Abstract
Background and Aim:
Solid organ (spleen and liver) injuries are dreaded by both surgeons and anesthesiologists because of associated high morbidity and mortality. The purpose of this review is to describe our experience of critical care concerns in solid organ injury, which otherwise has been poorly addressed in the literature.
Materials and Methods:
Retrospective cohort of solid organ injury (spleen and liver) patients was done from January 2010 to December 2011 in tertiary level trauma Center.
Results:
Out of 624 abdominal trauma patients, a total of 212 patients (70%) were admitted in intensive care unit (ICU). Their ages ranged from 6 to 74 years (median 24 years). Nearly 89% patients in liver trauma and 84% patients in splenic trauma were male. Mechanism of injury was blunt abdominal trauma in 96% patients and the most common associated injury was chest trauma. Average injury severity score, sequential organ failure assessment, lactate on admission was 16.84, 4.34 and 3.42 mmol/L and that of dying patient were 29.70, 7.73 and 5.09 mmol/L, respectively. Overall mortality of ICU admitted solid organ injury was 15.55%. Major issues of concern in splenic injury were hemorrhagic shock, overwhelming post-splenectomy infection and post-splenectomy vaccination. Issues raised in liver injury are damage control surgery, deadly triad, thromboelastography guided transfusion protocols and hemostatic agents. Conclusions: A protocol-based and multidisciplinary approach in high dependency unit can significantly reduce morbidity and mortality in patients with solid organ injury.
Keywords: Critical care, deadly triad, hepatic injury, liver trauma, solid organ injury, splenic trauma
INTRODUCTION
Solid organs-liver, spleen, and kidneys are the organs susceptible to tear or laceration by trauma to the abdomen, back or flank regions and can cause extensive bleeding. Both surgeons and anesthesiologists dread these injuries. This study reviews our 2-year experience of liver and spleen injury and identifies the critical care concerns and risk factors for morbidity and mortality. Issues pertaining to renal injury were not considered as kidneys are retroperitoneal and form a part of the genitourinary system.
We undertook this study in view of the limited literature available regarding critical care concerns of solid organ injury that constitute a major cause of abdominal trauma and morbidity in bleeding trauma patients. Issues related to solid organ injury also need to be addressed in view of development of new diagnostic as well as treating modalities and ever increasing incidence of solid organ injuries as a part of polytrauma.
MATERIALS AND METHODS
Retrospective case record analysis of solid organ injury from January 2010 to December 2011 was done in our level 1 trauma Center. There are about 6500 annual admissions in our institute and a large number of trauma referrals. Our intensive care unit (ICU) is a 12-bedded multidisciplinary unit headed by consultant anesthesiologist. It provides advanced airway support, mechanical and hemodynamic support along with advanced monitoring. Following due clearance from departmentally instituted ethical committee, case record analysis was carried out.
Patients were distributed according to admission pattern (ward/ICU), type of solid organ injured (liver/spleen/both) and mode of management used (surgical, non-operative management (NOM), angioembolization) [Figure 1]. Pre-established routine procedure carried out by the hospital included assessment for demographic profile (age and sex), mechanism of injury, associated injuries, hemodynamic parameters (e.g., systolic blood pressure and pulse rate), lactate levels on admission, trauma scores at admission - injury severity score (ISS) and sequential organ failure assessment (SOFA) score, standard American Association for the Surgery of Trauma grades of splenic and liver injury,[1] estimated blood loss, blood transfusion requirement, and treatment offered. Patient with hemodynamic instability were observed for response to initial fluid resuscitation with warm crystalloids (1-2 L for adults and 20 ml/kg for pediatric patients) or colloids. Blood was transfused to transient responders and non-responders; failure of which resulted in starting inotropes and indicated definitive treatment. Decision on the mode of management used was based upon treatment modality algorithm [Figure 2]. It is a pre-established routine procedure, which is carried out at our institution.
Figure 1.
