant Science. 1997;2:470–476. [Google Scholar]
- Leitch IJ, Bennett MD. Genome downsizing in polyploid plants. Biological Journal of the Linnean Society. 2004;82:651–663. [Google Scholar]
- Lihová J, Shimizu KK, Marhold K. Allopolyploid origin of Cardamine asarifolia (Brassicaceae): Incongruence between plastid and nuclear ribosomal DNA sequences solved by a single-copy nuclear gene. Molecular Phylogenetics and Evolution. 2006;39:759–786. doi: 10.1016/j.ympev.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Lim KY, Matyásek R, Lichtenstein CP, Leitch AR. Molecular cytogenetic analyses and phylogenetic studies in the Nicotiana section Tomentosae. Chromosoma. 2000;109:245–258. doi: 10.1007/s004120000074. [DOI] [PubMed] [Google Scholar]
- Lim KY, Soltis DE, Soltis PS, Tate J, Matyásek R, Srubarova H, Kovařík A, Pires JC, Xiong Z, Leitch AR. Rapid chromosome evolution in recently formed polyploids in Tragopogon (Asteraceae) PLoS ONE. 2008;3:e3353. doi: 10.1371/journal.pone.0003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wendel JF. Non-mendelian phenomenon in allopolyploid genome evolution. Current Genomics. 2002;3:489–505. [Google Scholar]
- Lysak MA, Koch MA, Beaulieu JM, Meister A, Leitch IL. The dynamic ups and downs of genome size evolution in Brassicaceae. Molecular Biology and Evolution. 2009;26:85–98. doi: 10.1093/molbev/msn223. [DOI] [PubMed] [Google Scholar]
- Mallet J. Hybridization as an invasion of the genome. Trends in Ecology and Evolution. 2005;20:229–237. doi: 10.1016/j.tree.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- Masterson J. Stomatal size in fossil plants: Evidence for polyploidy in majority of angiosperms. Science. 1994;264:421–424. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- Nei M, Li W-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution by gene duplication. George Allen and Unwin; London, UK: 1970. [Google Scholar]
- Otto FJ, Oldiges H, Göhde W, Jain VK. Flow cytometric measurement of nuclear DNA content variations as a potential in vivo mutagenicity test. Cytometry. 1981;2:189–191. doi: 10.1002/cyto.990020311. [DOI] [PubMed] [Google Scholar]
- Otto S. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. doi: 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Ozkan H, Tuna M, Galbraith DW. No DNA loss in autotetraploids of Arabidopsis thaliana. Plant Breeding. 2006;125:288–291. [Google Scholar]
- Parisod C, Holderegger R, Brochmann C. Evolutionary consequences of autopolyploidy. The New Phytologist. 2010;186:5–17. doi: 10.1111/j.1469-8137.2009.03142.x. [DOI] [PubMed] [Google Scholar]
- Park J-M, Manen J-F, Schneeweiss GM. Horizontal gene transfer of a plastid gene in the non-photosynthetic flowering plants Orobanche and Phelipanche (Orobanchaceae) Molecular Phylogenetics and Evolution. 2007;43:974–985. doi: 10.1016/j.ympev.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Paun O, Fay M, Forest F, Chase MW. Hybrid speciation in angiosperms: Parental divergence drives ploidy. The New Phytologist. 2009;182:507–518. doi: 10.1111/j.1469-8137.2009.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poczai P, Hyvönen J. Nuclear ribosomal spacer regions in plant phylogenetics: Problems and prospects. Molecular Biology Reports. 2009;37:1897–1912. doi: 10.1007/s11033-009-9630-3. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. Neopolyploidy in flowering plants. Annual Review of Ecology and Systematics. 2002;33:589–639. [Google Scholar]
- Rebernig CA, S Chneeweiss GM, Bardy KE, Schönswetter P, Villaseñor JL, Obermayer R, Stuessy TF, Weiss-Schneeweiss H. Multiple Pleistocene refugia and Holocene range expansion of an abundant southwestern American desert plant species (Melampodium leucanthum, Asteraceae) Molecular Ecology. 2010a;19:3421–3443. doi: 10.1111/j.1365-294X.2010.04754.x. [DOI] [PubMed] [Google Scholar]
- Rebernig CA, Weiss-Schneeweiss H, Schneeweiss GM, Schönswetter P, Obermayer R, Villaseñor JL, Stuessy TF. Quater nary range dynamics and polyploid evolution in an arid brushland plant species (Melampodium cinereum, Asteraceae) Molecular Phylogenetics and Evolution. 2010b;54:594–606. doi: 10.1016/j.ympev.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH. Polyploid evolution: Keeping the peace at genomic reunions. Current Biology. 2001;11:R925–R928. doi: 10.1016/s0960-9822(01)00556-5. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A, Ainouche ML, Wendel JF. Genetic and epigenetic consequences of recent hybridization and polyploidy in Spartina (Poaceae) Molecular Ecology. 2005;14:1163–1175. doi: 10.1111/j.1365-294X.2005.02488.x. [DOI] [PubMed] [Google Scholar]
- Sang T. Utility of low-copy nuclear gene sequences in plant phylogenetics. Critical Reviews in Biochemistry and Molecular Biology. 2002;37:121–147. doi: 10.1080/10409230290771474. [DOI] [PubMed] [Google Scholar]
- Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phylogeny, reticulate evolution and biogeography of Paeonia (Paeoniaceae) American Journal of Botany. 1997;84:1120–1136. [PubMed] [Google Scholar]
- Schlüter PM, Harris SA. Analysis of multilocus fingerprint data sets containing missing data. Molecular Ecology Notes. 2006;6:569–572. [Google Scholar]
