Abstract
Medullary thyroid cancer (MTC) is an aggressive form of thyroid cancer, which occurs in both heritable and sporadic forms. Discovery that mutations in the RET protooncogene predispose to familial cases of this disease has allowed for presymptomatic identification of gene carriers and prophylactic surgery to improve the prognosis of these patients. A significant number of patients with the sporadic type of MTC and even with familial disease, still present with nodal or distant metastases, making surgical cure difficult. Over the past several decades, many different types of therapy for metastatic disease have been attempted, with limited success. Improved understanding of the molecular defects and pathways involved in both familial and sporadic MTC has resulted in new hope for these patients with the development of drugs targeting the specific alterations responsible. This new era of targeted therapy with kinase inhibitors represents a significant step forward from previous trials of chemotherapy, radiotherapy, and hormonal therapy. Although much progress has been made, additional agents and strategies are needed to achieve durable, long-term responses in patients with metastatic MTC. This article reviews the history and results of medical management for metastatic MTC from the early 1970s up until the present day.
INTRODUCTION
Medullary thyroid cancer (MTC) comprises 5 to 10% of all thyroid cancers.1 MTC arises from the parafollicular C cells of the thyroid gland, which originate in the neural crest. The disease progresses from C cell hyperplasia (CCH), often with elevated calcitonin levels, to microscopically invasive carcinoma, then grossly evident carcinoma.2 Like other neuroendocrine tumors, MTC can elaborate a variety of products such as calcitonin (CT), carcinoembyonic antigen (CEA), serotonin, and chromogranin A that may cause symptoms such as diarrhea in patients with metastatic disease. In the context of CCH and MTC, the secretion of calcitonin predominates and can be used to confirm the diagnosis,3 indicate treatment efficacy,4 and monitor for disease progression or recurrence.5
Medullary thyroid cancer develops sporadically in 60 to 75% of cases,3,6 or as a result of a germline mutation in the rearranged during transfection (RET) protooncogene, as is seen in multiple endocrine neoplasia types 2A and 2B (MEN2), and familial MTC syndrome (FMTC). MTC often progresses in an indolent fashion with a 15-year survival of 85%, but has a tendency to spread to locoregional lymph nodes early, making surgical cure difficult.7 Total thyroidectomy and lymphadenectomy result in biochemical cure (normalization of calcitonin and CEA) only 40% of the time.7,8 Even when biochemical cure is achieved, approximately 9% of patients will later develop recurrent disease.8 For patients with sporadic MTC, total thyroidectomy and at minimum, central neck dissection, is performed upon histological confirmation of the disease. Patients with known RET mutations are offered prophylactic thyroidectomy and lymphadenectomy in childhood or upon discovery of the mutation.9 Due to the difficulty in achieving surgical cure, medical treatment for residual micrometastatic disease and recurrent disease are critical for long-term survival. Unfortunately, the relative rarity of the disease makes clinical trial design and patient accrual difficult. Thus, much of our knowledge about medical treatment of MTC rests upon small prospective series and retrospective reports.
The advent of targeted small-molecule kinase inhibitor drugs has revolutionized medical treatment of medullary thyroid cancer (MTC). Drugs such as vandetanib and cabozantinib produce disease regression in a significant portion of patients, and can extend progression-free survival in advanced, unresectable MTC.10,11 Other multikinase inhibitors such as sunitinib and sorafenib also offer hope to MTC patients progressing on other treatments, and ongoing clinical trials continue to evaluate additional agents. This review seeks to update readers on the recent developments in targeted small-molecule therapies for medical management of MTC. It also attempts to provide an overview of the major radioactive and chemotherapeutic regimens that preceded them, and remain as treatment options in MTC, as well as some of the many other therapies that have been tried with limited success in this previously treatment-refractory disease.
TYROSINE KINASE INHIBITORS
The first indication of the promise of small-molecule kinase inhibitors came from the class prototype, imatinib. Targeting the mutant BCR-ABL tyrosine kinase in chronic myeloid leukemia, imatinib dramatically improved response rates of CML patients in blast crisis, and significantly forestalled progression from the chronic phase in long-term studies.12,13 Imatinib also targets the mutated c-KIT receptor responsible for gastrointestinal stromal tumor (GIST), and use of imatinib after resection of high-risk GISTs had similarly impressive results, with 5-year survival improving from 35% to 83%.14 These encouraging studies suggested a role for small-molecule inhibitors in MTC.
Like CML and GIST, oncogenic transformation in MTC occurs due to a mutation causing constitutive activation of a signaling pathway. The causative genetic region for autosomal dominant MEN2A was mapped by genetic linkage to chromosome 10 in the late 1980s,15,16 and mutations in the (RET) gene were determined to cause MEN2A, MEN2B, and FMTC in the early 1990s.17–22 In sporadic MTC, somatic RET mutations occur in 40–65% of tumors.11,23 While many different RET mutations can lead to MEN2 syndromes, the most prevalent mutations include C634R in MEN2A and M918T in MEN2B.24 The M918T mutation also represents the most common somatically-occurring mutation in sporadic MTC.23 RET is a membrane-bound receptor tyrosine kinase involved in renal and enteric nervous development and is activated by any of four glial-derived neurotrophic factor (GDNF) molecules.25 While RET activation principally induces the RAS-RAF-MEK-ERK mitogen-activated protein kinase (MAPK) pathway, RET can also activate phosphatidylinositol-3-kinase/Akt (PI3K/Akt), janus-activated kinase/signal transducers and activators of transcription (JAK/STAT), and jun-N terminal kinase (JNK), among other pathways (Figure 1).25–27 In MTC, RET mutations lead to substrate-independent dimerization of the receptor causing constitutive activation, unrestricted signaling, and ultimately, cancer.25,28
Figure 1.
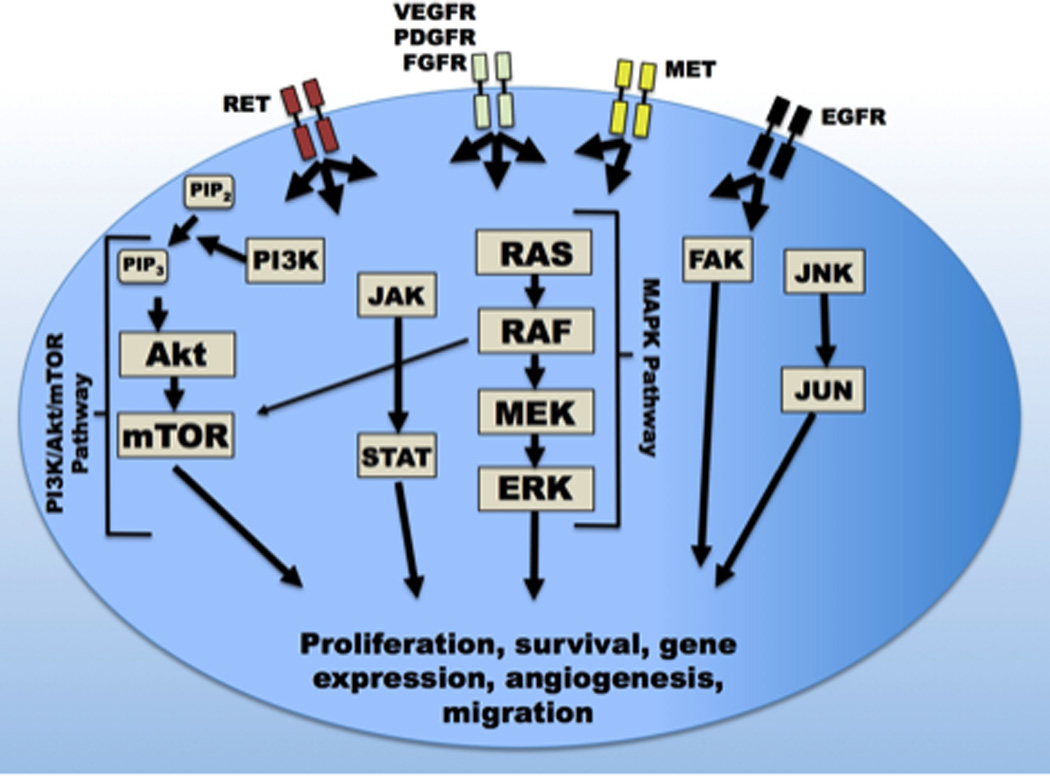
Receptors and pathways in medullary thyroid cancer. Kinase inhibitors block the activity of rearranged during transfection (RET), vascular endothelial growth factor receptor (VEGFR), and other receptors, inactivating the mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K), and other pathways. Extensive interpathway cross-talk exists. Arrows indicate pathways most commonly associated with each receptor, however, most receptors interact with additional pathways to varying extents. Abbreviations: mTOR: mammalian target of rapamycin; PIP2: phosphatidylinositol bisphosphate; PIP3 phosphatidylinositol trisphosphate; JAK: janus-activated kinase; STAT: signal transducers and activators of transcription; FAK: Focal adhesion kinase; JNK/JUN: jun-N-terminal kinase/JUN protein; RET: Rearranged during transfection kinase; PDGFR: Platelet-derived growth factor receptor; FGFR: Fibroblast growth factor receptor; MET: Mesenchymal epithelial transition factor/hepatocyte growth factor receptor; EGFR: Epidermal growth factor receptor.
Although it could be readily appreciated that MTC shared similar mechanisms of molecular pathogenesis to cancers treatable with imatinib, research into CML, melanoma, and papillary thyroid cancer helped spur development of MAPK and related pathway inhibitors.25,29 In addition to RET, interactors with the MAPK pathway include receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF).30 Gain-of-function mutations in BRAF kinase, a downstream target of RET and key effector in the MAPK pathway, occur in nearly 60% of melanoma cell lines and 45% of human papillary thyroid cancers.31–33 With aberrant activation of the MAPK and related pathways recognized as causative events in human cancers affecting a large number of patients, intensive research efforts have produced small molecule inhibitors with activity at multiple receptors and at multiple steps of these pathways, from receptor to effector kinases (Table 1). While much of this research has focused on papillary thyroid or other cancers, several kinase inhibitors have been evaluated for activity in medullary thyroid cancer, with two, vandetanib and cabozantinib, currently approved by the FDA for MTC.
Table 1.
