Abstract
RNA interference (RNAi) down-regulates gene expression post-transcriptionally, which is a therapeutically significant phenomenon that could potentially reduce the level of disease related proteins that are undruggable by conventional small molecular approaches. However, clinical application of small interference RNAs (siRNAs) requires design of potent siRNA sequences and development of safe and efficient delivery systems. To create a biocompatible siRNA delivery agent, we chemically modified natural polysaccharide curdlan in a regioselective manner to introduce amino group in the glucose units. The resulting 6-amino-curdlan (6AC) is water soluble and forms nanoparticles upon complexing with siRNAs. The novel curdlan-based nanoparticles efficiently delivered siRNAs to human cancer cells and mouse primary cells, and reduced 70–90% of target mRNA level. Moreover, 6AC nanoparticles delivered siRNA targeting eGFP to mouse embryonic stem (mES) cells stably expressing eGFP, and produced substantial reductions of eGFP protein level. The novel curdlan-based nanoparticle is a promising vehicle for delivery of short RNAs to knock down endogenous mRNAs.
Keywords: curdlan, siRNA delivery, nanoparticles, cancer cells, RT-qPCR
1. Introduction
Short interfering RNAs (siRNAs) are double stranded, 20–25 nucleotides RNAs that trigger hydrolysis of specific endogenous messenger RNA in sequence dependant manner (Fire, Xu, Montgomery, Kostas, Driver & Mello, 1998). SiRNAs that are either generated endogenously by enzymatic cleavage of long double strand alien RNAs (such as viral RNA), or chemically synthesized and forced introduced into the cells, are loaded into RNA-induced silencing complex (RISC) that recognizes and hydrolyzes a specific mRNA bearing complete complimentary sequence to the antisense strand of siRNA (Rana, 2007). Since the confirmation of RNAi effect of synthetic siRNAs on human cells, tremendous efforts have been made to introduce RNAi technique into clinic applications (Elbashir, Harborth, Lendeckel, Yalcin, Weber & Tuschl, 2001). Extensive studies have been carried out to stabilize and maximize RNAi effect by designing and screening for highly potent sequences with various modifications on nucleotides such as various 2’-modifications (Chiu & Rana, 2003), Locked Nucleic Acid (Elmen et al., 2005), Unlocked Nucleic Acid (Snead, Escamilla-Powers, Rossi & McCaffrey, 2013), and phosphodiester bond (Detzer, Overhoff, Mescalchin, Rompf & Sczakiel, 2008).
Meanwhile, development of safe and efficient siRNA delivery system has been greatly demanded to realize clinical application of siRNA. To protect siRNA from hydrolysis by nuclease and facilitate tissue targeting and cellular uptake, siRNAs can either be chemically attached to small molecules such as cholesterol (Soutschek et al., 2004) or N-acetylgalactosamine (GalNAc-siRNA), or be formulated with cationic molecules such as cationic lipids (Morrissey et al., 2005), proteins (Eguchi & Dowdy, 2010) or polymers (Rozema et al., 2007) to form nanoparticles with ideal size and surface characters. For instance, N-acetylgalactosamine binds with high affinity to asialoglycoprotein receptor (ASGPR) expressed on hepatocytes. Cluster of N-acetylgalactosamine that covalently linked to siRNA efficiently delivered siRNA to mouse liver (Akinc et al., 2010; Kanasty, Dorkin, Vegas & Anderson, 2013).
Sugar-containing cationic polymers have demonstrated great potentials for delivery of therapeutic RNA interference (RNAi). For instance, chitosan can readily bind to siRNA and enhance cellular uptake of siRNA (Yang et al., 2011). Remarkably, systemically administered cyclodextrin-based polymeric nanoparticles provided the first evidence of successful RNAi administration to humans, encouraging the prospect for RNAi-based therapies (Davis et al., 2010; Love et al., 2010; Semple et al., 2010). Curdlan is a non-pathogenic bacteria derived linear homopolymer of D-glucose bearing β(1→3) linkage. It has been widely used as a gelling agent in food industries since the approval from the U.S. Food and Drug Administration as a food additive. Curdlan can only dissolve in a few solvents such as dilute base (0.25 M NaOH), dimethyl sulfoxide (DMSO), and formic acid (McIntosh, Stone & Stanisich, 2005). Chemically modified water soluble curdlan has been explored in the pharmaceutical industry as a carrier of drugs and biotherapeutics (Jagodzinski et al., 1994; Li et al., 2010; Na, Park, Kim & Bae, 2000). Orally administered β-1,3-D-glucan encapsulated siRNA particles (termed GeRPs) efficiently delivered siRNA and silenced genes in mouse macrophages (Aouadi et al., 2009).We synthesized amine functionalized curdlan sulfate as an anti-HIV agent (Borjihan, 2003).