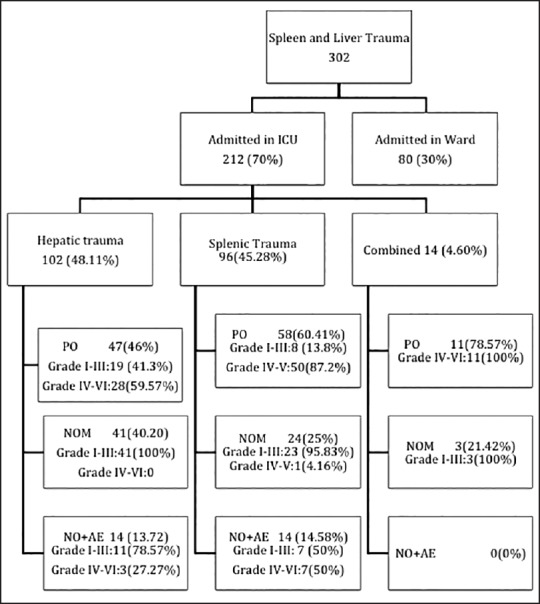
Distribution of solid organ injury patients according to admission pattern, type of organ injured and mode of management used. Intensive care unit. PO: post-operative; NOM: non-operative management; NOM + AE: non-operative management and angioembolization
Figure 2.
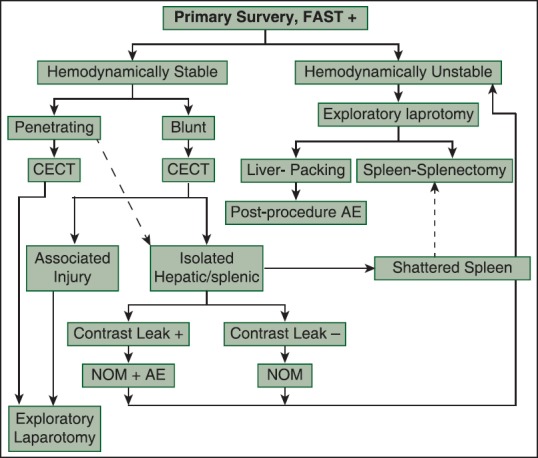
Treatment modality algorithm followed in solid organ injury. NOM: non-operative management; NOM + AE: non-operative management and angioembolization
Outcome variables were duration of ICU stay, average number of ventilator days, incidence of ventilator associated pneumonia (VAP), complications, causes of morbidity and mortality, incidence of complications (hemorrhagic shock, sepsis, coagulopathy, multiorgan dysfunction syndrome [MODS], abdominal compartment syndrome [ACS]), lactate, ISS, and SOFA of dying patients.
RESULTS
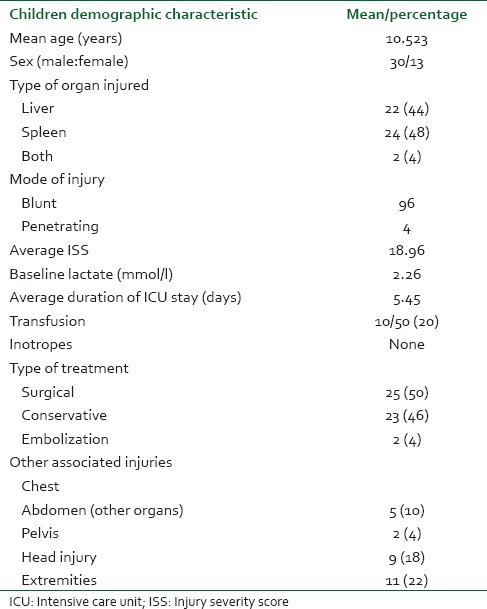
Out of 624 patients of abdominal trauma admitted, liver and spleen trauma constituted 48.40% (302). Distribution of patients having hepatic and splenic trauma according to age has been depicted in Figure 3a and b, respectively. Nearly 70% of such patients were admitted in ICU either post-operatively or for conservative management. During the study period, a total of 981 patients were admitted in ICU and solid organ injury constituted 212 (21.6%) of total admitted. Out of 212 patients admitted in ICU; 48.1% had liver trauma, 45.3% had splenic trauma and combined injury constituted the rest. Majority of patients admitted in ICU were post-operative patients - 46% hepatic trauma, 60.4% splenic trauma and 78.6% patients with combined hepatic and splenic trauma [Figure 1]. Evaluating demographic parameters, it was seen that their ages ranged from 6 to 74 years (median 24 years). Nearly 89% patients with liver trauma and 84% patients with splenic trauma were males. Mechanism of injury in 96% patients was blunt abdominal trauma, while the rest had penetrating injury. Most common associated injury was chest trauma - 69% in splenic and 51% in hepatic trauma [Table 1]. The relationship between issues of concern (hemorrhagic shock, hemodynamic instability, SOFA, lactate on admission, other organ involvement) has been summarized in Table 2 according to three different treatment plans (surgical, NOM and combined surgical and angioembolization). The ISS ranged from 16 to 62 with a mean of 16.8. Average SOFA on admission was 4.3 and baseline average lactate was 3.4 mmol/l. Out of 981 patients admitted in ICU, solid organ injury constituted 21.6% and average duration of ICU stay was 7.3 days. Average number of ventilator days was 3.93 and incidence of VAP was 9.3/1000 ventilator days. Average ISS, SOFA and lactate of expired patients were 29.7, 7.7 and 5.1 mmol/l, respectively. Complications included one case of ACS. Most common cause of mortality in splenic trauma was MODS while in hepatic trauma, it was hemorrhagic shock. Distribution of children (1-18 years) according to demographics and organs injured is depicted in Table 3. Overall mortality of ICU admitted patients with solid organ injury was 15.6% with hemorrhagic shock (45.5%) being the most common cause [Table 1].