Selected drugs targeting pathways involved in medullary thyroid cancer
| Drug | Alternative Name | Targets | Selected trials |
|---|---|---|---|
| Vandetanib | ZD6474 | VEGFR2, VEGFR3, EGFR, RET, PDGFR25,35 | MTC Early Phase37,38 Phase-III: 331 MTC patients10 |
| Cabozantinib | XL184 | MET, VEGFR2, RET, KIT, AXL, FLT341 | MTC Phase-I40 Phase-III: 330 MTC patients11 |
| Sorafenib | BAY 43–9006 | RAF, RET, VEGFR1-3, PDGFR, KIT, FLT327,39 | Phase-I: 1 MTC patient27 13 MTC patients46 Phase-II: Thyroid cancer; 1 MTC patient;141 21 MTC patients45 |
| Sunitinib | SU11248 | VEGFR1,2, PDGFR, RET, KIT, FLT3, CSF1R39,50 | Phase-II: Thyroid cancer including 7 MTC patients50 |
| Axitinib | AG-013736 | VEGFR1-3, much lower for KIT, PDGFR57 | Phase-II: Thyroid cancer including 11 MTC cases57 |
| Motesanib | AMG 706 | VEGFR1-3, PDGFR, KIT58 | Phase-I: 1 MTC patient142 Phase-II:91 MTC patients58 |
| Ponatinib | AP24534 | BCR-ABL, SRC, FLT3, KIT, FGFR1, PDGFR, VEGFR2, RET59 | MTC cell culture, animal model59 MTC cell culture, comparison with other MTC agents53 Phase-II: CML – High rate of arterial thrombotic events60 |
| Imatinib | STI571 | BCR-ABL, PDGFR, CSF1R, KIT, lower activity against RET54,55 | Phase-II: MTC54,55 |
| Gefitinib | ZD1839 | EGFR56 | Trial including 4 MTC patients56 |
Abbreviations: MTC: Medullary thyroid cancer; AXL: AXL receptor tyrosine kinase; BCR-ABL: Breakpoint cluster region-Abelson tyrosine-protein kinase; CML: Chronic myeloid leukemia; CSF1R: Colony stimulating factor-1 receptor (c-Fms); EGFR: Epidermal growth factor receptor; FGFR: Fibroblast growth factor receptor; FLT3: fms-related tyrosine kinase 3; KIT: c-Kit/Stem cell factor receptor; MET: Mesenchymal epithelial transition factor/hepatocyte growth factor receptor; PDGFR: Platelet-derived growth factor recptor; RAF: Rapidly accelerated fibrosarcoma kinase; RET: Rearranged during transfection; SRC: Avian sarcoma viral oncogene homolog; VEGFR: Vascular endothelial growth factor receptor
Vandetanib was the first kinase inhibitor approved for treatment of symptomatic metastatic medullary thyroid cancer.34 Initially developed as an orally-available VEGFR-inhibitor, vandetanib was found to prevent activation of RET receptors with common mutations and to block MTC tumor growth in mice.35 Two less-common RET mutants, V804M and V804L, demonstrate resistance to vandetanib inhibition of RET.36 In 2010, results of a phase-II study showed that treatment of with 300mg of vandetanib daily induced objective responses in 6 of 30 patients (20%) with unresectable locally advanced or metastatic hereditary MTC.37 Stable disease or objective responses occurred in 22 of 30 patients (73%), and 80% saw reductions in serum calcitonin. Estimated PFS was 27.9 months. A second study of 100mg daily vandetanib treatment in patients with advanced hereditary MTC reported similar results, with 13 of 19 patients (68%) having stable disease or partial objective responses.38 In both trials, diarrhea, rash, and fatigue were the most common adverse events, and QTc prolongation requiring dose-reduction occurred.37,38
Supported by the results of these initial trials, investigators initiated a phase-III randomized, double-blind trial of vandetanib 300mg daily versus placebo in unresectable locally advanced or metastatic medullary thyroid cancer.10 Included were 331 patients with sporadic or hereditary MTC. In this trial, vandetanib significantly prolonged PFS, with an estimated median PFS of 30.5 months in the vandetanib treatment group compared to 19.3 months in the placebo group (p<0.001). Objective radiologic responses occurred in 45% of treatment vs. 13% of placebo groups (p<0.001), and disease control rates were 87% in treatment vs. 71% in placebo groups (p=0.001). No overall survival benefit was noted due to inadequate event numbers, and possibly because 93% of patients who progressed while receiving placebo elected to crossover to open-label vandetanib. Additionally, all but one objective responses in the placebo group occurred after crossover. Toxicities were common, and 35% of patients required dose-reductions due to adverse events. Grade 3 and higher adverse events included diarrhea (11%), hypertension, (9%), QTc prolongation (8%), and fatigue (6%). Although not observed in the trial, torsades de pointes has been reported and QTc monitoring is essential during vandetanib treatment.39
A potential problem with VEGFR-inhibitor treatment is development of resistance or diminished response due to blockade-induced upregulation of related pathways.40 Cabozantinib, or XL184, is an orally-available multi-kinase inhibitor with activity against MET, VEGFR2, RET, and others.41 Its developers proposed its broader inhibition of important kinases, particularly MET, which can be upregulated in MTC, as offering potential to avoid treatment failures due to targeting VEGFR alone.40,41 In cell culture and animal models, cabozantinib prevented phosphorylation of its target kinases, reduced cell proliferation, and limited angiogenesis, tumor invasiveness, and metastasis of multiple cancer cell lines.41 In a phase-I trial of cabozantinib, of 37 patients with advanced or recurrent medullary thyroid cancer, 10 (29%) demonstrated objective partial responses, and 68% had stable disease or objective response at 6 months.40
The efficacy of cabozantinib for prolonging progression-free survival was shown in a phase-III randomized, double-blind trial of cabozantinib in progressive medullary thyroid cancer.11 In this manufacturer-sponsored trial, 330 patients with advanced or recurrent MTC were randomized to cabozantinib 140mg daily or placebo until intolerable toxicity or progression. Patients receiving cabozantinib had significantly longer progression-free survival than those receiving placebo (median PFS 11.2 vs. 4.0 months, p<0.001), and this advantage applied both to patients with and without RET mutations. Objective radiographic responses were observed in 28% of cabozantinib versus 0% of placebo patients (p<0.001). Although not enough deaths had occurred to fully evaluate overall survival, despite the large advantage in progression-free survival among treated patients, investigators observed no difference in overall survival, with death occurring in 21 (10%) patients in the cabozantinib arm and 10 (9%) of patients in the placebo arm. This was not due to crossover, as crossover upon progression was forbidden by the study protocol, and patients receiving any other additional cancer therapy were censored. Adverse events were frequent with cabozantinib treatment. Grade 3 or 4 adverse events, defined as severe or life-threatening/disabling,42 including hemorrhage, fistulas, and gastrointestinal perforation occurred in 69% of cabozantinib patients versus 33% in the placebo group, while “serious adverse events,” defined as imminently life-threating or resulting in death (which were reported separately from grade 3 and 4 events),43 occurred in 42.1% of cabozantinib patients versus 22.9% of placebo patients. Overall, 79% of cabozantinib-treated patients required dose-reductions due to adverse events. Due to a requirement for radiographically-evident disease progression for inclusion in this trial, which was not required for the phase-III trial of vandetanib, patients in the cabozantinib trial may have had more advanced disease, although this too was influenced by crossover (placebo group PFS 4.0 months vs. 19.3 months in the vandetanib trial placebo group).10,11 This difference and the protocol difference regarding whether treatment crossover was allowed, complicate direct comparison of the two trials, both in terms of outcomes and side-effect profiles.
Current National Comprehensive Cancer Network (NCCN) guidelines now recommend that clinicians consider either vandetanib or cabozantinib for medical therapy of advanced, unresectable MTC.44 After failure of these agents, the guidelines recommend considering two additional agents, sorafenib or sunitinib, or enrollment in a clinical trial.44 The evidence for sorafenib and sunitinib in MTC is not as robust as for the other drugs, but small studies have demonstrated efficacy of both in medullary thyroid cancer.
Sorafenib is a multi-kinase inhibitor that is FDA-approved for treatment of renal cell and hepatocellular cancers.45 Although developed for B-RAF and C-RAF inhibition, sorafenib also potently inhibits several other pathways and kinases including VEGFR, PDGFR, and both wild-type, mutant, and V804M and V804L resistant-mutant RET.27 A phase-I study evaluating treatment with sorafenib and the farnesyl-transferase inhibitor (affecting RAS kinase) tipifarnib reported a dramatic objective response in a sporadic, RET-mutation-positive MTC patient.27 Later follow-up with 13 MTC patients enrolled in the same study revealed a 38% rate of partial responses and 69% overall disease control (partial response + 6 month stable disease) rate.46 A phase-II study evaluated 21 MTC patients treated with 400mg twice-daily sorafenib without tipifarnib. All patients experienced stable disease and some tumor shrinkage, with 2 having objective partial responses.45 Durable stable disease of at least 6 months occurred in 11 patients (52%).45 Adverse events were frequent with 76% of patients requiring dose-reductions and grade 3 or higher complications occurring in 13 patients (62%). The most common serious adverse events were hand-foot-skin reaction, hypertension, diarrhea, and infection.45 A non-randomized, retrospective study of Spanish thyroid cancer patients treated with sorafenib found a 47% response rate among 15 MTC patients.47 Additional small studies and case-reports support the consideration of sorafenib in MTC, although additional randomized studies are needed.34,48,49
Sunitinib is a multi-tyrosine kinase inhibitor with activity against VEGFR1 and 2, c-KIT, FLT3, PDGFR, and RET.50 On the basis of its inhibitory spectrum, Kelleher and McDermott in 2008 reported treating a patient with metastatic MTC with sunitinib, leading to marked reduction of tumor size by imaging and improvement in clinical symptoms51. Another group reported a patient with advanced MTC whose dramatic response to sunitinib permitted surgical resection of his disease, despite the absence of a detectable RET mutation.52 A phase-II trial testing 37.5mg daily sunitinib included 7 patients with metastatic MTC and found objective responses in 3 of 6 with radiologically-evaluable lesions (50%) and stable disease in 2 for a disease control rate of 71% in this small sample.50 More than 10% of all treated patients experienced grade 3 or higher toxicities of fatigue, diarrhea, hand/foot syndrome, and leuko/neutropenia.50 Both sorafenib and sunitinib effectively block phosphorylation of the RET V804M mutant, which confers resistance to vandetanib and reduced effectiveness of cabozantinib.53
Other tyrosine kinase inhibitors have been assessed for treatment of MTC. The class-prototype imatinib has limited inhibitory activity against RET, and two open-label trials treated a total of 24 MTC patients with imatinib. In these trials, no objective responses were observed, whereas treatment incurred significant toxicity, and use of imatinib has stalled in MTC.54,55 Gefitinib represents another drug with efficacy in a different cancer type that was explored in MTC. Gefitinib is an EGFR inhibitor with activity in EGFR-mutant non-small cell lung cancer, however when tested in a phase-II trial which enrolled four patients with MTC, no objective responses were noted.56