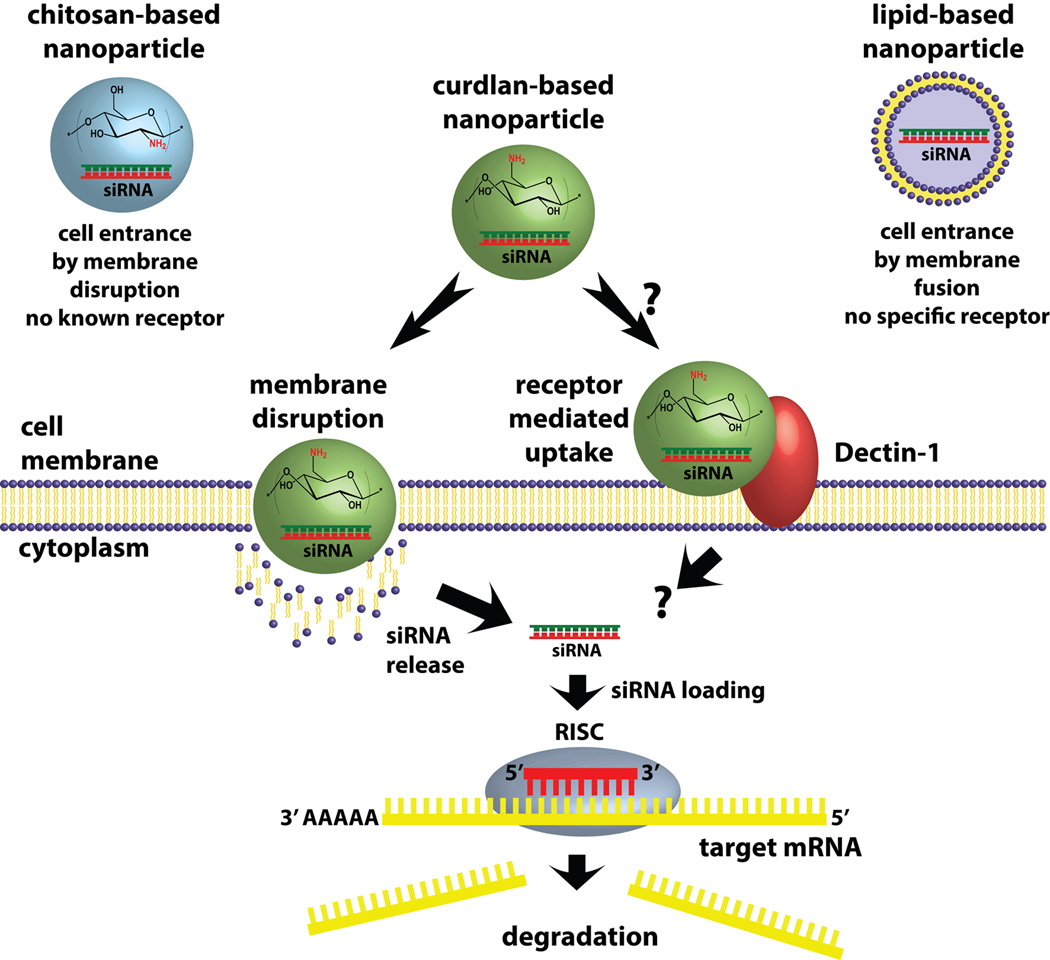
However, nucleic acid delivery by curdlan-based cationic polymer has not been reported so far. Chitosan has been extensively investigated for in vitro and in vivo siRNA delivery in experimental animals. However, tissue specific delivery by chitosan has proved to be difficult because of the unavailability of specific cell surface receptors for chitosan (Figure 1). Curdlan has unique β(1,3)-linkage and binds to Dectin-1 expressed on dendritic cells, monocytes, macrophages and B-cells. Unmodified curdlan was reported to deliver antisense oligodeoxynucleotides to mouse peritoneal macrophages (Mochizuki & Sakurai, 2011). Amination of curdlan has several advantages including increased water solubility and increased nucleic acid affinity. Moreover, fully or partially aminated curdlan could be explored for Dectin-1 targeted tissue specific delivery of biotherapeutics including siRNA, although the impact of amination to curdlan-Dectin-1 binding needs further confirmation. Here we report the synthesis and characterization of 6-amino-6-deoxy-curdlan (6AC), and siRNA delivery efficiency of 6AC polymer-based nanoparticles to various cell lines.
