Abstract
Context:
Many techniques are used for sedation of colonoscopies. Patient-controlled sedation (PCS) is utilizing many drugs or drug combinations.
Aims:
The aim of this study is to compare the safety and feasibility of propofol/remifentanil versus propofol/alfentanil given to sedate patients undergoing outpatient colonoscopies through a patient-controlled technique.
Settings and Design:
Controlled randomized and double-blind study.
Materials and Methods:
A total of 80 patients were randomly divided into two groups; PA group received a combination of propofol/alfentanil and PR group received propofol/remifentanil combination. Patients were monitored for heart rate (HR), blood pressure (BP), oxygen saturation, and Ramsay sedation scale (RSS). Times of the following events were recorded; initiation of sedation, insertion and removal of the colonoscope, recovery and discharge. Five intervals were calculated; time to sedation, procedure time, postprocedure time, procedure room time, and postanesthesia care unit (PACU) time. Endoscopist and patient satisfaction scores were obtained.
Statistical Analysis Used:
Unpaired Student's t-test was used to compare between the two groups. Paired Student's t-test was used to compare baseline readings with readings after 30 min of sedation in the same group when needed.
Results:
Both groups showed slowing of the HR and decrease in mean arterial BP. HR and mean arterial BP were significantly lower 5 and 10 min after initiation of sedation in PR group when compared with PA group. Both HR and mean arterial BP returned to presedation readings 30 min after initiation of sedation in PR group but not in PA group. No differences between the two groups concerning oxygen saturation, RSS, endoscopist and patient satisfaction scores. Postprocedure and PACU times were significantly prolonged in PA group.
Conclusion:
PCS with either remifentanil/propofol or alfentanil/propofol for patients undergoing outpatient colonoscopy is safe and feasible. Remifentanil/proofol has more beneficial advantages in this setting secondary to its more rapid clearance.
Keywords: Alfentanil, colonoscopy, patient-controlled sedation, remifentanil
INTRODUCTION
Colorectal cancer is the third most common cancer worldwide constituting about 9% of all cancer incidence.[1] Colonoscopy is a sensitive screening tool and has the potential to prevent about 65% of colorectal cancer cases.[2] In 2002, it was estimated that about 14.2 million colonoscopies were performed in US.[3]
In a study conducted over 1187 patients subjected to colonoscopies, although 94% of patients were sedated; however, 23% of patients judged that colonoscopy was more uncomfortable than expected with 10% reported it as very uncomfortable.[4] Many sedation techniques were utilized during colonoscopies including intermittent bolus administration,[5,6] intravenous (IV) continuous infusion[7] and patient-controlled administration. For patient-controlled sedation (PCS) propofol,[8,9] midazolam/fentanyl,[10] propofol/alfentanil[6,11,12,13,14] and propofol/remifentanil[10] were all tried with different levels of success.
Adding propofol to alfentanil increases sedation, potentiates analgesia, and decreases postoperative nausea and vomiting, with no increase in respiratory depression.[15] Alfentanil has a relatively long half-life compared with propofol. Remifentanil has a shorter half-life, comparative duration of effect to propofol, and lacks residual postoperative sedation.[16] These properties may render remifentanil more suitable than alfentanil when given with propofol in settings of outpatient colonoscopy.
This study was aimed to compare the safety and feasibility of a combination of propofol/remifentanil with that of propofol/alfentanil for patients undergoing outpatient colonoscopy utilizing PCS.
MATERIALS AND METHODS
After obtaining approval of Research Ethics Committee, this controlled randomized double-blinded study was conducted. Inclusion criteria included patients aged 18-65 years, American Society of Anesthesiologists classification I, II or III and scheduled for elective outpatient colonoscopy. Written consents were obtained from all patients. Exclusion criteria included known allergy to study drugs, neuropsychiatric disease, advanced heart or respiratory disease, alcohol or drug abuse, gastrointestinal bleeding, pregnancy, patients with history of colonic surgery and patients unable to use PCS.