Axitinib is an orally-available kinase inhibitor with relative specificity to VEGFR1–3.57 A phase-II trial treated 60 patients, including 11 with MTC, with 5mg of axitinib twice daily. Partial response or stable disease occurred in 5 (45%). Adverse events included fatigue and diarrhea, which were common. Twelve percent of all treated patients experienced grade 3 or higher hypertension, and no other serious toxicity occurred in more than 5% of patients. Interestingly, axitinib’s activity in MTC occurs without inhibition of RET. Another orally-available multi-kinase inhibitor, motesanib, demonstrates activity against VEGFR1–3, PDGFR, and KIT, but does not inhibit mutant RET.58 A phase-II trial enrolling 91 MTC patients found that although only 2 patients achieved objective partial radiographic responses, 76% of treated patients achieved “clinical benefit”, defined as objective response or durable stable disease.58 Both RET mutation-positive and negative patients achieved durable stable disease, with higher rates (62%) among RET mutation-negative than mutation-positive (42%) patients.58 Grade 3 and 4 adverse events occurred in 38% and 3% of patients, with gallbladder toxicity occurring in 8 of 91 patients (9%).58
Ponatinib, a newer multikinase inhibitor, shows broad inhibitory activity across a wide range of kinases. In particular, ponatinib shows strong inhibition at low drug concentrations of BCR-ABL and RET, with anti-RET potency at least 100–1000 times greater than those for vandetanib, cabozantinib, or motesanib.53 In vitro studies demonstrate that ponatinib inhibits phosphorylation and signaling through multiple pathways, and besides BCR-ABL and RET, ponatinib blocks VEGFR2, PDGFRα, SRC, KIT, FGFR1, and FLT3.59 Two recent studies support ponatinib’s potential for efficacy in MTC. In cell culture, low concentrations of ponatinib prevent phosphorylation of RET and its downstream target ERK1/2,53 and mice injected with MTC cell line tumors show significantly inhibited tumor growth with ponatinib treatment.59 Notably, ponatinib shows in vitro activity against kinases with common inhibitor-resistance mutations, including the BCR-ABL T315I mutant, FLT3 F691I mutant, and RET V804 and Y806 mutants.53,59 Additionally, although rare, some patients do carry RET V804 and Y806 mutations, which confer resistance to vandetanib and reduce cabozantinib effectiveness by blocking the RET ATP-ase active site where these inhibitors bind.53 By exploiting a slightly different mechanism of activity – binding and stabilizing the inactive receptor state – ponatinib could offer an alternative after initial treatment options have failed.53 While such a broad spectrum of activity could theoretically lead to greater efficacy, recent clinical trials of ponatinib in CML raised serious safety concerns. The phase-II PACE trial observed high rates of arterial thrombotic events, including cardiovascular (7.1%), cerebrovascular (3.6%), and peripheral vascular (4.9%) thrombosis.60 Ponatinib sales and clinical trials were suspended in late 2013 after United States Prescribing Information adverse event surveillance identified a 17.1% rate of arterial thrombotic events,60 and the current drug label contains a black-box warning citing a rate of arterial and venous occlusion of “at least 27%” in ponatinib-treated patients.61 Although ponatinib can once again be prescribed for CML under a risk evaluation and mitigation strategy, reports of fatal arterial thrombotic events continue, and its future in MTC appears doubtful.62
Neither vandetanib nor cabozantinib shows a definitive correlation between RET mutational status and efficacy.63 Phase-II trials of cabozantinib identified patients with and without RET mutations who showed tumor shrinkage.40 In the phase-III vandetanib trial, patients with sporadic MTC who had the RET M918T mutation had a higher response rate than those who did not (54.5 vs. 30.9%), but the investigators’ inability to determine the mutational status of 45% of the study patients due to inadequate tissue specimens, limited overarching conclusions regarding genotype and response to treatment.10 Patients receiving motesanib, which functions principally through VEGFR inhibition, had a higher rate of partial responses and stable disease (8% and 62%) in RET mutation-negative patients than in mutation-positive patients (0% and 42%).58 Further complicating genotype-phenotype correlations, axitinib offers minimal if any inhibition of RET,57 yet still achieved stable disease or objective responses in 45% of MTC patients, while gefitinib achieved no responses through targeting EGFR.56 Although RET mutations are sufficient to induce neoplasia in MEN2 patients, aberrant activity of VEGFR and MET is also observed in MTC.40,58 Lack of a strong connection between the presence of an activating RET mutation and response to treatment, or drug activity against RET, highlights the contributions that blockade of additional receptors besides RET, especially VEGFR, make to clinical efficacy of these drugs63. Thus, despite the appeal of “rational” treatment, the pathways representing the most important targets for clinical effectiveness may not be the same as those presumed based on our understanding of MEN2. Further research could improve understanding of signal transduction when considered as complex networks, rather than as discrete and sequentially proceeding pathways, improving future efforts to target therapeutics.
Although there are now options for patients with metastatic MTC, the question of optimal medical management will likely remain open for the time being. No randomized trial has yet demonstrated an overall survival benefit in MTC with targeted agents, and their toxicities and expense are considerable.63 Head-to-head studies directly comparing therapies are lacking, but could elucidate the relative benefits of different agents. Over the next several years, longer follow-up of patients included in recent trials will become available to determine effects on overall survival, studies of additional agents will accrue, and continuing research will further illuminate the cellular networks involved in response to treatment.
CYTOTOXIC CHEMOTHERAPY
Prior to introduction of current kinase inhibitor-based therapies, chemotherapy and radiation formed the mainstay of medical MTC treatment. While recent advances in small-molecule therapies have largely supplanted these regimens, they remain in consideration for refractory cases, and research continues to improve the sensitivity of MTC cells to them. As kinase inhibitors display mostly cytostatic, rather than cytocidal effects, a need persists for effective agents to eliminate cancerous cells.
Doxorubicin and dacarbazine-Standard cytotoxic chemotherapy agents have been employed in management of metastatic MTC for more than 30 years with mixed results. Doxorubicin (Adriamycin) and dacarbazine (DTIC) are the two agents with the most extensive track record, and dacarbazine is currently recommended as a treatment option for patients with disseminated, symptomatic disease.44
Doxorubicin was first tested in MTC patients in the 1970s. Two case series64,65 and one small prospective study66 documented the response of advanced MTC to doxorubicin administered as a single agent. The results of the case series were split. One reported a robust anti-tumor effect64 but the other only minor tumor shrinkage.65 The prospective study was more encouraging, as 3 of 5 MTC patients responded to treatment and demonstrated significantly better overall survival than those patients that did not respond.66
Thereafter, doxorubicin was studied in combination with a number of other agents. In 1985, Shimaoka et al. combined doxorubicin with cisplatin in a multi-institutional, prospective, randomized controlled trial of advanced thyroid cancer. Ten of 84 patients had MTC—4 of which were treated with doxorubicin alone, and 6 treated with the combination regimen. Disappointingly, only 1 MTC patient in the doxorubicin-only arm, and 2 in the combination arm demonstrated partial tumor responses, defined as decrease in total tumor volume of more than 50%. There were no complete responses.67 Sridhar et al. also tested doxorubicin in combination with cisplatin, but included only 1 MTC patient in a study of neuroendocrine cancers. The patient with MTC did relatively well—demonstrating a partial response lasting 58 weeks—but treatment was eventually discontinued due to doxorubicin-mediated cardiac toxicity.68
In 1990, Scherubl et al. attempted to build on previous work by testing doxorubicin combination regimens in a phase II clinical trial. In this study, vindesine and cisplatin were added to doxorubicin and administered to 20 patients with advanced thyroid cancer, 10 of whom had MTC. Of MTC patients, 1 had a partial tumor response, 6 had stable disease and 3 progressed. The authors declared this combination ineffective for treatment of advanced thyroid cancers.69 Droz et al. used a range of chemotherapeutics in patients with advanced non-anaplastic thyroid cancers but included only a small number of MTC patients in each group. Investigators observed partial tumor responses in the MTC patients in the doxorubicin-only arm, but these were short lived and many patients experienced cardiac toxicity.70 Although doxorubicin induces partial morphologic tumor responses in a reasonable proportion of MTC patients, its use is severely limited by cardiac toxicity, and thus it is not an ideal chemotherapeutic agent for patients with advanced MTC.
Dacarbazine (DTIC), an alkylating agent, was first used as a single agent chemotherapeutic for MTC in the 1980s. The first case reports boasted dramatic reductions in metastatic tumor burdens (though these lasted less than 1 year) and thus encouraged further investigation of this drug’s efficacy in advanced MTC.71,72 The first DTIC/5-FU combination trial achieved partial responses in 3 of the 5 MTC patients included in the study. These responses lasted 8 to 10 months. A fourth patient achieved stable disease. Given the encouraging tumor responses and a limited number of grade I and II side effects, this study served as the basis for testing DTIC combinations in larger numbers of patients with MTC.73
The DTIC combination trials that followed failed to achieve results that would suggest the drug’s utility as a standard adjuvant treatment of advanced MTC. DTIC was combined in various ways with cyclophosphamide, vincristine, streptozocin, and epirubicin. Though no trial included more than 20 MTC patients, most reported moderate success with one-third to one-half of patients achieving partial tumor responses or stable disease for an average of 1 year.74–78 Despite the results of these trials, of the major antineoplastic agents studied, DTIC is the only one currently recommended for patients with disseminated symptomatic MTC by the National Comprehensive Cancer Network.44
Capecitabine-Capecitabine is a 5-FU prodrug that is converted in target tissues by thymidylate phosphorylase and selectively accumulates in tumors, rather than plasma or muscle tissue. Capecitabine interferes with DNA synthesis by inhibiting thymidylate synthase, preventing tumor cell proliferation.79 Widespread experimentation with 5-FU in other neuroendocrine cancers, availability of capecitabine via oral administration, and the promise of trials evaluating capecitabine in colorectal and breast cancers, has led to use of this drug in metastatic MTC.80 Thus far, only case reports exist, and in these, concurrent use of other agents obscures capecitabine’s effects. Nevertheless, disease stabilization in most patients has been reported.80–82
EXTERNAL BEAM RADIATION
External beam radiotherapy (EBRT) has been used sporadically in the treatment of MTC for more than 40 years, but is not currently considered standard treatment. As with other methodologies, there are limited clinical trials to guide decision-making about the indications for EBRT. Most of what is known about the efficacy and safety of EBRT for MTC is derived from small, single institution experiences.