- Schubert I, Lysak MA. Interpretation of karyotype evolution should consider chromosome structural constraints. Trends in Genetics. 2011;27:207–216. doi: 10.1016/j.tig.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Shimizu-Inatsugi R, Lihová J, Iwanaga H, Kudoh H, Marhold K, Savolainen O, Watanabe K, Yakubov VV, Shimizu KK. The allopolyploid Arabidopsis kamchatica originated from multiple individuals of Arabidopsis lyrata and Arabidopsis halleri. Molecular Ecology. 2009;18:4024–4048. doi: 10.1111/j.1365-294X.2009.04329.x. [DOI] [PubMed] [Google Scholar]
- Small RL, Cronn RC, Wendel JF. Use of nuclear genes for phylogeny reconstruction in plants. Australian Systematic Botany. 2004;17:145–170. [Google Scholar]
- Soltis DE, Soltis PS, Schemske DW, Hancock J, Thompson J, Husband B, Judd WS. Autopolyploidy and sympatric speciation in angiosperms: Have we grossly underestimated the number of species? Taxon. 2007;56:13–30. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Longevity, habitat, and release of genetic variability in the higher plants. Cold Spring Harbor Symposia on Quantitative Biology. 1958;23:365–378. doi: 10.1101/sqb.1958.023.01.035. [DOI] [PubMed] [Google Scholar]
- Stothard P. The Sequence Manipulation Suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques. 2000;28:1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- Stuessy TF. A new variety and new combination in Melampodium (Compositae-Heliantheae) Sida. 1969;3:348–349. [Google Scholar]
- Stuessy TF. Chromosome studies in Melampodium (Compositae, Heliantheae) Madroño. 1971a;20:365–372. [Google Scholar]
- Stuessy TF. Systematic relationships in the white-rayed species of Melampodium (Compositae) Brittonia. 1971b;23:177–190. [Google Scholar]
- Stuessy TF. Revision of the genus Melampodium (Compositae: Heliantheae) Rhodora. 1972;74:1–70. 161–219. [Google Scholar]
- Stuessy TF, Blöch C, Villaseñor JL, Rebernig CA, Weiss-Schneeweiss H. Phylogenetic analyses of DNA sequences with chromosomal and morphological data confirm and refine sectional and series classification within Melampodium (Asteraceae, Millerieae) Taxon. 2011;60:436–449. [Google Scholar]
- Stuessy TF, Weiss-Schneeweiss H, Keil DJ. Diploid and polyploid cytotype distribution in Melampodium cinereum and M. leucanthum (Asteraceae, Heliantheae) American Journal of Botany. 2004;91:889–898. doi: 10.3732/ajb.91.6.889. [DOI] [PubMed] [Google Scholar]
- Tate JA, Soltis DE, Soltis PS. Polyploidy in plants. In: Gregory TR, editor. The evolution of the genome, 371-426. Elsevier Academic Press; New York, New York, USA: 2005. [Google Scholar]
- Turner BL, King RM. Chromosome numbers in the Compositae. VIII. Mexican and Central American Species. The Southwestern Naturalist. 1964;9:27–39. [Google Scholar]
- Turpeinen T, Kulmala J, Nevo E. Genome size variation in Hordeum spontaneum populations. Genome. 1999;42:1094–1099. doi: 10.1139/g99-066. [DOI] [PubMed] [Google Scholar]
- Van Depeer Y, Dewachter R. TREECON for Windows: A software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Computer Applications in the Biosciences. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- Vitte C, Panaud O. LTR retrotransposons and flowering plant genome size: Emergence of the increase/decrease model. Cytogenetic and Genome Research. 2005;110:91–107. doi: 10.1159/000084941. [DOI] [PubMed] [Google Scholar]
- Weiss H, Maluszyńska J. Chromosomal rearrangement in autotetraploid plants of Arabidopsis thaliana. Hereditas. 2000;133:255–261. doi: 10.1111/j.1601-5223.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- Weiss-Schneeweiss H, Blöch C, Turner B, Villaseñor JL, Stuessy TF, Schneeweiss GM. The promiscuous and the chaste: Frequent allopolyploid speciation and its genomic consequences in American daisies (Melampodium sect. Melampodium; Asteraceae) Evolution. 2012;66:211–228. doi: 10.1111/j.1558-5646.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- Weiss-Schneeweiss H, Greilhuber J, Schneeweiss GM. Genome size evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. American Journal of Botany. 2006;93:148–156. doi: 10.3732/ajb.91.3.439. [DOI] [PubMed] [Google Scholar]
- Weiss-Schneeweiss H, Tremetsberger K, Schneeweiss GM, Parker JS, Stuessy TF. Karyotype diversification and evolution in diploid and polyploid South American Hypochaeris (Asteraceae) inferred from rDNA localization and genetic fingerprint data. Annals of Botany. 2008;101:909–918. doi: 10.1093/aob/mcn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss-Schneeweiss H, Villaseñor JL, Stuessy TF. Chromosome numbers, karyotypes, and evolution in Melampodium (Asteraceae) International Journal of Plant Sciences. 2009;170:1168–1182. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
- Wolfe KH. Yesterday’s polyploids and the mystery of diploidisation. Nature Reviews Genetics. 2001;2:333–341. doi: 10.1038/35072009. [DOI] [PubMed] [Google Scholar]
- Wu CI. The genic view of the process of speciation. Journal of Evolutionary Biology. 2001;14:851–865. [Google Scholar]