Eighty patients were randomly assigned to one of two groups; PA group received a combination of propofol/alfentanil and PR group received propofol/remifentanil combination for PCS during colonoscopy. Patients were randomized by sequentially numbered envelopes. The anesthesiologist, nurse and endoscopist were all blind to the drug mixed with propofol. All procedures were conducted by the same endoscopist. The mixture was prepared by the hospital pharmacist just before the start of the procedure. The mixture was prepared in a 50-ml syringe that is connected to a separate IV cannula with primed IV tubing. Each syringe contained 48 ml of propofol 1% plus 2 ml of either 1 mg of alfentanil in PA group or 0.5 mg of remifentanil in PR group. A patient-controlled analgesia pump (Perfusor fmTM, B. Braun, Germany) was used to deliver PCS. The machine was adjusted to give no background infusion, bolus of 0.75 ml over 10 s with no lockout time. Patients were instructed to push the demand button 4 times before the colonoscope is inserted then whenever they feel any discomfort.
All patients received 3 L/min of oxygen via nasal cannula. The anesthesiologist intervened if the patient experienced bradycardia (heart rate [HR] <50/min), hypotension (systolic blood pressure [BP] <90 mmHg), desaturation (oxygen saturation [SpO2] <90%) or the patient is not responsive to tactile stimulation. Patients were monitored by a trained nurse that collected data just before the start of sedation (time 0), 1 min after the start of sedation and then every 5 min for HR, mean arterial BP, SpO2 and level of sedation using Ramsay sedation scale (RSS) [Table 1].
Table 1.
Ramsay sedation scale

Times of the following events were recorded; initiation of sedation (time of the patient pushing demand button 4 times), insertion of colonoscope (judged when RSS reach 3-4), removal of colonoscope, recovery time (RSS become 2, HR, BP and oxygen saturation are within 20% of basal readings), and discharge time (patient reaches Aldrete score [Table 2] of 9 or more). From these five events, five intervals were calculated; time to sedation (from initiation of sedation to insertion of colonoscope), procedure time (from insertion to removal of colonoscope), postprocedure time (from removal of colonoscope to discharge time), procedure room time (from initiation of sedation to recovery time), and postanesthesia care unit (PACU) time (from recovery time to discharge time).
Table 2.
Aldrete score
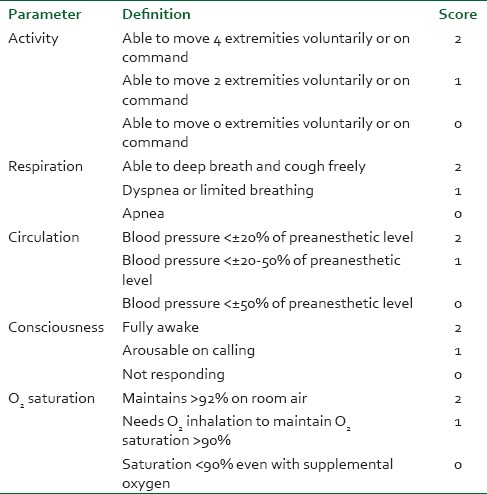
Immediately after the procedure ended, the endoscopist was asked to report his satisfaction in a scale from 1 to 10 (1: Not satisfied and 10: Very satisfied). Just before discharge, patients were asked to report their satisfaction clearly concerning only sedation and analgesia in a scale from 1 to 10 (1: Not satisfied and 10: Very satisfied).
Data were presented as mean ± standard deviation. Unpaired Student's t-test was used for comparison between the two groups. Paired Student's t-test was used to compare baseline readings (time 0) to readings after 30 min from initiation of sedation within the same group for both HR and mean arterial BP. Statistical analysis was performed using SPSS statistical package (SPSS Inc., Chicago, Il, USA) version 15 for windows. P < 0.05 was considered to be statistically significant.
RESULTS
There were no differences between groups concerning demographic data and number of patients subjected to polypectomies [Table 3].