Prior to 1990, a number of small series reported contradictory conclusions about the utility of EBRT as adjuvant treatment for MTC. In 1977, Steinfeld treated 4 MTC patients with EBRT and achieved local control of their disease lasting 3 to 6 years. He recommended that EBRT be used to treat MTC, and further suggested that the modality be tested in combination with chemotherapy.83 Samaan et al. published a contradictory retrospective study in 1988. In this series, 57 patients with advanced MTC treated with total or subtotal thyroidectomy followed by EBRT were compared to patients treated with surgery only. The group treated with EBRT had worse survival than those treated with thyroidectomy only.84
The largest series examining EBRT in MTC was published in 1990 by Jensen et al. This multicenter retrospective review included 5,287 patients, 200 of whom had MTC. Approximately 30% of MTC patients were offered some sort of adjuvant treatment, although the specific number receiving EBRT was not reported. The authors found that nearly equal proportions of patients with MTC at each disease stage were offered EBRT. Analysis of the entire MTC cohort demonstrated that patients undergoing surgery followed by EBRT had 100% 5-year survival, whereas patients who only had surgery had a 91% 5-year survival. When the cohort was divided by disease stage, EBRT conferred a survival advantage in patients with regional disease, but not in patients with distant metastases.85 Given their large sample size, this study serves as one of the foundations for the ATA recommendation to offer EBRT to post-thyroidectomy MTC patients at high risk for locoregional recurrence.9
Another large series examining EBRT in patients with MTC was published in 1996. This was a retrospective, single center series looking at patients diagnosed and treated for MTC from 1954 to 1992. The study included 72 patients with MTC, 43 of whom received EBRT. At this institution, the indications for EBRT in the context of MTC included (1) residual microscopic disease after thyroidectomy, (2) presence of tumor within 2 mm of the resection margin, (3) extraglandular invasion or lymph node involvement, and (4) elevated post-operative CT (in the absence of distant metastases). Patients received a variety of radiation protocols. The most important finding in this series was that in patients at high risk for locoregional recurrence, EBRT seemed to confer a significantly longer 10-year relapse-free rate compared to those that only had surgery (p = 0.049).86 This study provided support for Jensen et al’s suggestion that EBRT is indicated in those patients at high risk for locoregional recurrence.
Nearly a decade later, Schwartz et al. published a single institution series that included 34 patients with MTC treated with total thyroidectomy, appropriate neck dissection and EBRT between the years 1995 and 2004. Indications for adjuvant EBRT were gross or microscopic residual disease, soft tissue extension, nodal disease, or mediastinal involvement. There were 4 locoregional failures in this cohort, all occurring within 26 months of completion of EBRT. Five-year disease specific survival was 62%, and overall survival was 56%. The 5-year relapse-free rate was 87%. These results supported a role for EBRT in patients at high risk for local recurrence in the adjuvant setting. The authors emphasized that this type of control was especially important in patients with MTC, even those with distant metastases, as they tend to live a long time with disease and good locoregional control can confer a better quality of life.87
The most recent study examining the use and utility of EBRT in MTC patients was published in 2010 by Martinez et al. This analysis was conducted using the SEER cohort (1988 – 2004), and included 534 patients with MTC. All patients had been treated with total thyroidectomy with at least one lymph node excised at the time of surgery. Patients with distant metastases were excluded. Interestingly, EBRT did not affect overall survival when all patients were examined, nor when the subset of patients with lymph node-positive disease were analyzed.88
Currently, the American Thyroid Association suggests that EBRT be used in patients post-operatively who are at high risk for locoregional recurrence, as well as be offered to patients with extensive M1 disease as a palliative treatment.9
RADIOACTIVE IODINE
Radioactive iodine (RAI) has been useful in the treatment of differentiated thyroid cancer since the 1940s,89 but is largely ineffective in MTC. In differentiated thyroid cancers, follicular cells concentrate radioactive 131I through a sodium iodide symporter on the cell surface, permitting damage to the cancer cell by the emitted β particle. Though C cells do not concentrate iodine,90 they can be damaged by exposure to radioactive 131I by virtue of the bystander effect.91–94 RAI treatment has been offered to MTC patients with positive post-operative thyroid scans or persistently elevated calcitonin, as these are signs that thyroid tissue was left behind after surgery. It is possible that the residual tissue is a mixture of C cells and iodine-concentrating follicular cells, and thus small foci of MTC may be eradicated by the β particles emitted from neighboring follicular cells.
Though initial case reports were promising,95,96 the utility of this modality has not been borne out in modern practice. Saad et al. published the M.D. Anderson experience with RAI in MTC in 1983. In this series, there was no difference in disease course, recurrence rate, or overall survival between 15 MTC patients who underwent RAI and 84 patients treated with surgery alone.97 In 2006, Erdogan et al. published a series (n=7) suggesting that RAI may be useful as an adjuvant treatment for MTC in patients with persistently elevated calcitonin and residual microscopic disease. Their conclusions were based on the finding that 2 of 3 patients with localized disease and 1 of 4 patients with locoregional disease achieved biochemical cure after treatment with RAI. However, their conclusions were limited by their small study population and short follow up interval, which was a maximum of five years.98
The most recent RAI series by Meijer et al.99 is the largest and supports the suggestion made 30 years earlier that RAI is not an effective adjuvant treatment for MTC. This multicenter, retrospective analysis compared 232 patients with local or locoregional MTC treated with surgery only to 61 matched patients that were also given post-operative RAI. Investigators attempted to standardize the surgical treatment received by the patients by only including those patients that underwent surgery according to the American Thyroid Association guidelines set forth in 2009.9 In these groups, they found no difference in disease-free or disease-specific survival. These findings held when they performed the same analysis for the subgroup of patients with hereditary, clinically apparent MTC. Patients with MTC still occasionally receive RAI when medical therapies, such as cytotoxic chemotherapy fail, but current evidence indicates this is unlikely to provide much benefit. There has been renewed interest in “resensitizing” thyroid cells refractory to treatment with RAI by using kinase inhibitors in follicular-origin thyroid cancer, but these experiments have not yet gained ground in MTC.100,101
SOMATOSTATIN ANALOGUE-BASED REGIMENS
Somatostatin is an endogenous peptide that inhibits many secretory or proliferative cellular functions by binding to a somatostatin receptor (SSTR) expressed on cells of neural crest origin. The endogenous peptide has a short half-life, but longer-lasting synthetic analogues allow imaging and treatment of a variety of neuroendocrine tumors (NETs). Octreotide, the most widely used analogue, binds primarily to SSTR type 2 (SSTR2), which is the most commonly expressed receptor subtype on gastrointestinal (GI) NETs and on MTC cells.102 Octreotide suppresses hormonal secretion by NETs, thereby alleviating symptoms, reducing biomarker levels, and occasionally causing tumor stabilization.103 Given the drug’s success in treating patients with GI NETs, it has also been tried in patients with MTC.
In 1990 Mahler et al. reported 3 patients with metastatic MTC treated daily with escalating doses of octreotide delivered via a subcutaneous pump. In 2 patients, both with MEN2A, CT levels dropped below baseline and remained at normal levels for 15 and 17 months. In addition, these patients reported improvement in symptoms, providing support for the use of the drug in MTC patients with symptoms refractory to other treatments.104 Two years later, Modigliani et al. published a trial including 14 patients with MTC and persistently elevated CT. These patients were stratified into high or low risk groups based on their pre-treatment CEA levels and given a daily infusion of 0.5 mg of octreotide for 90 days. In contrast to Mahler et al., this group found that treatment with octreotide was not associated with a sustained decrease in CT levels, nor did the drug cause significant tumor stabilization or regression. In fact, in the high risk group of patients, only 1 patient had a slight drop in CT, and all demonstrated progressive disease by imaging.105
These early disappointing results led investigators to experiment with octreotide combined with other antineoplastic therapies. Two different trials from the same Italian group combined octreotide with recombinant interferon-alpha 2b (IFNα2b), as this adjunct had been tried in other neuroendocrine tumors with positive results.106 The first trial, published in 1996, treated MTC patients for a total of 12 months with three times daily injections of octreotide and three times weekly injections of IFNα2b. Two of 8 patients enrolled in the study dropped out due to the side effects from IFNα2b treatment. The remaining 6 had no significant tumor response, but did have decreases in their CEA levels.107 Their second trial was published in 2000 and included 7 patients with symptomatic and advanced MTC. This time, patients were treated with lanreotide, another long-acting somatostatin analogue, in combination with IFNα2b. Tumor responses were better in this trial, with 3 patients demonstrating disease stabilization after 6 months of treatment, and 2 patients showing minor (< 25%) tumor regression. The two remaining patients continued to progress throughout treatment. Six of 7 patients had improvement of their symptoms. Despite the slightly improved outcomes compared to the previous trial, the authors concluded that IFNα2b was not a helpful adjunct to MTC treatment with somatostatin analogues as it caused unpleasant side effects in most patients and failed to have much effect on tumor growth.108
