Abstract
Background:
Significant increase in serum triglyceride (ST) concentration have been described in adult population after prolonged administration of propofol formulation containing long chain triglyceride (LCT). Though, medium chain triglyceride-LCT (MCT-LCT) propofol when compared with LCT propofol for long-term sedation in adults resulted in identical triglyceride levels, the elimination of triglyceride was faster in patients administered MCT-LCT propofol.
Materials and Methods:
A total of 40 children were randomized into two groups of 20 each; Group I were induced with 1% LCT propofol (3 mg/kg) and Group II with 1% medium and LCT propofol and maintained with descalating dose of 20.15 and 10 mg/kg/h at 10 min intervals. Blood samples for ST concentration were obtained before induction of anesthesia, at the end of propofol infusion and 4 h after terminating propofol infusion.
Results:
ST levels were raised significantly above the basal values in both the groups but the rise was significantly higher in Group I (P < 0.05). Four hours after stopping propofol infusion the triglyceride levels were similar to the basal values in Group II, whereas in Group I the values were significantly greater than the baseline (P < 0.05) as well as those of Group II (P < 0.05). No clinically significant adverse effect of hypertriglyceridemia was observed.
Conclusion:
Even short term anesthesia with LCT and MCT-LCT propofol (1%) leads to elevated ST levels. The increase in ST levels is less with MCT-LCT propofol and elimination of triglyceride is also rapid after terminating MCT-LCT propofol infusion.
Keywords: Long chain triglyceride propofol, medium chain triglyceride-long chain triglyceride propofol, pediatric, serum triglyceride
INTRODUCTION
Propofol, a sedative and hypnotic agent has become increasingly popular for induction and maintenance of anesthesia in children and adults.[1] It is also used extensively as a sedative agent in the intensive care unit for short and long-term sedation during mechanical ventilation.[1,2] Significant increase in serum triglyceride (ST) concentration have also been described in adult population particularly after prolonged administration which is attributed to propofol formulation containing long chain triglyceride (LCT).[3,4,5,6] Though, medium chain triglyceride-LCT (MCT-LCT) propofol when compared with LCT propofol for long-term sedation in adults (at least 48 h) resulted in identical triglyceride levels, the elimination of triglyceride was faster in patients administered MCT-LCT propofol.[7] Similarly, short term administration of LCT propofol in standard doses for sedation and anesthesia have led to a significant increase in triglyceride levels in children.[8,9] Furthermore, anesthetic doses of propofol have transiently increased pancreatic and hepatic enzymes in pediatric patients undergoing craniotomy and receiving phenytoin for antiepileptic prophylaxis.[10] The availability of propofol in two different fat emulsion based intravenous (IV) formulations necessitate a comparison of ST levels after short term administration of LCT and MCT-LCT propofol for induction and maintenance of anesthesia in children.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Ethics Committee. After obtaining written informed consent from the parents or legal guardian, 40 American Society of Anesthesiologists I pediatric patients aged 3-12 years scheduled for elective surgery of 1-2 h duration were enrolled in this prospective randomized double-blind study. The children were randomly allocated to either Group I (1% LCT propofol) or Group II (1% MCT-LCT propofol) by computer generated random number tables. All the children fasted for 8 h for solid food and 2 h for clear fluid preoperatively and were premedicated with oral midazolam 0.5 mg/kg 30 min before induction of anesthesia. An IV line was secured by preparing the puncture site with lidocaine-prilocaine cream in all the children. Anesthesiologist loading the study drug was not involved in the management of patients. In Group I (n = 20) anesthesia was induced and maintained with 3 mg/kg and descalating dose of 20.15 and 10 mg/kg/h of 1% LCT propofol at 10 min intervals respectively. In Group II (n = 20) induction and maintenance of anesthesia was done with 1% MCT-LCT propofol in the same dose as in Group I.