Table 3.
Demographic data and number of patients subjected to polypectomies in the two groups

Figures 1 and 2 show changes in HR and mean arterial pressure (MAP) consecutively. Both groups showed slowing in HR and decrease in MAP after the start of sedation. HR and MAP were significantly lower in PR group when compared with PA group 5 and 10 min after the start of sedation. HR and MAP returned to presedation values 30 min after the start of sedation in PR group while stayed at significantly lower values in PA group when compared with presedation values. Figure 3 shows changes in arterial oxygen saturation. There were no statistically significant changes between groups at anytime. Eight patients in PA group and six in PR group suffered desaturation but no one needed bag-mask ventilation or endotracheal intubation. Figure 4 shows changes in RSS. There were no statistically significant changes between groups at anytime.
Figure 1.
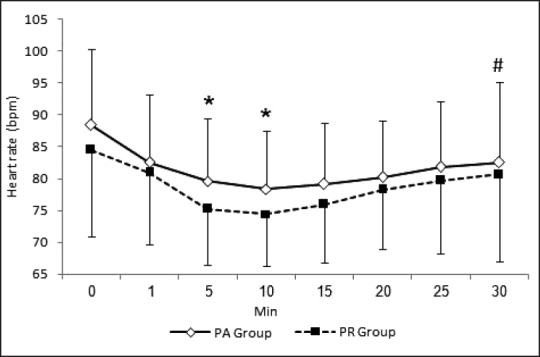
Heart rate changes during procedure. *P < 0.05, significant inter-group differences. #P < 0.05, significant changes compared with time 0 reading in the same group
Figure 2.
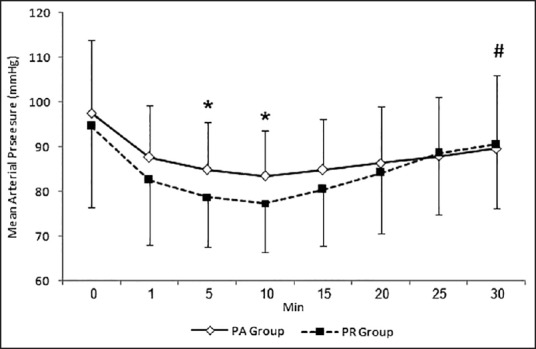
Mean arterial pressure changes during procedure. *P < 0.05, significant inter-group differences. #P < 0.05, significant changes compared with time 0 reading in the same group
Figure 3.
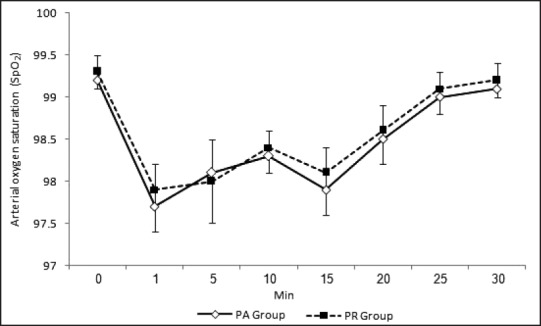
Arterial oxygen saturation changes during procedure
Figure 4.
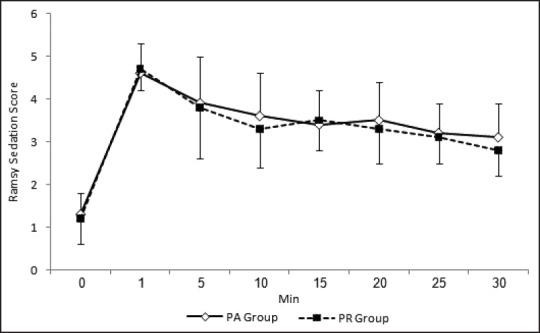
Ramsay sedation scale changes during procedure
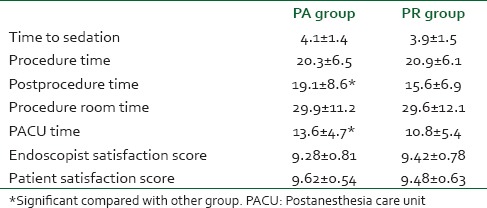
Table 4 shows different times calculated during the study. There were statistically significant differences in postprocedure and PACU times favoring shorter times in PR group. Table 4 also contains satisfaction scores of the endoscopist and patients. Both showed no differences.