The most recent trial examining the utility of a somatostatin analogue for MTC treatment enrolled 22 patients with advanced MTC and treated all with either octreotide or octreotide LAR (the long-acting formulation). Thirteen patients in this group also received chemotherapy (n = 6), EBRT (n =2), or a combination of the two (n = 5). In the group of 13 patients treated with a somatostatin analogue plus additional treatments, 12 had subjective tumor responses—5 partial responses and 7 had stabilizations. In the group treated only with somatostatin analogues (n = 9), 6 had objective tumor responses—3 partial responses, and 3 stabilizations. There was no significant difference in the overall survival seen in the 2 different groups, and thus the authors reiterated what others had already found: somatostatin analogues may be helpful controlling symptoms in advanced MTC, but they do not appear to significantly affect tumor burden.109
PEPTIDE RECEPTOR RADIATION THERAPY
Peptide receptor radionuclide therapy (PRRT) was first developed to treat somatostatin receptor-expressing tumors, but has now expanded to use other receptors, such as cholecystokinin (CCK) receptors A and B. This treatment relies on the binding of a radiolabeled ligand to its respective receptor expressed on a tumor’s surface to produce a cytotoxic effect. Patients eligible for this treatment should have advanced cancer with inoperable metastases.110 In MTC, PRRT targeting SSTR2 and CCK-A or –B has been tried, as MTC expresses both receptors at high levels.102,111
The only prospective study using CCK-B/gastrin receptor-based PPRT treated 8 patients with advanced MTC with the radioligand 90Y-DTPT-D-Glu-minigastrin.112 Patients were injected with the radioligand at 4 to 6 week intervals and given escalating doses until toxicity limited further increases. Overall, 2 patients developed partial remissions and 4 had stable disease. Unfortunately, the regimen proved relatively toxic as 1 patient (the patient demonstrating the best response) went on to develop chronic myelogenous leukemia as well as grade I nephrotoxicity, and another developed chronic renal failure. Other PRRT trials in patients with MTC have all used radiolabeled somatostatin analogues that bind to SSTR2. Bodei et al. published their experience with SSTR PRRT in 21 MTC patients in 2004.113 Patients received varying cumulative doses of 90Y-DOTATOC, but regardless of dose, all were pretreated with amino acid solutions to prevent nephrotoxicity. They found that 67% of their patients derived a clinical benefit from PRRT—manifest as either an objective tumor response or stabilization of tumor burden. Two patients had complete responses, with the duration of clinical benefits ranging from 3 to 40 months. None of these patients suffered permanent renal toxicity. The authors were encouraged by the moderate responses in their patients, but noted that the patients treated in this study were the least likely to derive benefit from the treatment, as their tumor burdens were too great for optimal 90Y radiation effect.113
In 2007, a group in Basel, Switzerland published results from a phase II trial examining the efficacy of 90Y-DOTATOC treatment in 31 patients with stage IV MTC (M1 disease) who had progressed within the last 12 months and had visible radiolabeled somatostatin analogue uptake on Octreoscan.114 Patients were treated with 90Y-DOTATOC until they failed to respond to treatment. Treatment response was gauged based on alteration of CT doubling time, toxicity, and overall survival. Patients who demonstrated significant lengthening of their CT doubling time were characterized as ‘responders.” In this cohort, there were 18 responders (58.1%) and 13 nonresponders (41.9%). PRRT responders demonstrated significantly longer survival than nonresponders as measured both from the time of MTC diagnosis (108 months vs. 80 months) and from the time of 90Y-DOTATOC treatment (74.5 months vs. 10.8 months). Interestingly, there was no correlation between PRRT response and uptake on pretreatment Octreoscan, suggesting that Octreoscan may exclude patients from treatment that could actually benefit. As in other PRRT trials, some patients developed nephrotoxicity as a consequence of treatment, 4 of which were permanent toxicities. Though this study used changes in CT doubling time rather than objective tumor response by imaging to gauge benefit, the difference in survival between responders and nonresponders support the conclusion that PRRT has a positive effect on advanced MTC and is thus worthy of further study.114
The most recent report of PRRT for metastatic MTC evaluated both 90Y and 177Lu labeled somatostatin analogues.115 A total of 16 patients with advanced non-radioiodine-avid thyroid cancer were enrolled, 8 of whom had metastatic MTC. Patients received up to 5 treatments of PRRT. Of the patients with MTC, 4 maintained stable disease, 1 developed a partial remission, and 1 continued to progress. Two patients were lost to follow up. The patient with partial remission also had decreased CT levels, whereas those that progressed saw their CT levels increase. In this trial, only mild hematologic toxicity was seen, and all cases were reversible. Of the entire cohort, patients with MTC demonstrated the most promising responses to treatment, supporting the Swiss group’s conclusion that PRRT is a worthwhile addition to MTC treatment and warrants a phase III study.115
EXPERIMENTAL TREATMENTS
A number of other treatments are in the early stages of development for the treatment of advanced MTC, and will hopefully impact future management. Histone deacetylase inhibitors increase 125I accumulation in follicular cells and cause apoptosis. Reports of its use in 3 patients with MTC have been published, and in each case only disease stabilization was achieved. It is now being tried as a chemosensitizing agent.116–123 Thalidomide is known for its antiangiogenic properties and has been tried in 7 MTC patients in a phase II trial. Due to severe side effects, only 5 completed the study. A partial tumor response was achieved in only 1 patient.124–130 Plitidepsin is an antibiotic that can induce apoptosis and has been used to treat 1 patient with MTC. The patient had a partial response to treatment, with a 52% reduction in their nodal disease. A phase II trial is now underway.131 Radioimmunotherapy, a targeted treatment that uses bispecific anti-CEA monoclonal antibodies which also bind a radiolabeled hapten (131I or90Y-DOTA), has been reported in approximately 100 MTC patients. Most patients have stable disease with treatment, but a handful of minor tumor responses have been reported, as well as 1 complete response.132–138 Vaccination with dendritic cells pulsed with MTC-specific antigens has also been attempted. A trial in 7 MTC patients resulted in 1 objective tumor response and 2 biological responses.139,140
SUMMARY
For much of its history, medullary thyroid cancer has proven refractory to most medical therapies. Although DTIC or doxorubicin can produce tumor responses in a subset of MTC patients, no large trials demonstrating survival benefits exist. Nevertheless, until recently they were the only options showing any effectiveness, and they still may play a role when MTC fails with other treatments. Other therapies such as somatostatin analogues may relieve symptoms in some patients, but do not alter the disease course. External beam radiation and radioactive iodine are mostly ineffective. Additional chemotherapeutic combinations, novel agents, immunotherapy, and other drugs have all been tried in MTC, but were ineffective or remain in the early phases of development. Given the long record of failures in managing this frustrating disease, the high rates of partial response and disease stabilization with small-molecule kinase inhibitors mark an important shift in MTC treatment. Evidence from large randomized trials showing improved progression-free survival supports using drugs such as vandetanib and cabozantinib as first-line agents for symptomatic metastatic MTC. Sorafenib and sunitinib may also produce improvement in patients failing first-line drugs. Despite better progression-free survival, however, cytostatic rather than cytotoxic effects predominate with these kinase inhibitors, and their impact on overall survival remains to be demonstrated. Moreover, their adverse events are frequent and often serious. While the challenge posed by metastatic MTC has not been solved by currently-available kinase inhibitors, patients have more options than ever before. Research in the next few years will likely add additional kinase-inhibitors for patients progressing on or developing resistance to existing drugs, and the search continues for agents with greater efficacy and cytotoxicity for MTC cells.
Acknowledgments
Supported by NIH 5T32#CA148062-04 (JEM, SKS)
Footnotes
None of the authors has any potential conflicts of interest to disclose.
REFERENCES
- 1.Baloch ZW, LiVolsi VA. Prognostic Factors in Well-Differentiated Follicular-Derived Carcinoma and Medullary Thyroid Carcinoma. Thyroid. 2001;11(7):637–645. doi: 10.1089/105072501750362709. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe HJ, Melvin KEW, Cervi-Skinner SJ, et al. C-Cell Hyperplasia Preceding Medullary Thyroid Carcinoma. New England Journal of Medicine. 1973;289(9):437–441. doi: 10.1056/NEJM197308302890901. [DOI] [PubMed] [Google Scholar]
- 3.Pelizzo MR, Boschin IM, Bernante P, et al. Natural History, Diagnosis, Treatment and Outcome of Medullary Thyroid Cancer: 37 Years Experience on 157 Patients. Eur J Surg Oncol. 2007;33(4):493–497. doi: 10.1016/j.ejso.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Fugazzola L, Pinchera A, Luchetti F, et al. Disappearance Rate of Serum Calcitonin after Total Thyroidectomy for Medullary Thyroid Carcinoma. Int J Biol Markers. 1994;9(1):21–24. doi: 10.1177/172460089400900104. [DOI] [PubMed] [Google Scholar]
- 5.Meijer JA, le Cessie S, van den Hout WB, et al. Calcitonin and Carcinoembryonic Antigen Doubling Times as Prognostic Factors in Medullary Thyroid Carcinoma: A Structured Meta-Analysis. Clin Endocrinol (Oxf) 2010;72(4):534–542. doi: 10.1111/j.1365-2265.2009.03666.x. [DOI] [PubMed] [Google Scholar]
- 6.Kebebew E, Ituarte PHG, Siperstein AE, et al. Medullary Thyroid Carcinoma: Clinical Characteristics, Treatment, Prognostic Factors, and a Comparison of Staging Systems. Cancer. 2000;88:1139–1148. doi: 10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Rendl G, Manzl M, Hitzl W, et al. Long-Term Prognosis of Medullary Thyroid Carcinoma. Clin Endocrinol (Oxf) 2008;69(3):497–505. doi: 10.1111/j.1365-2265.2008.03229.x. [DOI] [PubMed] [Google Scholar]
- 8.Modigliani E, Cohen R, Campos J, et al. Prognostic Factors for Survival and for Biochemical Cure in Medullary Thyroid Carcinoma: Results in 899 Patients. Clinical Endocrinology. 1998;48:265–273. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 9.Kloos RT, Eng C, Evans DB, et al. Medullary Thyroid Cancer: Management Guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 10.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase Iii Trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J Clin Oncol. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a Specific Inhibitor of the Bcr-Abl Tyrosine Kinase in the Blast Crisis of Chronic Myeloid Leukemia and Acute Lymphoblastic Leukemia with the Philadelphia Chromosome. N Engl J Med. 2001;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 13.Hochhaus A, Druker B, Sawyers C, et al. Favorable Long-Term Follow-up Results over 6 Years for Response, Survival, and Safety with Imatinib Mesylate Therapy in Chronic-Phase Chronic Myeloid Leukemia after Failure of Interferon-Alpha Treatment. Blood. 2008;111(3):1039–1043. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 14.DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-Term Results of Adjuvant Imatinib Mesylate in Localized, High-Risk, Primary Gastrointestinal Stromal Tumor: Acosog Z9000 (Alliance) Intergroup Phase 2 Trial. Ann Surg. 2013;258(3):422–429. doi: 10.1097/SLA.0b013e3182a15eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew CG, Chin KS, Easton DF, et al. A Linked Genetic Marker for Multiple Endocrine Neoplasia Type 2a on Chromosome 10. Nature. 1987;328(6130):527–528. doi: 10.1038/328527a0. [DOI] [PubMed] [Google Scholar]