All the children received fentanyl 2 μg/kg for intraoperative analgesia. Tracheal intubation was facilitated by 0.5 mg/kg IV atracurium. Ventilation was controlled using 50% N2O in O2 (Datex-Ohmeda Aestiva-5). Propofol infusion was stopped after completion of surgical procedure. Residual neuromuscular blockade was reversed and patients were extubated. Intraoperative monitoring consisted of noninvasive blood pressure (BP), heart rate (HR), electrocardiogram, oxygen saturation (SpO2) and end-tidal carbon dioxide. Total dose of propofol administered, duration of anesthesia and surgery were noted. A volume of 1 ml of venous blood sample was drawn for ST estimation just before induction of anesthesia triglyceride (TG I), at the end of propofol infusion (TG II) and 4 h after stopping propofol infusion (TG III). ST levels were measured by enzymatic in-vitro test using Automated Roche Clinical Chemistry Analyzer, Roche, Germany. Statistical analysis was performed using SPSS 16 software. Independent t-test was used to compare age, weight, duration of anesthesia and surgery and total dose of propofol used. General linear model analysis with repeated measure analysis of variance was used to assess trend for triglyceride values measured in two groups. Intragroup comparison of triglyceride values was done with paired t-test. Independent t-test was used for inter group comparison. All tests were two-tailed and P < 0.05 was considered as significant.
RESULTS
There were no significant differences between the groups with regard to demographic data, duration of anesthesia and surgery and total dose of propofol used [Table 1]. The baseline ST values were comparable between the groups. At the end of propofol infusion, triglyceride levels were significantly higher than the baseline values in both the groups (P < 0.05), but the values in Group I were significantly higher than in Group II (P < 0.05). Four hours after stopping propofol infusion triglyceride levels were similar to the basal values in Group II but in Group I patients had triglyceride levels higher than the baseline as well as the levels in Group II (P < 0.05) [Table 2]. Mean TG level at different time intervals were not following the significantly different trend in the two propofol groups and no significant interaction was noted between the two groups. The patients showed rapid rise as well as rapid fall at baseline and 4-h postinfusion. They follow similar ascending and descending trends in both groups. Intraoperative BP, HR and SpO2 were similar in the two groups [Figure 1].
Table 1.
Demographic data, duration of anesthesia and surgery and total dose of propofol used
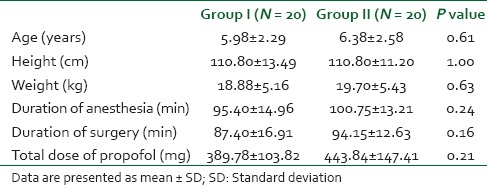
Table 2.
Serum TG levels

Figure 1.
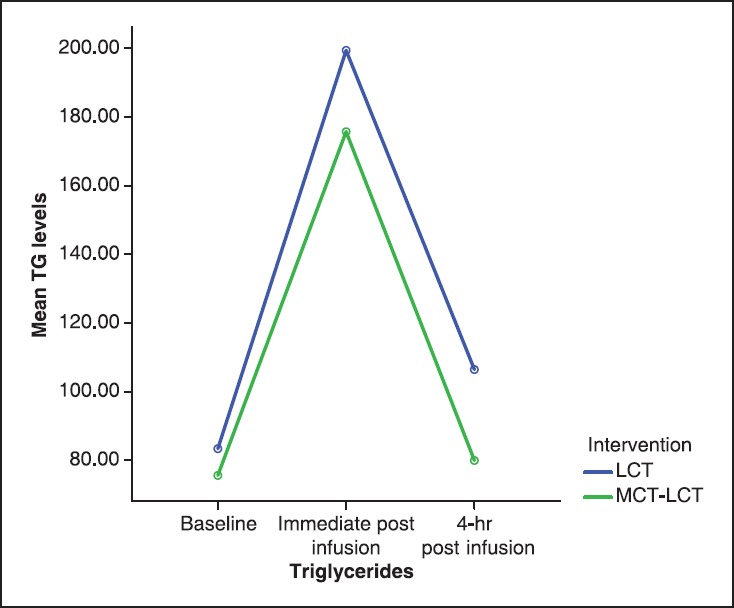
Mean triglyceride at different time points in two propofol groups
DISCUSSION
This clinical study demonstrated that both LCT and MCT-LCT propofol cause significant rise in triglyceride levels after short term administration in children for induction and maintenance of anesthesia. However, children in MCT-LCT group had lower triglyceride levels than children in LCT group at the end of propofol infusion and 4 h after termination. Propofol 1% contains 10% soyabean oil as the main component with LCTs, which are considered responsible for the hypertriglyceridemia. The use of 2% propofol with double the concentration of active drug and half the lipid load has reduced, although not eliminated the occurrence of hypertrigyceridemia.[11,12] Partial substitution of LCT with MCT in propofol emulsion has also been done with the aim to reduce the risk of hypertriglyceridemia attributed to propofol administration. Theilen et al.[7] observed similar increase in triglyceride levels during administration of 2% MCT-LCT and 2% LCT propofol in adult patients mechanically ventilated for 48 h, though total amount of propofol and thus the amount of fat was higher in MCT-LCT group than LCT group because of longer sedation time in MCT-LCT group. After stopping propofol infusion, a trend toward more rapid decrease in triglyceride levels was observed in MCT-LCT propofol group.