Figure 3.
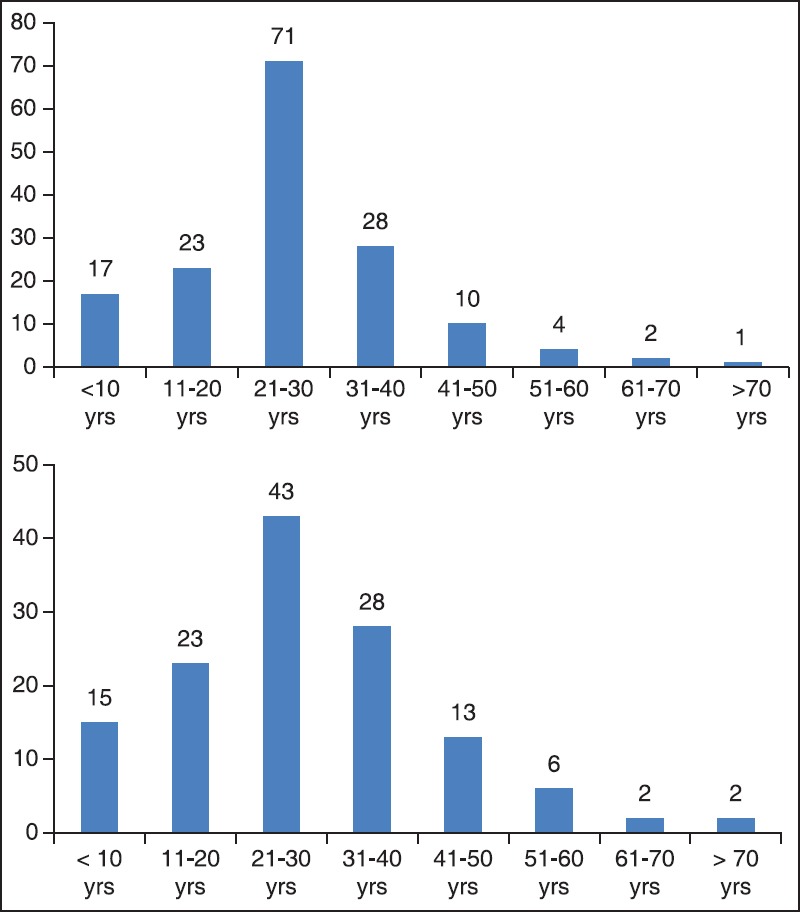
(a and b) Distribution of subjects according to age
Table 1.
Distribution of patients according to different demographic parameters and baseline variables
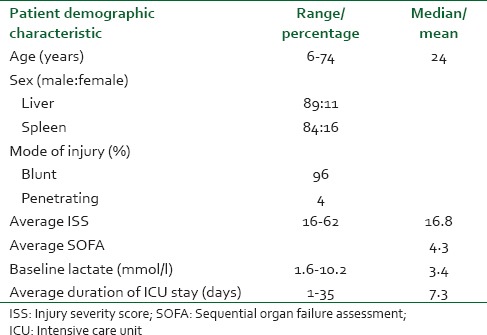
Table 2.