- 16.Simpson NE, Kidd KK, Goodfellow PJ, et al. Assignment of Multiple Endocrine Neoplasia Type 2a to Chromosome 10 by Linkage. Nature. 1987;328(6130):528–530. doi: 10.1038/328528a0. [DOI] [PubMed] [Google Scholar]
- 17.Lairmore TC, Dou S, Howe JR, et al. A 1.5-Megabase Yeast Artificial Chromosome Contig from Human Chromosome 10q11.2 Connecting Three Genetic Loci (Ret, D10s94, and D10s102) Closely Linked to the Men2a Locus. Proc Natl Acad Sci U S A. 1993;90(2):492–496. doi: 10.1073/pnas.90.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan LM, Kwok JB, Healey CS, et al. Germ-Line Mutations of the Ret Proto-Oncogene in Multiple Endocrine Neoplasia Type 2a. Nature. 1993;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 19.Donis-Keller H, Dou S, Chi D, et al. Mutations in the Ret Proto-Oncogene Are Associated with Men 2a and Fmtc. Hum Mol Genet. 1993;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 20.Hofstra RM, Landsvater RM, Ceccherini I, et al. A Mutation in the Ret Proto-Oncogene Associated with Multiple Endocrine Neoplasia Type 2b and Sporadic Medullary Thyroid Carcinoma. Nature. 1994;367(6461):375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 21.Eng C, Smith DP, Mulligan LM, et al. Point Mutation within the Tyrosine Kinase Domain of the Ret Proto-Oncogene in Multiple Endocrine Neoplasia Type 2b and Related Sporadic Tumours. Hum Mol Genet. 1994;3(2):237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- 22.Carlson KM, Dou S, Chi D, et al. Single Missense Mutation in the Tyrosine Kinase Catalytic Domain of the Ret Protooncogene Is Associated with Multiple Endocrine Neoplasia Type 2b. Proc Natl Acad Sci U S A. 1994;91(4):1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elisei R, Cosci B, Romei C, et al. Prognostic Significance of Somatic Ret Oncogene Mutations in Sporadic Medullary Thyroid Cancer: A 10-Year Follow-up Study. J Clin Endocrinol Metab. 2008;93(3):682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 24.Eng C, Clayton D, Schuffenecker I, et al. The Relationship between Specific Ret Proto-Oncogene Mutations and Disease Phenotype in Multiple Endocrine Neoplasia Type 2. International Ret Mutation Consortium Analysis. JAMA. 1996;276(19):1575–1579. [PubMed] [Google Scholar]
- 25.Wells SA, Jr, Santoro M. Targeting the Ret Pathway in Thyroid Cancer. Clin Cancer Res. 2009;15(23):7119–7123. doi: 10.1158/1078-0432.CCR-08-2742. [DOI] [PubMed] [Google Scholar]
- 26.Houvras Y. Completing the Arc: Targeted Inhibition of Ret in Medullary Thyroid Cancer. J Clin Oncol. 2012;30(2):200–202. doi: 10.1200/JCO.2011.38.7639. [DOI] [PubMed] [Google Scholar]
- 27.Hong D, Ye L, Gagel R, et al. Medullary Thyroid Cancer: Targeting the Ret Kinase Pathway with Sorafenib/Tipifarnib. Mol Cancer Ther. 2008;7(5):1001–1006. doi: 10.1158/1535-7163.MCT-07-2422. [DOI] [PubMed] [Google Scholar]
- 28.Santoro M, Carlomagno F, Romano A, et al. Activation of Ret as a Dominant Transforming Gene by Germline Mutations of Men2a and Men2b. Science. 1995;267(5196):381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 29.Sherman SK, Howe JR. Translational Research in Endocrine Surgery. Surg Oncol Clin N Am. 2013;22(4):857–884. doi: 10.1016/j.soc.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapiteijn E, Schneider TC, Morreau H, et al. New Treatment Modalities in Advanced Thyroid Cancer. Ann Oncol. 2012;23(1):10–18. doi: 10.1093/annonc/mdr117. [DOI] [PubMed] [Google Scholar]
- 31.Davies H, Bignell GR, Cox C, et al. Mutations of the Braf Gene in Human Cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 32.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of Activation of the Raf-Erk Signaling Pathway by Oncogenic Mutations of B-Raf. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 33.Tufano RP, Teixeira GV, Bishop J, et al. Braf Mutation in Papillary Thyroid Cancer and Its Value in Tailoring Initial Treatment: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2012;91(5):274–286. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 34.Nixon IJ, Shaha AR, Tuttle MR. Targeted Therapy in Thyroid Cancer. Curr Opin Otolaryngol Head Neck Surg. 2013;21(2):130–134. doi: 10.1097/MOO.0b013e32835aa2c2. [DOI] [PubMed] [Google Scholar]
- 35.Carlomagno F, Vitagliano D, Guida T, et al. Zd6474, an Orally Available Inhibitor of Kdr Tyrosine Kinase Activity, Efficiently Blocks Oncogenic Ret Kinases. Cancer Res. 2002;62(24):7284–7290. [PubMed] [Google Scholar]
- 36.Carlomagno F, Santoro M. Identification of Ret Kinase Inhibitors as Potential New Treatment for Sporadic and Inherited Thyroid Cancer. J Chemother. 2004;16(Suppl 4):49–51. doi: 10.1179/joc.2004.16.Supplement-1.49. [DOI] [PubMed] [Google Scholar]
- 37.Wells SA, Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the Treatment of Patients with Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. J Clin Oncol. 2010;28(5):767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson BG, Paz-Ares L, Krebs A, et al. Vandetanib (100 Mg) in Patients with Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. J Clin Endocrinol Metab. 2010;95(6):2664–2671. doi: 10.1210/jc.2009-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haraldsdottir S, Shah MH. An Update on Clinical Trials of Targeted Therapies in Thyroid Cancer. Curr Opin Oncol. 2014;26(1):36–44. doi: 10.1097/CCO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 40.Kurzrock R, Sherman SI, Ball DW, et al. Activity of Xl184 (Cabozantinib), an Oral Tyrosine Kinase Inhibitor, in Patients with Medullary Thyroid Cancer. J Clin Oncol. 2011;29(19):2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yakes FM, Chen J, Tan J, et al. Cabozantinib (Xl184), a Novel Met and Vegfr2 Inhibitor, Simultaneously Suppresses Metastasis, Angiogenesis, and Tumor Growth. Mol Cancer Ther. 2011;10(12):2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute Common Terminology Criteria for Adverse Events V3.0. National Cancer Institute; 2006. [Accessed 2/14/2014]. http://www.ctep.cancer.gov. [Google Scholar]
- 43.International Conference on Harmonisation Guideline. Clinical Saftey Data Management: Definitions and Standards for Expedited Reporting. [Accessed 2/14/2014];1995 http://www.fda.gov.
- 44.Tuttle RM, Ball DW, Byrd D, et al. Thyroid Carcinoma. [Accessed January 29, 2014, 2014];NCCN Clinical Practice Guidelines in Oncology. 2013 2.2013. [Google Scholar]
- 45.Lam ET, Ringel MD, Kloos RT, et al. Phase Ii Clinical Trial of Sorafenib in Metastatic Medullary Thyroid Cancer. J Clin Oncol. 2010;28(14):2323–2330. doi: 10.1200/JCO.2009.25.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong DS, Cabanillas ME, Wheler J, et al. Inhibition of the Ras/Raf/Mek/Erk and Ret Kinase Pathways with the Combination of the Multikinase Inhibitor Sorafenib and the Farnesyltransferase Inhibitor Tipifarnib in Medullary and Differentiated Thyroid Malignancies. J Clin Endocrinol Metab. 2011;96(4):997–1005. doi: 10.1210/jc.2010-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capdevila J, Iglesias L, Halperin I, et al. Sorafenib in Metastatic Thyroid Cancer. Endocr Relat Cancer. 2012;19(2):209–216. doi: 10.1530/ERC-11-0351. [DOI] [PubMed] [Google Scholar]
- 48.Frank-Raue K, Ganten M, Kreissl MC, Raue F. Rapid Response to Sorafenib in Metastatic Medullary Thyroid Carcinoma. Exp Clin Endocrinol Diabetes. 2011;119(3):151–155. doi: 10.1055/s-0030-1262836. [DOI] [PubMed] [Google Scholar]
- 49.Aller S, Popescu A, Rao S, et al. Transient Partial Response to Sorafenib Treatment in an Adolescent Patient with Men2b Syndrome and End-Stage Medullary Thyroid Carcinoma. Pediatr Blood Cancer. 2012;58(1):98–100. doi: 10.1002/pbc.23032. [DOI] [PubMed] [Google Scholar]
- 50.Carr LL, Mankoff DA, Goulart BH, et al. Phase Ii Study of Daily Sunitinib in Fdg-Pet-Positive, Iodine-Refractory Differentiated Thyroid Cancer and Metastatic Medullary Carcinoma of the Thyroid with Functional Imaging Correlation. Clin Cancer Res. 2010;16(21):5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelleher FC, McDermott R. Response to Sunitinib in Medullary Thyroid Cancer. Ann Intern Med. 2008;148(7):567. doi: 10.7326/0003-4819-148-7-200804010-00027. [DOI] [PubMed] [Google Scholar]
- 52.Cleary JM, Sadow PM, Randolph GW, et al. Neoadjuvant Treatment of Unresectable Medullary Thyroid Cancer with Sunitinib. J Clin Oncol. 2010;28(23):e390–e392. doi: 10.1200/JCO.2009.27.4225. [DOI] [PubMed] [Google Scholar]
- 53.Mologni L, Redaelli S, Morandi A, et al. Ponatinib Is a Potent Inhibitor of Wild-Type and Drug-Resistant Gatekeeper Mutant Ret Kinase. Mol Cell Endocrinol. 2013;377(1–2):1–6. doi: 10.1016/j.mce.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Frank-Raue K, Fabel M, Delorme S, et al. Efficacy of Imatinib Mesylate in Advanced Medullary Thyroid Carcinoma. Eur J Endocrinol. 2007;157(2):215–220. doi: 10.1530/EJE-06-0695. [DOI] [PubMed] [Google Scholar]
- 55.de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, et al. A Phase Ii Trial of Imatinib Therapy for Metastatic Medullary Thyroid Carcinoma. J Clin Endocrinol Metab. 2007;92(9):3466–3469. doi: 10.1210/jc.2007-0649. [DOI] [PubMed] [Google Scholar]
- 56.Pennell NA, Daniels GH, Haddad RI, et al. A Phase Ii Study of Gefitinib in Patients with Advanced Thyroid Cancer. Thyroid. 2008;18(3):317–323. doi: 10.1089/thy.2007.0120. [DOI] [PubMed] [Google Scholar]
- 57.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib Is an Active Treatment for All Histologic Subtypes of Advanced Thyroid Cancer: Results from a Phase Ii Study. J Clin Oncol. 2008;26(29):4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlumberger MJ, Elisei R, Bastholt L, et al. Phase Ii Study of Safety and Efficacy of Motesanib in Patients with Progressive or Symptomatic, Advanced or Metastatic Medullary Thyroid Cancer. J Clin Oncol. 2009;27(23):3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 59.De Falco V, Buonocore P, Muthu M, et al. Ponatinib (Ap24534) Is a Novel Potent Inhibitor of Oncogenic Ret Mutants Associated with Thyroid Cancer. J Clin Endocrinol Metab. 2013;98(5):E811–E819. doi: 10.1210/jc.2012-2672. [DOI] [PubMed] [Google Scholar]
- 60.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome-Positive Leukemias. N Engl J Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ponatinib Prescribing Information. [Accessed 4/22/2014];2013 http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203469s007s008lbl.pdf.