In our study, the triglyceride levels in 1% LCT propofol group were significantly higher than 1% MCT-LCT propofol group after short term administration for induction and maintenance of anesthesia in children. The elimination of triglycerides in MCT-LCT group was faster with triglyceride concentrations returning to preoperative values 4 h after termination of propofol administration, whereas in LCT group the values were significantly higher than the baseline at the same time. Ozlü et al.[8] in their study have reported significant rise in triglyceride levels in children administered propofol for induction and maintenance of anesthesia for short term. A significant rise in triglycerides has also been reported in children receiving short term propofol sedation for magnetic resonance imaging by Gottschling et al.[9] Reports of propofol infusion leading to slightly increased hepatic and pancreatic enzyme levels in pediatric patients receiving antiepileptic drug phenytoin and undergoing craniotomy for tumor resection exist. However, there was no significant clinical effect on the acid-base status or any clinical sign of hepatitis or pancreatitis.[10] Limitation of this study was that there was no control group to differentiate the effects of phenytoin and propofol on hepatic and pancreatic enzymes. No comparison was made between the two different formulations of propofol available (LCT vs. MCT-LCT).
Studies report that neither the type of emulsion nor the concentration of propofol in the IV formulation affect the pharmacokinetics and pharmacodynamics of propofol to a large extent.[13] However, the rapid clearance of triglycerides in MCT-LCT group in our study and in the study of Theilen et al.[7] could be associated with well-known characteristic of MCT-LCT, which are more readily hydrolyzed and more quickly eliminated from the circulation than are LCT.[14,15] We used 1% MCT-LCT and 1% LCT propofol, whereas Theilen et al.[7] used 2% MCT-LCT and 2% LCT propofol, which has 50% less lipid load. Whether using 2% MCT-LCT and 2% LCT propofol in children would lead to any difference in triglyceride levels needs further investigations. Pretreatment with 1% lidocaine was used with 60 s of tourniquet time prior to injection of propofol and we report no difference in incidence of injection pain between the two groups.
Limitation of this study is that we did not measure hepatic and pancreatic enzymes and acid-base status between the two groups. We have excluded children with preexisting diabetes mellitus, cardiac, hepatic, or renal disease, coagulopathy or any suspicion of previous pancreatitis, or any abnormalities in the biochemical workup. The results of this study cannot be extrapolated in such patients. Another limitation of this study is that we used fixed doses of propofol according to body weight and not according to the depth of anesthesia. Further study is needed to measure the level of TG with depth of anesthesia monitoring.
CONCLUSION
We conclude that even short term anesthesia with LCT and MCT-LCT propofol (1%) leads to elevated ST levels. The increase in ST levels is less with MCT-LCT propofol and elimination of triglyceride is also rapid after terminating MCT-LCT propofol infusion. However in this study, only four children in each group had triglyceride level above the normal limits (>200 mg/dl) at the termination of propofol infusion. Neither did any patient have triglyceride levels >200 mg/dl 4 h after stopping propofol nor did any child report any adverse effects related to rise in triglyceride levels.