Distribution of patients in three different treatment modalities according to outcome influencing variables
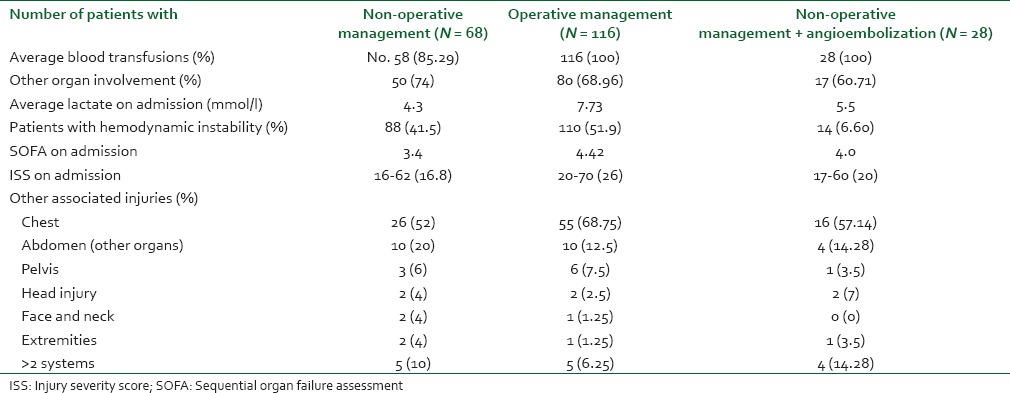
Table 3.
Distribution of children (1-18 years) according to demographics and organs injured
DISCUSSION
This review describes our experience of critical care concerns in solid organ injury, which otherwise has been poorly addressed in the literature. A patient can be admitted in ICU either post-operatively or for observation as a part of conservative management [Figure 1]. Management of a patient with solid organ injury is governed by hemodynamic status of the patient. A hemodynamically unstable patient or patient with penetrating injury undergoes surgical intervention. However, there has been a shift from operative to NOM in hemodynamically stable patients due to the advent of evidence guided approach and developments in radiology.
American Association for the Surgery of Trauma Organ Injury Scale grading system forms the basis for surgery, angioembolization or observation. Grading system for solid organ injuries is based on computed tomography (CT) appearance of hematoma, parenchyma and vascular injury.[1] It is graded as Grade (I to V) in splenic injury and Grade (I to VI) in hepatic injury. A patient with lower grade of injury is more suitable for NOM and patient with higher grade is associated with failure of NOM and more likely to undergo surgical intervention.[2] Observation should be done in a high dependency environment with well-developed critical care unit. This is in consensus with our data as seen in Figure 1, which indicates that the majority of blunt abdominal trauma patients admitted in ICU were post-operative patients with higher grade of injury and higher ISS, which usually require surgical intervention.
The number of blood transfusions required, the total of transfusion performed, reoperations, concomitant liver and splenic injuries, and SOFA, lactate, etc., measure the trauma impact [Table 4]. Multiplicity of solid organ injury results in higher failure rate of NOM as seen in our study 78.57% (11 0f 14) of patients with combined hepatic and splenic injury underwent surgical interventions.[3] Failure of NOM can be judged by lactate levels at admission; solid viscus grading score associated other injuries, a drop in the hematocrit in the 1st h after admission and need for transfusions.[4] Overall mortality rate for solid organ injury was 15.55% that was significantly less than international data in view of well-organized protocol based approach in our setup. A patient admitted to ICU with solid organ injury undergoes the following stepwise logistic approach [Figure 4]. Early initiation of thromboprophylaxis even in non-operative solid organ injury should be the goal as it does not increase failure rates or blood transfusion requirements.[5] Post-operative ICU considerations include monitoring for and prevention of ongoing bleeding and shock, coagulopathy, hypothermia, ACS, acute lung injury, deep venous thrombosis and pulmonary emboli, and sepsis.[6]
Table 4.
Distribution of solid organ injury patients according to the cause of mortality
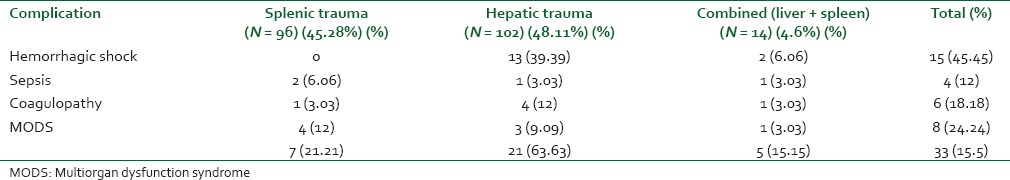
Figure 4.