Table 4.
Times calculated during the study and satisfaction scores of the endoscopist and patients
DISCUSSION
Colonoscopy is considered the most accurate and the most commonly used procedure to image the large bowel, especially when colorectal cancer screening and surveillance of adenomas are considered.[17,18,19] Propofol is widely used for sedation during the performance of different procedures. When propofol is used as a sole agent, large doses may be needed to tolerate some invasive procedures. Myocardial depression and peripheral vasodilation may follow. With higher doses, hypotension, respiratory depression, and decreased upper airway protective reflex activity can be life-threatening.[20] Moreover, propofol lacks analgesic properties.
Adding opioids to propofol has many advantages. It decreases pain and discomfort during the performance of the procedure. It also decreases pain arising from propofol injection. Propofol in its turns decreases nausea and vomiting due to opioids. Moreover, total amounts of both drugs decrease and recovery is more rapid.[21]
Many opioids were added to propofol with different degrees of success. Fentanyl,[20] alfentanil,[11,12,20] sufentanil,[22,23] and remifentanil[10,24] were all used. Alfentanil is less lipid soluble than fentanyl. This allows less tissue accumulation and therefore greater binding of plasma concentrations to opioid receptors and more rapid onset of effects.[25,26] Remifentanil may have advantages over alfentanil because of its shorter half-life, comparative duration of effect to propofol, and lack of residual postoperative sedation.[16]
Egan et al. stated that alfentanil and remifentanil have many similarities. Pharmacokinetically, the two drugs are similar in terms of steady-state distribution volume but remifentanil's central clearance is substantially greater (2.9 vs. 0.36 L/min). Terminal half-life for remifentanil in the same study was 35.1 min compared with 94.5 min for alfentanil resulting in faster decline of blood concentration after discontinuation of an infusion. Pharmacodynamically, the drugs are similar in terms of the time required for equilibration between blood and the effect-site concentrations.[27] This similarity explains the overall few statistically significant differences between the two groups in the present study. Still, differences in pharmacokinetics and pharmacodynamics between the two drugs can explain most of results in the present study.
When effects of a drug could be terminated by the end of the procedure, this drug will be very beneficial in states of outpatient settings. This goes very well with remifentanil when concerning performance of colonoscopies. Known that colonoscopy is usually not inducing postprocedural pain except if complicated, makes remifentanil a more suitable choice when mixed with propofol for PCS of cases of outpatient colonoscopy.