- 62.Mayer K, Gielen GH, Willinek W, et al. Fatal Progressive Cerebral Ischemia in Cml under Third-Line Treatment with Ponatinib. Leukemia. 2014;28(4):976–977. doi: 10.1038/leu.2013.320. [DOI] [PubMed] [Google Scholar]
- 63.Sherman SI. Lessons Learned and Questions Unanswered from Use of Multitargeted Kinase Inhibitors in Medullary Thyroid Cancer. Oral Oncol. 2013;49(7):707–710. doi: 10.1016/j.oraloncology.2013.03.442. [DOI] [PubMed] [Google Scholar]
- 64.Gottlieb JA, Hill CS, Ibanez ML, Clark RL. Chemotherapy of Thyrod Cancer: An Evaluation of Experience with 37 Patients. Cancer. 1972;30(3):848–853. doi: 10.1002/1097-0142(197209)30:3<848::aid-cncr2820300336>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 65.Husain M, Alsever RN, Lock JP, et al. Failure of Medullary Carcinoma of the Thyroid to Respond to Doxorubicin Therapy. Hormone Research. 1978;9(1):22–25. doi: 10.1159/000178893. [DOI] [PubMed] [Google Scholar]
- 66.Gottlieb JA, Hill CS. Chemotherapy of Thyroid Cancer with Adriamycin: Experience with 30 Patients. NEJM. 1974;290(4):193–197. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 67.Shimaoka K, Schoenfeld DA, DeWys WD, et al. A Randomized Trial of Doxorubicin Versus Doxorubicin Plus Cisplatin in Patients with Advanced Thyroid Carcinoma. Cancer. 1985;56:2155–2160. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 68.Sridhar KS, Holland JF, Brown JC, et al. Doxorubicin Plus Cisplatin in the Treatment of Apudomas. Cancer. 1985;55:2634–2637. doi: 10.1002/1097-0142(19850601)55:11<2634::aid-cncr2820551117>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 69.Scherubl H, Raue F, Ziegler R. Combination Chemotherapy of Advanced Medullary and Differentiated Thyroid Cancer: Phase Ii Study. J Cancer Res Clin Oncol. 1990;116:21–23. doi: 10.1007/BF01612635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Droz J, Schlumberger M, Rougier P, et al. Chemotherapy in Metastatic Nonanaplastic Thyroid Cancer: Experience at the Institut Gustave-Roussy. Tumori. 1990;76:480–483. doi: 10.1177/030089169007600513. [DOI] [PubMed] [Google Scholar]
- 71.Kessinger A, Foley JF, Lemon HM. Therapy of Malignant Apud Cell Tumors: Effectiveness of Dtic. Cancer. 1983;51:790–794. doi: 10.1002/1097-0142(19830301)51:5<790::aid-cncr2820510507>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 72.Petursson SR. Metastatic Medullary Thyroid Carcinoma: Complete Response to Combination Chemotherapy with Dacarbazine and 5-Fluorouracil. Cancer. 1988;62:1899–1903. doi: 10.1002/1097-0142(19881101)62:9<1899::aid-cncr2820620905>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 73.Orlandi F, Caraci P, Berruti A, et al. Chemotherapy with Dacarbazine and 5-Fluorouracil in Advanced Medullary Thyroid Cancer. Annals of Oncology. 1994;5:763–765. doi: 10.1093/oxfordjournals.annonc.a058984. [DOI] [PubMed] [Google Scholar]
- 74.Wu L, Averbuch SD, Ball DW, et al. Treatment of Advanced Medullary Thyroid Carcinoma with a Combination of Cyclophosphamide, Vincristine, and Dacarbazine. Cancer. 1994;73:432–436. doi: 10.1002/1097-0142(19940115)73:2<432::aid-cncr2820730231>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 75.Schlumberger M, Abdelmoumene N, Delisle MJ, Couette JE. Treatment of Advanced Medullary Thyroid Cancer with an Alternating Combination of 5 Fu-Streptozocin and 5 Fu-Dacarbazine. Br J Cancer. 1995;71:363–365. doi: 10.1038/bjc.1995.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nocera M, Baudin E, Pellegriti G, et al. Treatment of Advanced Medullary Thyroid Cancer with an Alternating Combination of Doxorubicin-Streptozocin and 5 Fu-Dacarbazine. British Journal of Cancer. 2000;83(6):715–718. doi: 10.1054/bjoc.2000.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bajetta E, Rimassa L, Carnaghi C, et al. 5-Fluorouracil, Dacarbazine, and Epirubicin in the Treatment of Patients with Neuroendocrine Tumors. Cancer. 1998;83:372–378. doi: 10.1002/(sici)1097-0142(19980715)83:2<372::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 78.Di Bartolomeo M, Bajetta E, Bochicchio AM, et al. A Phase Ii Trial of Dacarbazine, Fluorouracil and Epirubicin in Patients with Neuroendocrine Tumors. Annals of Oncology. 1995;6:77–79. doi: 10.1093/oxfordjournals.annonc.a059049. [DOI] [PubMed] [Google Scholar]
- 79.Ishikawa T, Utoh M, Sawada N, et al. Tumor Selective Delivery of 5-Fluorouracil by Capecitabine, a New Oral Fluoropyrimidine Carbamate, in Human Cancer Xenografts. Biochemical Pharmacology. 1998;55:1091–1097. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- 80.Labidi SI, Gravis G, Tarpin C, et al. Medullary Thyroid Cancer Treated by Capecitabine. Anti-Cancer Drugs. 2007;18:831–834. doi: 10.1097/CAD.0b013e3280adc8f3. [DOI] [PubMed] [Google Scholar]
- 81.Gilliam LK, Kohn AD, Lalani T, et al. Capecitabine Therapy for Refractory Metastatic Thyroid Carcinoma: A Case Series. Thyroid. 2006;16(8):801–810. doi: 10.1089/thy.2006.16.801. [DOI] [PubMed] [Google Scholar]
- 82.Paiva CE. Use of Capecitabine in Refractory Metastatic Medullary Thyroid Carcinoma. Thyroid. 2008;18(5):587–588. doi: 10.1089/thy.2007.0220. [DOI] [PubMed] [Google Scholar]
- 83.Steinfeld AD. The Role of Radiation Therapy in Medullary Carcinoma of the Thyroid. Radiology. 1977;123:745–746. doi: 10.1148/123.3.745. [DOI] [PubMed] [Google Scholar]
- 84.Samaan NA, Schultz PN, Hickey RC. Medullary Thyroid Carcinoma: Prognosis of Familial Versus Sporadic Disease and the Role of Radiotherapy. Journal of Clinical Endocrinology and Metabolism. 1988;67(4):801–805. doi: 10.1210/jcem-67-4-801. [DOI] [PubMed] [Google Scholar]
- 85.Jensen MH, Davis K, Derrick L. Thyroid Cancer: A Computer-Assisted Review of 5287 Cases. Otolaryngology--Head and neck surgery. 1990;102(1):51–65. doi: 10.1177/019459989010200109. [DOI] [PubMed] [Google Scholar]
- 86.Brierly J, Tsang R, Simpson WJ, et al. Medullary Thyroid Cancer: Analyses of Survival and Prognostic Factors and the Role of Radiation Therapy in Local Control. Thyroid. 1996;6(4):305–310. doi: 10.1089/thy.1996.6.305. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz DL, Rana V, Shaw S, et al. Postoperative Radiotherapy for Advanced Medullary Thyroid Cancer--Local Disease Control in the Modern Era. Head Neck. 2008;30(7):883–888. doi: 10.1002/hed.20791. [DOI] [PubMed] [Google Scholar]
- 88.Martinez SR, Beal SH, Chen A, et al. Adjuvant External Beam Radiation for Medullary Thyroid Carcinoma. J Surg Oncol. 2010;102(2):175–178. doi: 10.1002/jso.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maheshwari YK, Hill CS, Haynie TP, et al. 131i Therapy in Differentiated Thyroid Cancer: M.D. Anderson Hospital Experience. Cancer. 1981;47:664–671. doi: 10.1002/1097-0142(19810215)47:4<664::aid-cncr2820470408>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 90.Ljungberg O. Medullary Carcinoma of the Human Thyroid Gland: Autoradiographic Localization of Radioiodine. Acta Pathologica et Microbiologica Scandinavica. 1966;68(4):476–480. doi: 10.1111/apm.1966.68.4.476. [DOI] [PubMed] [Google Scholar]
- 91.Feinstein RE, Gimeno EJ, El-Salhy M, et al. Evidence of C-Cell Destruction in the Thyroid Gland of Mice Exposed to High 131i Doses. Acta Radiologica Oncology. 1986;25(3):199–202. doi: 10.3109/02841868609136405. [DOI] [PubMed] [Google Scholar]
- 92.Thurston V, Williams ED. The Effect of Radiation on Thyroid C Cells. Acta Endocrinologica. 1982;99(1):72–78. doi: 10.1530/acta.0.0990072. [DOI] [PubMed] [Google Scholar]
- 93.Shah KH, Oslapas R, Calandra DB, et al. Effects of Radiation on Parafollicular C Cells of the Thyroid Gland. Surgery. 1983;94(6):989–994. [PubMed] [Google Scholar]
- 94.Ott RA, Hofmann C, Oslapas R, et al. Radioiodine Sensitivity of Parafollicular C Cells in Aged Long-Evans Rats. Surgery. 1987;102(6):1043–1048. [PubMed] [Google Scholar]
- 95.Deftos LJ, Stein MF. Radioiodine as an Adjunct to the Surgical Treatment of Medullary Thyroid Carcinoma. Journal of Clinical Endocrinology and Metabolism. 1980;50(5):967–968. doi: 10.1210/jcem-50-5-967. [DOI] [PubMed] [Google Scholar]
- 96.Hellman DE, Kartchner M, Van Antwerp JD, et al. Radioiodine in the Treatment of Medullary Carcinoma of the Thyroid. Journal of Clinical Endocrinology and Metabolism. 1979;48(3):451–455. doi: 10.1210/jcem-48-3-451. [DOI] [PubMed] [Google Scholar]
- 97.Saad MF, Guido JJ, Samaan NA. Radioactive Iodine in the Treatment of Medullary Carcinoma of the Thyroid. Journal of Clinical Endocrinology and Metabolism. 1983;57(1):124–128. doi: 10.1210/jcem-57-1-124. [DOI] [PubMed] [Google Scholar]
- 98.Erdogan MF, Gursoy A, Erdogan G, Kamel N. Radioactive Iodine Treatment in Medullary Thyroid Carcinoma. Nuclear Medicine Communications. 2006;27:359–362. doi: 10.1097/01.mnm.0000202860.30274.e4. [DOI] [PubMed] [Google Scholar]
- 99.Meijer JA, Bakker LE, Valk GD, et al. Radioactive Iodine in the Treatment of Medullary Thyroid Carcinoma: A Controlled Multicenter Study. Eur J Endocrinol. 2013;168(5):779–786. doi: 10.1530/EJE-12-0943. [DOI] [PubMed] [Google Scholar]
- 100.Liu D, Hu S, Hou P, et al. Suppression of Braf/Mek/Map Kinase Pathway Restores Expression of Iodide-Metabolizing Genes in Thyroid Cells Expressing the V600e Braf Mutant. Clin Cancer Res. 2007;13(4):1341–1349. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]
- 101.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-Enhanced Radioiodine Uptake in Advanced Thyroid Cancer. N Engl J Med. 2013;368(7):623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mato E, Matias-Guiu X, Chico A, et al. Somatostatin and Somatostatin Receptor Subtype Gene Expression in Medullary Thyroid Carcinoma. Journal of Clinical Endocrinology and Metabolism. 1998;83(7):2417–2420. doi: 10.1210/jcem.83.7.4955. [DOI] [PubMed] [Google Scholar]