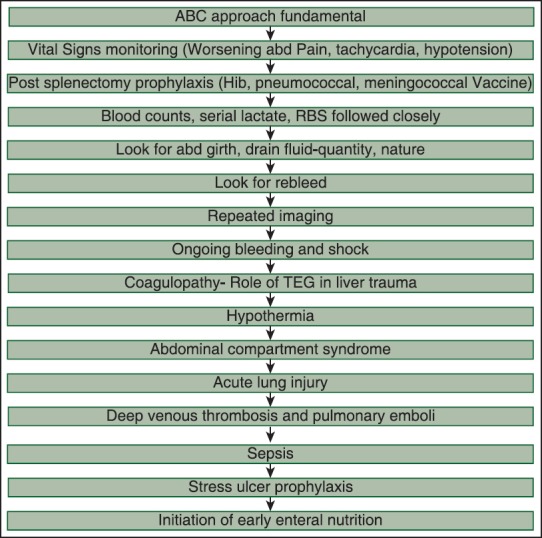
Protocol followed in our intensive care unit highlighting issues of concern in solid organ injury. Abd is abdominal, thromboelastography is thromboelastography
Concerns in splenic trauma
Missed splenic injury is the most common cause of preventable trauma deaths.[7] Spleen is very susceptible to injury and blood loss due to its position under left lower ribs, fragile capsule, multiple ligamentous insertions and high vascularity.[8] Blunt splenic injuries are most commonly (80-90%) due to road traffic accidents with most vulnerable age being young adults.[9] Penetrating injuries as a cause of splenic trauma is on the rise in civilized population.[10] In the present study, splenic injuries were most commonly found in second and third decade and affected more males than females that are similar to other studies.[11] Most patients sustained blunt injuries, which is similar to study results of Agbakwuru et al.[12] and in contrast to Edino[13] With the advent of coordinated trauma services, there is a shift from mandatory laparotomy toward “Selective Non-operative Management” and splenic conservation.[11,14] This change is because spleen is an integral part of reticuloendothelial system and combats encapsulated organisms such as (streptococcus pneumonia, Salmonella typhi, Neisseria meningitidis, Escherichia coli, Haemophilus influenzae, Streptococcus agalactiae, Klebsiella pneumoniae) and protozoal agents, which include the malarial parasite and babesia. Concept of splenic salvage is upcoming in view of its role in cellular and humoral immunity and danger of overwhelming post-splenectomy infection (OPSI) in patients with asplenia. When the spleen is no longer present; IgG and C3b are still bound to bacteria, but they cannot be removed from the blood circulation due to the loss of the splenic macrophages. Estimated incidence of OPSI is 0.23-0.42%/year, with a lifetime risk of 5%. OPSI carries a high mortality of 38-69%.[15] Triple vaccination with Pneumococcal, H. influenzae and meningococcal vaccines are highly recommended at least 2 weeks before an elective splenectomy that is not practical in splenectomy for trauma. Hence, all patients with asplenia should be vaccinated before discharge from the hospital and re-vaccination every 5-10 years.[15] At our institution, all patients undergoing splenectomy are administered triple vaccination post-operatively. These patients should be given additional antibiotic prophylaxis to compensate for occasional vaccination failures. In our patients, we did not see any patients with OPSI. Asplenic individuals should be issued with a form of medical alert, such as a card or a bracelet.