In the present study, both mixtures seemed safe and feasible. It is well-known that both alfentanil and remifentanil induce bradycardia and hypotension.[28] These changes were evident in both groups with significant slowing of the HR and lowering of BP with PR group when compared with PA group. However, no medications were used to treat bradycardia or hypotension. Although lockout was zero and bolus injection time was only 10 s which might allow easy overdosing, respiratory depression did not happen. Oxygen desaturation was recorded is the present study in 14 cases. Both groups were almost equally affected. Patients did not need positive pressure ventilation reflecting the feasibility of sedation with these drugs. This was reflected on the endoscopist satisfaction for both groups. Other factors affected the endoscopist satisfaction were a short time needed for sedation and considerable procedure room time. Patient satisfaction was high in both groups. Administration of drugs through PCS is an important factor to increase patient satisfaction.[29] It allows the patient to self-administer the exact amount of anesthetics required to treat varying degrees of pain and discomfort.[21]
CONCLUSION
Patient-controlled sedation with either propofol/remifentanil or propofol/alfentanil is safe and feasible technique of sedation in settings of outpatient colonoscopy. Propofol/remifentanil has more beneficial advantages over propofol/alfentanil due to more rapid clearance of remifentanil compared with alfentanil.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Boyle P, Langman JS. ABC of colorectal cancer: Epidemiology. BMJ. 2000;321:805–8. doi: 10.1136/bmj.321.7264.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner H, Chang-Claude J, Seiler CM, Stürmer T, Hoffmeister M. Potential for colorectal cancer prevention of sigmoidoscopy versus colonoscopy: Population-based case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:494–9. doi: 10.1158/1055-9965.EPI-06-0460. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LC, Richards TB, Shapiro JA, Nadel MR, Manninen DL, Given LS, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge V, Sint Nicolaas J, Lalor EA, Wong CK, Walters B, Bala A, et al. A prospective audit of patient experiences in colonoscopy using the Global Rating Scale: A cohort of 1,187 patients. Can J Gastroenterol. 2010;24:607–13. doi: 10.1155/2010/724924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinar K, Yakut M, Ozden A. Sedation with midazolam versus midazolam plus meperidine for routine colonoscopy: A prospective, randomized, controlled study. Turk J Gastroenterol. 2009;20:271–5. doi: 10.4318/tjg.2009.0025. [DOI] [PubMed] [Google Scholar]
- 6.Bright E, Roseveare C, Dalgleish D, Kimble J, Elliott J, Shepherd H. Patient-controlled sedation for colonoscopy: A randomized trial comparing patient-controlled administration of propofol and alfentanil with physician-administered midazolam and pethidine. Endoscopy. 2003;35:683–7. doi: 10.1055/s-2003-41519. [DOI] [PubMed] [Google Scholar]
- 7.Martínez JF, Aparicio JR, Compañy L, Ruiz F, Gómez-Escolar L, Mozas I, et al. Safety of continuous propofol sedation for endoscopic procedures in elderly patients. Rev Esp Enferm Dig. 2011;103:76–82. doi: 10.4321/s1130-01082011000200005. [DOI] [PubMed] [Google Scholar]
- 8.Heiman DR, Tolliver BA, Weis FR, O’Brien BL, DiPalma JA. Patient-controlled anesthesia for colonoscopy using propofol: Results of a pilot study. South Med J. 1998;91:560–4. doi: 10.1097/00007611-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ng JM, Kong CF, Nyam D. Patient-controlled sedation with propofol for colonoscopy. Gastrointest Endosc. 2001;54:8–13. doi: 10.1067/mge.2001.116110. [DOI] [PubMed] [Google Scholar]
- 10.Mandel JE, Tanner JW, Lichtenstein GR, Metz DC, Katzka DA, Ginsberg GG, et al. A randomized, controlled, double-blind trial of patient-controlled sedation with propofol/remifentanil versus midazolam/fentanyl for colonoscopy. Anesth Analg. 2008;106:434–9. doi: 10.1213/01.ane.0000297300.33441.32. [DOI] [PubMed] [Google Scholar]
- 11.Roseveare C, Seavell C, Patel P, Criswell J, Kimble J, Jones C, et al. Patient-controlled sedation and analgesia, using propofol and alfentanil, during colonoscopy: A prospective randomized controlled trial. Endoscopy. 1998;30:768–73. doi: 10.1055/s-2007-1001419. [DOI] [PubMed] [Google Scholar]