- 103.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide Lar in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report from the Promid Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 104.Mahler C, Verhelst J, De Longueville M, Harris A. Long-Term Treatment of Metastatic Medullary Thyroid Carcinoma with the Somatostatin Analogue Octreotide. Clinical Endocrinology. 1990;33:261–269. doi: 10.1111/j.1365-2265.1990.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 105.Modigliani E, Cohen R, Joannidis S, et al. Results of Long-Term Continuous Subcutaneous Octreotide Administration in 14 Patients with Medullary Thyroid Carcinoma. Clinical Endocrinology. 1992;36:183–186. doi: 10.1111/j.1365-2265.1992.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 106.Janson ET, Oberg K. Long-Term Management of the Carcinoid Syndrome: Treatment with Octreotide Alone and in Combination with Alpha-Interferon. Acta Oncologica. 1993;32(2):225–229. doi: 10.3109/02841869309083916. [DOI] [PubMed] [Google Scholar]
- 107.Lupoli G, Cascone E, Arlotta F, et al. Treatment of Advanced Medullary Thyroid Carcinoma with a Combination of Recombinant Interferon Α-2b and Octreotide. Cancer. 1996;78:1114–1118. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1114::AID-CNCR23>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 108.Vitale G, Tagliaferrie P, Caraglia M, et al. Slow Release Lanreotide in Combination with Interferon-Α2b in the Treatment of Symptomatic Advanced Medullary Thyroid Cancer. Journal of Clinical Endocrinology and Metabolism. 2000;85(3):983–988. doi: 10.1210/jcem.85.3.6435. [DOI] [PubMed] [Google Scholar]
- 109.Vainas I, Koussis C, Pazaitou-Panayiotou K, et al. Somatostatin Receptor Expression in Vivo and Response to Somatostatin Analog Therapy with or without Other Antineoplastic Treatments in Advanced Medullary Thyroid Carcinoma. J. Exp. Clin. Cancer Res. 2004;23(4):549–559. [PubMed] [Google Scholar]
- 110.Reubi JC, Macke HR, Krenning E. Candidates for Peptide Receptor Radiotherapy Today and in the Future. J Nucl Med. 2005;46:67S–75S. [PubMed] [Google Scholar]
- 111.Reubi JC, Schaer J, Waser B. Cholecystokinin(Cck)-a and Cck-B/Gastrin Receptors in Human Tumors. Cancer Research. 1997;57:1377–1386. [PubMed] [Google Scholar]
- 112.Behe M, Behr TM. Cholecystokinin-B (Cck-B)/Gastrin Receptor Targeting Peptides for Staging and Therapy of Medullary Thyroid Cancer and Other Cck-B Receptor Expressing Malignancies. Biopolymers. 2002;66(6):399–418. doi: 10.1002/bip.10356. [DOI] [PubMed] [Google Scholar]
- 113.Bodei L, Handkiewicz-Junak D, Grana C, et al. Receptor Radionuclide Therapy with 90y-Dotatoc in Patients with Medullary Thyroid Carcinomas. Cancer biotherapy and radiopharmaceuticals. 2004;19(1):65–71. doi: 10.1089/108497804773391694. [DOI] [PubMed] [Google Scholar]
- 114.Iten F, Muller B, Schindler C, et al. Response to [90yttrium-Dota]-Toc Treatment Is Associated with Long-Term Survival Benefit in Metastasized Medullary Thyroid Cancer: A Phase Ii Clinical Trial. Clin Cancer Res. 2007;13(22 Pt 1):6696–6702. doi: 10.1158/1078-0432.CCR-07-0935. [DOI] [PubMed] [Google Scholar]
- 115.Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide Receptor Radionuclide Therapy of Treatment-Refractory Metastatic Thyroid Cancer Using (90)Yttrium and (177)Lutetium Labeled Somatostatin Analogs: Toxicity, Response and Survival Analysis. Am J Nucl Med Mol Imaging. 2013;4(1):39–52. [PMC free article] [PubMed] [Google Scholar]
- 116.Richon VM, Webb Y, Merger R, et al. Second Generation Hybrid Polar Compounds Are Potent Inducers of Transformed Cell Differentiation. Proc Natl Acad Sci. 1996;93:5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Richon VM, Zhou X, Rifkind RA, Marks PA. Histone Deacetylase Inhibitors: Development of Suberoylanilide Hydroxamic Acid (Saha) for the Treatment of Cancers. Blood Cells Mol Dis. 2001;27(1):260–264. doi: 10.1006/bcmd.2000.0376. [DOI] [PubMed] [Google Scholar]
- 118.Furuya F, Shimura H, Suzuki H, et al. Histone Deacetylase Inhibitors Restore Radioiodide Uptake and Retention in Poorly Differentiated and Anaplastic Thyroid Cancer Cells by Expression of the Sodium/Iodide Symporter Thyroperoxidase and Thyroglobulin. Endocrinology. 2004;145(6):2865–2875. doi: 10.1210/en.2003-1258. [DOI] [PubMed] [Google Scholar]
- 119.Kitazono M, Robey R, Zhan Z, et al. Low Concentrations of the Histone Deacetylase Inhibitor, Depsipeptide (Fr901228), Increase Expression of the Na+/I- Symporter and Iodine Accumulation in Poorly Differentiated Thyroid Carcinoma Cells. J Clin Endocrinol Metab. 2001;86:3430–3435. doi: 10.1210/jcem.86.7.7621. [DOI] [PubMed] [Google Scholar]
- 120.Cohen LA, Marks PA, Rifkind RA, et al. Suberoylanilide Hydroxamic Acid (Saha), a Histone Deacetylase Inhibitor, Suppresses the Growth of Carcinogen-Induced Mammary Tumors. Anticancer Research. 2002;22:1497–1504. [PubMed] [Google Scholar]
- 121.Mitsiades CS, Poulaki V, McMullan C, et al. Novel Histone Deacetylase Inhibitors in the Treatment of Thyroid Cancer. Clin Cancer Res. 2005;11:3958–3965. doi: 10.1158/1078-0432.CCR-03-0776. [DOI] [PubMed] [Google Scholar]
- 122.Kelly WK, O'Connor OA, Krug LM, et al. Phase I Study of an Oral Histone Deacetylase Inhibitor, Suberoylanilide Hydroxamic Acid, in Patients with Advanced Cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woyach JA, Kloos RT, Ringel MD, et al. Lack of Therapeutic Effect of the Histone Deacetylase Inhibitor Vorinostat in Patients with Metastatic Radioiodine-Refractory Thyroid Carcinoma. J Clin Endocrinol Metab. 2009;94(1):164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide Is an Inhibitor of Angiogenesisi. Proc Natl Acad Sci. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amato RJ. Thalidomide Therapy for Renal Cell Carcinoma. Critical Reviews in Oncology/Hematology. 2003;46:59–65. doi: 10.1016/s1040-8428(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 126.Drake MJ, Robson W, Mehta P, et al. An Open-Label Phase Ii Study of Low-Dose Thalidomide in Androgen-Independent Prostate Cancer. Br J Cancer. 2003;88(6):822–827. doi: 10.1038/sj.bjc.6600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fine HA, Figg WD, Jaeckle K, et al. Phase Ii Trial of the Antiangiogenic Agent Thalidomide in Patients with Recurrent High-Grade Gliomas. J Clin Oncol. 2000;18(708–715) doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 128.Singhal S, Mehta J, Desikan R, et al. Antitumor Activity of Thalidomide in Refractory Multiple Myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 129.Hsu C, Chen C, Chen L, et al. Low-Dose Thalidomide Treatment for Advanced Hepatocellular Carcinoma. Oncology. 2003;65(3):242–249. doi: 10.1159/000074477. [DOI] [PubMed] [Google Scholar]
- 130.Ain KB, Lee C, Williams KD. Phase Ii Trial of Thalidomide for Therapy of Radioiodine-Unresponsive and Rapidly Progressive Thyroid Carcinomas. Thyroid. 2007;17(7):663–670. doi: 10.1089/thy.2006.0289. [DOI] [PubMed] [Google Scholar]
- 131.Izquierdo MA, Bowman A, Garcia M, et al. Phase I Clinical and Pharmacokinetic Study of Plitidepsin as a 1-Hour Weekly Intravenous Infusion in Patients with Advanced Solid Tumors. Clin Cancer Res. 2008;14(10):3105–3112. doi: 10.1158/1078-0432.CCR-07-1652. [DOI] [PubMed] [Google Scholar]
- 132.Kraeber-Bodere F, Salaun PY, Ansquer C, et al. Pretargeted Radioimmunotherapy (Prait) in Medullary Thyroid Cancer (Mtc) Tumour Biol. 2012;33(3):601–606. doi: 10.1007/s13277-012-0359-6. [DOI] [PubMed] [Google Scholar]
- 133.Juweid ME, Hajjar G, Swayne LC, et al. Phase I/Ii Trial of 131i-Mn-14 F(Ab)2 Anti-Carcinoembryonic Antigen Monoclonal Antibody in the Treatment of Patients with Metastatic Medullary Thyroid Carcinoma. Cancer. 1999;85:1828–1842. doi: 10.1002/(sici)1097-0142(19990415)85:8<1828::aid-cncr25>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 134.Kraeber-Bodere F, Bardet S, Hoefnagel CA. Radioimmunotherapy in Medullary Thyroid Cancer Using Bispecific Antibody and Iodine 131-Labeled Bivalent Hapten: Preliminary Results of a Phase I/Ii Clinical Trial. Clinical Cancer Research. 1999;5:3190–3198. [PubMed] [Google Scholar]
- 135.Chatal JF, Campion L, Kraeber-Bodere F, et al. Survival Improvement in Patients with Medullary Thyroid Carcinoma Who Undergo Pretargeted Anti-Carcinoembryonic-Antigen Radioimmunotherapy: A Collaborative Study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24(11):1705–1711. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 136.Salaun PY, Campion L, Bournaud C, et al. Phase Ii Trial of Anticarcinoembryonic Antigen Pretargeted Radioimmunotherapy in Progressive Metastatic Medullary Thyroid Carcinoma: Biomarker Response and Survival Improvement. J Nucl Med. 2012;53(8):1185–1192. doi: 10.2967/jnumed.111.101865. [DOI] [PubMed] [Google Scholar]
- 137.Kraeber-Bodere F, Sai-Maurel C, Campion L, et al. Enhanced Antitumor Activity of Combined Pretargeted Radioimmunotherapy and Paclitaxel in Medullary Thyroid Cancer Xenograft. Molecular Cancer Therapeutics. 2002;1:267–274. [PubMed] [Google Scholar]
- 138.Stein R, Chen S, Reed L, et al. Combining Radioimmunotherapy and Chemotherapy for Treatment of Medullary Thyroid Carcinoma: Effectiveness of Dacarbazine. Cancer. 2002;94(1):51–61. doi: 10.1002/cncr.10157. [DOI] [PubMed] [Google Scholar]
- 139.Schott M, Feldkamp J, Klucken M, et al. Calcitonin-Specific Antitumor Immunity in Medullary Thyroid Carcinoma Following Dendritic Cell Vaccination. Cancer Immunol Immunother. 2002;51(11–12):663–668. doi: 10.1007/s00262-002-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schott M, Seissler J, Lettmann M, et al. Immunotherapy for Medullary Thyroid Carcinoma by Dendritic Cell Vaccination. J Clin Endocrinol Metab. 2001;86:4965–4969. doi: 10.1210/jcem.86.10.7949. [DOI] [PubMed] [Google Scholar]
- 141.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase Ii Trial of Sorafenib in Advanced Thyroid Cancer. J Clin Oncol. 2008;26(29):4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rosen LS, Kurzrock R, Mulay M, et al. Safety, Pharmacokinetics, and Efficacy of Amg 706, an Oral Multikinase Inhibitor, in Patients with Advanced Solid Tumors. J Clin Oncol. 2007;25(17):2369–2376. doi: 10.1200/JCO.2006.07.8170. [DOI] [PubMed] [Google Scholar]