Concerns in hepatic trauma
Major hepatic trauma remains an important cause of exsanguation and death after solid organ injury.[16] It is most frequently injured abdominal organ in view of its large size, high vascularity, anterior placement, delicate glissons capsule and fragile parenchyma.[17] Its management includes resuscitation, angioembolization and operative strategies. CT remains the gold standard investigational tool for follow-up in hemodynamically stable patients, but has limitations for patients who are too unstable for transportation. In such patients raised serum alanine aminotransferase serves as a sensitive diagnostic marker for blunt liver injury and its levels may determine prognosis and guide the management.[18] Operative management remains the mainstay of treatment in high-grade injury and hemodynamic instability with ongoing hemorrhage.[19] Operative management of liver injuries, even in experienced hands, carries high mortality and morbidity.[20]
The deadly triad of hypothermia, coagulopathy and acidosis can exacerbate potentially preventable cause of mortality in hepatic trauma.[21] Mortality of trauma patients with the “triad of death” is 50-60%.[22] The “triad of death” is defined as an international normalized ratio of >1.5, serum pH of <7.2 and a core temperature of <35°C. Hypothermia causes increase in oxygen debt, increases lactic acid production further potentiating coagulopathy. Both consumptive and dilution coagulopathy occur because of loss of coagulation proteins and platelets during ongoing bleeding and movement of interstitial water into circulation to maintain intravascular volume. Acidosis is due to underlying hypo perfusion that can be corrected by active control of hemorrhage.
The concept of damage control resuscitation addresses early coagulopathy and advocates minimizing crystalloid use in patients who are predicted to require a massive transfusion. Early transfusion of plasma and platelets along with the first few units of red blood cells is encouraged. Thromboelastography (TEG) based transfusion protocols are helpful in managing coagulopathy in such extensively bleeding hepatic trauma patients.[23,24] We utilized TEG guided resuscitation with blood products and found it as accurate and rapid tool for resuscitation. Trauma exsanguinations protocols allow effective use of plasma and platelets pre-operatively, intra-operatively and post-operatively resulting in a significant reduction of morbidity and mortality.[25,26]
Hemostatic adjuncts like recombinant factor VIIa (rFVIIa) that limit transfusion requirements and normalize coagulation process can be considered as a treatment option. However, in actively bleeding liver patients concerns of its off label use, usefulness, optimal timing, dose, potential to induce serious adverse effects like thromboembolic events, inability to monitor hemostatic potential and expensive nature makes its use controversial.[27,28,29,30] The CONTROL trial provided some evidence on the efficacy and safety of rFVIIa post trauma showing reduction of blood transfusion, but there was no overall outcome benefit.[31] It has been used as a late rescue therapy after other methods to control massive bleeding have been exhausted. Actively bleeding liver trauma patients might have already developed the lethal triad when rFVIIa administration may not be beneficial. It is because acidosis severely impairs the activity of rFVIIa by reducing the activity of proteases in the coagulation system limiting its use in exsanguinating trauma patients.[32]
Several Food and Drug Administration-approved hemostatic drugs (aprotinin, desmopressin, tranexamic acid, epsilon-aminocaproic acid) have not been found to effect blood loss, survival time or mortality rate in uncontrolled hemorrhage due to liver injury in rat models.[33] Hence, we did not use rFVIIa and above mentioned hemostatic agents in our patients.
A patient undergoing conservative treatment should be especially watched for ACS while a post-operative patient should be watched for peri-operative sepsis. Early control of an identifiable source of infection provides the best results with sepsis following liver trauma.[34] Septic complications may go unnoticed because many of the signs of sepsis mimic features of uncomplicated liver injuries. A sudden increase in serum bilirubin level or a temperature spike in a patient with progressively falling bilirubin levels and total leucocyte count (TLC) warrants need to investigate for a septic focus in the abdomen. Therefore, intravenous antibiotics are frequently administered in the immediate peri-operative period. After major hepatic resection, glucose infusions are required to treat hypoglycemia, while plasma and albumin infusions are used to treat hypoalbuminemia until the improvement of nutritional status of the patient. Coagulation defects are treated with vitamin K supplements and TEG guided corrections. Most of these patients also develop transient jaundice that lasts from several days to several weeks. Serial hemoglobin, TLC, liver function test and CT abdomen are necessary to know the outcome of the patient. Another complication that may arise in hepatic injury is hemobilia, which requires immediate investigation in the form of hepatic angiography and CT or ultrasound.
Other ICU considerations and complications
ACS
ACS is defined as a sustained intraabdominal pressure of more than 20 mmHg, with or without an abdominal partial pressure of 60 mmHg that is associated with new organ dysfunction or failure.[35] Initiation of goal-directed resuscitation strategies consists of both non-operative and surgical measures. Non-operative measures include optimizing organ perfusion and fluid volume, reducing abdominal wall tension, and evacuating intraluminal contents as well as space-occupying lesions in the abdominal cavity. Surgical decompression is reserved for failure of non-operative measures.[36] We had only one case of ACS which is in sharp contrast with the current literature where up to 20-30% patients have ACS. Such a low incidence of ACS is because in our institution mesh laprostomy is being widely practiced.