Thus, there is no definitive clinically significant advantage of using MCT-LCT propofol over LCT propofol for short duration of anesthesia in children with no comorbidities. However, in view of the significant difference in ST levels further studies are needed to clarify the situation in children with preexisting hepatic, pancreatic, and metabolic disturbances.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marik PE. Propofol: Therapeutic indications and side-effects. Curr Pharm Des. 2004;10:3639–49. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 2.Fulton B, Sorkin EM. Propofol. An overview of its pharmacology and a review of its clinical efficacy in intensive care sedation. Drugs. 1995;50:636–57. doi: 10.2165/00003495-199550040-00006. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston JM, Shelly MP. The effect on serum lipid concentrations of a prolonged infusion of propofol — Hypertriglyceridaemia associated with propofol administration. Intensive Care Med. 1991;17:424–6. doi: 10.1007/BF01720682. [DOI] [PubMed] [Google Scholar]
- 4.Kunst G, Böhrer H. Serum triglyceride levels and propofol infusion. Anesthesia. 1995;50:1101. doi: 10.1111/j.1365-2044.1995.tb05980.x. [DOI] [PubMed] [Google Scholar]
- 5.Barrachina F, Mateu-de Antonio J. Propofol and hypertriglyceridemia: No problem? Crit Care Med. 1999;27:224–5. doi: 10.1097/00003246-199901000-00058. [DOI] [PubMed] [Google Scholar]
- 6.Mateu J, Barrachina F. Hypertriglyceridaemia associated with propofol sedation in critically ill patients. Intensive Care Med. 1996;22:834–5. doi: 10.1007/BF01709533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theilen HJ, Adam S, Albrecht MD, Ragaller M. Propofol in a medium- and long-chain triglyceride emulsion: Pharmacological characteristics and potential beneficial effects. Anesth Analg. 2002;95:923–9. doi: 10.1097/00000539-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Ozlü O, Ozkara HA, Eris S, Ocal T. Propofol anesthesia and metabolic acidosis in children. Paediatr Anesth. 2003;13:53–7. doi: 10.1046/j.1460-9592.2003.00965.x. [DOI] [PubMed] [Google Scholar]
- 9.Gottschling S, Meyer S, Krenn T, Kleinschmidt S, Reinhard H, Graf N, et al. Effects of short-term propofol administration on pancreatic enzymes and triglyceride levels in children. Anesthesia. 2005;60:660–3. doi: 10.1111/j.1365-2044.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- 10.Türe H, Mercan A, Koner O, Aykac B, Türe U. The effects of propofol infusion on hepatic and pancreatic function and acid-base status in children undergoing craniotomy and receiving phenytoin. Anesth Analg. 2009;109:366–71. doi: 10.1213/ane.0b013e3181a89641. [DOI] [PubMed] [Google Scholar]
- 11.Sandiumenge Camps A, Sanchez-Izquierdo Riera JA, Toral Vazquez D, Sa Borges M, Peinado Rodriguez J, Alted Lopez E. Midazolam and 2% propofol in long-term sedation of traumatized critically ill patients: Efficacy and safety comparison. Crit Care Med. 2000;28:3612–9. doi: 10.1097/00003246-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Barrientos-Vega R, Sánchez-Soria MM, Morales-Garcia C, Cuena-Boy R, Castellano-Hernández M. Pharmacoeconomic assessment of propofol 2% used for prolonged sedation. Crit Care Med. 2001;29:317–22. doi: 10.1097/00003246-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Cox EH, Knibbe CA, Koster VS, Langemeijer MW, Tukker EE, Lange R, et al. Influence of different fat emulsion-based intravenous formulations on the pharmacokinetics and pharmacodynamics of propofol. Pharm Res. 1998;15:442–8. doi: 10.1023/a:1011980432646. [DOI] [PubMed] [Google Scholar]
- 14.Wicklmayr M, Rett K, Dietze G, Mehnert H. Comparison of metabolic clearance rates of MCT/LCT and LCT emulsions in diabetics. JPEN J Parenter Enteral Nutr. 1988;12:68–71. doi: 10.1177/014860718801200168. [DOI] [PubMed] [Google Scholar]
- 15.Jeevanandam M, Holaday NJ, Voss T, Buier R, Petersen SR. Efficacy of a mixture of medium-chain triglyceride (75%) and long-chain triglyceride (25%) fat emulsions in the nutritional management of multiple-trauma patients. Nutrition. 1995;11:275–84. [PubMed] [Google Scholar]