- 12.Roseveare C, Seavell C, Patel P, Criswell J, Shepherd H. Patient-controlled sedation with propofol and alfentanil during colonoscopy: A pilot study. Endoscopy. 1998;30:482–3. doi: 10.1055/s-2007-1001312. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Li AC, Ko CW, Chu DW, Chan KC, Poon CM, et al. Use of a variable-stiffness colonoscope decreases the dose of patient-controlled sedation during colonoscopy: A randomized comparison of 3 colonoscopes. Gastrointest Endosc. 2007;65:424–9. doi: 10.1016/j.gie.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Lee DW, Chan AC, Wong SK, Li AC, Sze TS, Chung SC. The safety, feasibility, and acceptability of patient-controlled sedation for colonoscopy: Prospective study. Hong Kong Med J. 2004;10:84–8. [PubMed] [Google Scholar]
- 15.Pavlin DJ, Coda B, Shen DD, Tschanz J, Nguyen Q, Schaffer R, et al. Effects of combining propofol and alfentanil on ventilation, analgesia, sedation, and emesis in human volunteers. Anesthesiology. 1996;84:23–37. doi: 10.1097/00000542-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Sá Rêgo MM, Inagaki Y, White PF. Remifentanil administration during monitored anesthesia care: Are intermittent boluses an effective alternative to a continuous infusion? Anesth Analg. 1999;88:518–22. doi: 10.1097/00000539-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 18.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2:72–7. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: Results from a national consortium. Gastrointest Endosc. 2005;62:875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Türk HŞ, Aydoğmuş M, Ünsal O, Köksal HM, Açik ME, Oba S. Sedation-analgesia in elective colonoscopy: Propofol-fentanyl versus propofol-alfentanil. Rev Bras Anestesiol. 2013;63:352–7. doi: 10.1016/j.bjane.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Joo HS, Perks WJ, Kataoka MT, Errett L, Pace K, Honey RJ. A comparison of patient-controlled sedation using either remifentanil or remifentanil-propofol for shock wave lithotripsy. Anesth Analg. 2001;93:1227–32. doi: 10.1097/00000539-200111000-00037. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Wang M, Xiang H, Lin X, Cao D, Ye L. Prediction of effect-site concentration of sufentanil by dose-response target controlled infusion of sufentanil and propofol for analgesic and sedation maintenance in burn dressing changes. Burns. 2014;40:455–9. doi: 10.1016/j.burns.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Akarsu Ayazoğlu T, Polat E, Bolat C, Yasar NF, Duman U, Akbulut S, et al. Comparison of propofol-based sedation regimens administered during colonoscopy. Rev Med Chil. 2013;141:477–85. doi: 10.4067/S0034-98872013000400009. [DOI] [PubMed] [Google Scholar]
- 24.Joo JD, In JH, Kim DW, Jung HS, Kang JH, Yeom JH, et al. The comparison of sedation quality, side effect and recovery profiles on different dosage of remifentanil patient-controlled sedation during breast biopsy surgery. Korean J Anesthesiol. 2012;63:431–5. doi: 10.4097/kjae.2012.63.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiPalma JA, Herrera JL, Weis FR, Dark-Mezick DL, Brown RS. Alfentanil for conscious sedation during colonoscopy. South Med J. 1995;88:630–4. doi: 10.1097/00007611-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Jabbour-Khoury SI, Dabbous AS, Rizk LB, Abou Jalad NM, Bartelmaos TE, El-Khatib MF, et al. A combination of alfentanil-lidocaine-propofol provides better intubating conditions than fentanyl-lidocaine-propofol in the absence of muscle relaxants. Can J Anaesth. 2003;50:116–20. doi: 10.1007/BF03017841. [DOI] [PubMed] [Google Scholar]
- 27.Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: Comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84:821–33. doi: 10.1097/00000542-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson LB, Viby-Mogensen J, Møller J, Fonsmark L, Østergaard D. Remifentanil vs. alfentanil for direct laryngoscopy: A randomized study comparing two total intravenous anaesthesia techniques. TIVA for direct laryngoscopy. Acta Anaesthesiol Belg. 2002;53:213–9. [PubMed] [Google Scholar]
- 29.Osborne GA, Rudkin GE, Curtis NJ, Vickers D, Craker AJ. Intra-operative patient-controlled sedation. Comparison of patient-controlled propofol with anaesthetist-administered midazolam and fentanyl. Anaesthesia. 1991;46:553–6. doi: 10.1111/j.1365-2044.1991.tb09654.x. [DOI] [PubMed] [Google Scholar]