Sepsis
Post-operative sepsis can occur from peritoneal soiling, prolonged ventilation, intravascular lines and catheter related infections. Following a protocol based approach and adhering to control of disease (Centers for Disease Control) guidelines can limit such complications.
Other causes of morbidity include missed injuries, anastomotic breakdown with peritonitis, wound infection or dehiscence, bowel ischemia or obstruction, and abscess or fistula formation, which all should be aware of.
One of the drawbacks of the study was that we did not address renal injuries which certainly contribute to mortality, but addressing it would have resulted in complicated results. Another drawback is use of SOFA in all the patients which is however validated only in adult patients and not children. However, there were only two pediatric patients; hence, the severity and outcome analysis of adult and pediatric patients performed in a single sample did not make the results unreliable.
CONCLUSION
Solid organ injury constitutes a major cause of preventable morbidity and mortality in abdominal trauma patients. It is responsible for major ICU resource utilization and consumes a significant amount of health budget. Rapid assessment and treatment together with the protocol based approach can salvage such patients
ACKNOWLEDGMENT
Mr. Naresh Kumar for his technical support and help.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: Spleen and liver (1994 revision) J Trauma. 1995;38:323–4. doi: 10.1097/00005373-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Notash AY, Amoli HA, Nikandish A, Kenari AY, Jahangiri F, Khashayar P. Non-operative management in blunt splenic trauma. Emerg Med J. 2008;25:210–2. doi: 10.1136/emj.2007.054684. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra AK, Latifi R, Fabian TC, Ivatury RR, Dhage S, Bee TK, et al. Multiplicity of solid organ injury: Influence on management and outcomes after blunt abdominal trauma. J Trauma. 2003;54:925–9. doi: 10.1097/01.TA.0000066182.67385.86. [DOI] [PubMed] [Google Scholar]
- 4.Yanar H, Ertekin C, Taviloglu K, Kabay B, Bakkaloglu H, Guloglu R. Nonoperative treatment of multiple intra-abdominal solid organ injury after blunt abdominal trauma. J Trauma. 2008;64:943–8. doi: 10.1097/TA.0b013e3180342023. [DOI] [PubMed] [Google Scholar]
- 5.Eberle BM, Schnüriger B, Inaba K, Cestero R, Kobayashi L, Barmparas G, et al. Thromboembolic prophylaxis with low-molecular-weight heparin in patients with blunt solid abdominal organ injuries undergoing nonoperative management: Current practice and outcomes. J Trauma. 2011;70:141–6. doi: 10.1097/TA.0b013e3182032f45. [DOI] [PubMed] [Google Scholar]
- 6.Smith CE. Trauma anesthesia. In: Wilson WC, editor. Anesthesia Considerations for Abdominal Trauma. 1st ed. New York: Cambridge University Press, New York; 2008. p. 167. [Google Scholar]
- 7.Küçük ON, Gültekin SS, Aras G. Evaluation of a traumatic spleen laceration with spontaneous regression by selective spleen scintigraphy. Clin Nucl Med. 2007;32:141–4. doi: 10.1097/01.rlu.0000251950.88850.79. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield CG, Shiwani MH, Garner JP. The modern management of splenic injury: A model for coordinated trauma services. J Pak Med Assoc. 2009;59:503–4. [PubMed] [Google Scholar]
- 9.Khan JS, Iqbal N, Gardezi JR. Pattern of visceral injuries following blunt abdominal trauma in motor vehicular accidents. J Coll Physicians Surg Pak. 2006;16:645–7. doi: 10.2006/JCPSP.645647. [DOI] [PubMed] [Google Scholar]
- 10.Chalya PL, Mabula JB, Giiti G, Chandika AB, Dass RM, McHembe MD, et al. Splenic injuries at Bugando Medical Centre in NorthWestern Tanzania: A tertiary hospital experience. BMC Res Notes. 2012;5:59. doi: 10.1186/1756-0500-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan KK, Chiu MT, Vijayan A. Management of isolated splenic injuries after blunt trauma: An institution's experience over 6 years. Med J Malaysia. 2010;65:304–6. [PubMed] [Google Scholar]
- 12.Agbakwuru EA, Akinkuolie AA, Sowande OA, Adisa OA, Alatise OI, Onakpoya UU, et al. Splenic injuries in a semi urban hospital in Nigeria. East Cent Afr J Surg. 2008;13:95–100. [Google Scholar]
- 13.Edino ST. Pattern of abdominal injuries in Aminu Kano Teaching Hospital, Kano. Niger Postgrad Med J. 2003;10:56–9. [PubMed] [Google Scholar]
- 14.Haan JM, Bochicchio GV, Kramer N, Scalea TM. Nonoperative management of blunt splenic injury: A 5-year experience. J Trauma. 2005;58:492–8. doi: 10.1097/01.ta.0000154575.49388.74. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7:657–60. doi: 10.1046/j.1198-743x.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 16.Beal SL. Fatal hepatic hemorrhage: An unresolved problem in the management of complex liver injuries. J Trauma. 1990;30:163–9. [PubMed] [Google Scholar]
- 17.Badger SA, Barclay R, Campbell P, Mole DJ, Diamond T. Management of liver trauma. World J Surg. 2009;33:2522–37. doi: 10.1007/s00268-009-0215-z. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava AR, Kumar S, Agarwal GG, Ranjan P. Blunt abdominal injury: Serum ALT-A marker of liver injury and a guide to assessment of its severity. Injury. 2007;38:1069–74. doi: 10.1016/j.injury.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Trunkey DD. Hepatic trauma: Contemporary management. Surg Clin North Am. 2004;84:437–50. doi: 10.1016/S0039-6109(03)00228-7. [DOI] [PubMed] [Google Scholar]
- 20.Gaarder C, Naess PA, Eken T, Skaga NO, Pillgram-Larsen J, Klow NE, et al. Liver injuries – Improved results with a formal protocol including angiography. Injury. 2007;38:1075–83. doi: 10.1016/j.injury.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–9. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 22.Mitra B, Cameron PA, Parr MJ, Phillips L. Recombinant factor VIIa in trauma patients with the ‘triad of death’. Injury. 2012;43:1409–14. doi: 10.1016/j.injury.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 24.Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100:792–7. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 25.Zaydfudim V, Dutton WD, Feurer ID, Au BK, Pinson CW, Cotton BA. Exsanguination protocol improves survival after major hepatic trauma. Injury. 2010;41:30–4. doi: 10.1016/j.injury.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr, et al. Damage control hematology: The impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–82. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 27.Duchesne JC, Mathew KA, Marr AB, Pinsky MR, Barbeau JM, McSwain NE. Current evidence based guidelines for factor VIIa use in trauma: The good, the bad, and the ugly. Am Surg. 2008;74:1159–65. [PubMed] [Google Scholar]
- 28.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: The need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–6. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 29.Stein DM, Dutton RP, Hess JR, Scalea TM. Low-dose recombinant factor VIIa for trauma patients with coagulopathy. Injury. 2008;39:1054–61. doi: 10.1016/j.injury.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Knudson MM, Cohen MJ, Reidy R, Jaeger S, Bacchetti P, Jin C, et al. Trauma, transfusions, and use of recombinant factor VIIa: A multicenter case registry report of 380 patients from the Western Trauma Association. J Am Coll Surg. 2011;212:87–95. doi: 10.1016/j.jamcollsurg.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial: Efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 32.Meng ZH, Wolberg AS, Monroe DM, 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: Implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–91. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- 33.Ryan KL, Cortez DS, Dick EJ, Jr, Pusateri AE. Efficacy of FDA-approved hemostatic drugs to improve survival and reduce bleeding in rat models of uncontrolled hemorrhage. Resuscitation. 2006;70:133–44. doi: 10.1016/j.resuscitation.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Bender JS, Geller ER, Wilson RF. Intra-abdominal sepsis following liver trauma. J Trauma. 1989;29:1140–4. doi: 10.1097/00005373-198908000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–32. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher JJ. Intra-abdominal hypertension: Detecting and managing a lethal complication of critical illness. AACN Adv Crit Care. 2010;21:205–19. doi: 10.1097/NCI.0b013e3181d94fd5. [DOI] [PubMed] [Google Scholar]