Figure 1.
A schematic of cellular uptake of siRNA-loaded nanoparticles formulated by chitosan, aminated curdlan and lipid materials.
2. Materials and methods
2.1. Chemicals and general methods
Curdlan was purchased from Wako Pure Chemical Industries, Itd. (Catalog number: 034-09901, Osaka, Japan). All other chemicals were purchase from Aladdin (Shanghai, China). Dimethylformamide (DMF) was distilled after drying. Dimethyl sulfoxide (DMSO) was dried over 4 Å molecular sieve. Dialysis tube (cut-off molecular weight of 3000; Spectrum Laboratories, Inc.) was used for the purification of curdlan derivatives. Gel permeation chromatography (GPC) was performed on a Waters HPLC instrument equipped with Ultrahydrogel™ 500 (7.8x300 mm) and Ultrahydrogel™ 250 (7.8x300 mm) columns (Waters) using acetate buffer (pH4.5) as eluent. 13C NMR was recorded on a Brucker 500 NMR spectrometer. Chemical shifts (δ = 0 ppm) were referred to TMS with the residual proton of the deuterated solvent.
2.2. Amine functionalization of curdlan and determination of degree of substitution
The hydroxyl group at the C6 position of glucose unit of curdlan was substituted by amine group in a similar way that we reported previously (Borjihan, 2003). Briefly, a solution of curdlan in dry DMSO was reacted with CBr4 in the presence of triphenylphosphine (PPh3) and NaN3 under N2 protection. The resulting 6-azido-6-deoxy curdlan was reduced by NaBH4 in DMSO at 60°C for 4 h. 6-Amino-curdlan with different degree of substitution (DS) were synthesized in the same way by reducing the amount of reactants and the reaction time in the step of 6-azido substitution, and then reduced in the same method described above. Then the product was purified by precipitation and extensive washing with methanol, followed by dialysis and freeze-drying to give 6-amino-curdlan as white powder.
The DS of 6-amino-curdlan was determined by 13C NMR. The C6 (C6-OH) of unmidified glucose unit of curdlan showed at 61 ppm. After amination, C6 (C6-NH2) shifted to 41 ppm. In the case of complete substitution (6AC-100), the signal of C6-OH disappeared and the signal of C6-NH2 appeared; partially amine substituted curdlan derivatives showed peaks for both C6-OH and C6-NH2, the intensity ratio of which was used to determine the DS. In the case of 6AC-70, the ratio of C6-OH: C6-NH2 intensity was about 3: 7, and in the case 6AC-50, it was 1:1.
2.3. Characterization of 6AC nanoparticles
Size distribution of nanoparticles was determined by Dynamic Light Scattering (DLS) with Zetasizer Nano-ZS90 (Malvern Instrument, UK).The scattering angle was fixed at 90°, and the measurement was carried out at a constant temperature of 25°. The sample solution was diluted in 1×PBS (pH=7.4) prepared with RNase free water prior to analysis. Zetal potential of nanoparticle dispersion system was measured by Laser Doppler Microelectrophoresis (LDM) with Zetasizer Nano-ZS90 at 25° . The sample was dissolved in 1×PBS (pH=7.4) prepared with RNase free water prior to analysis.
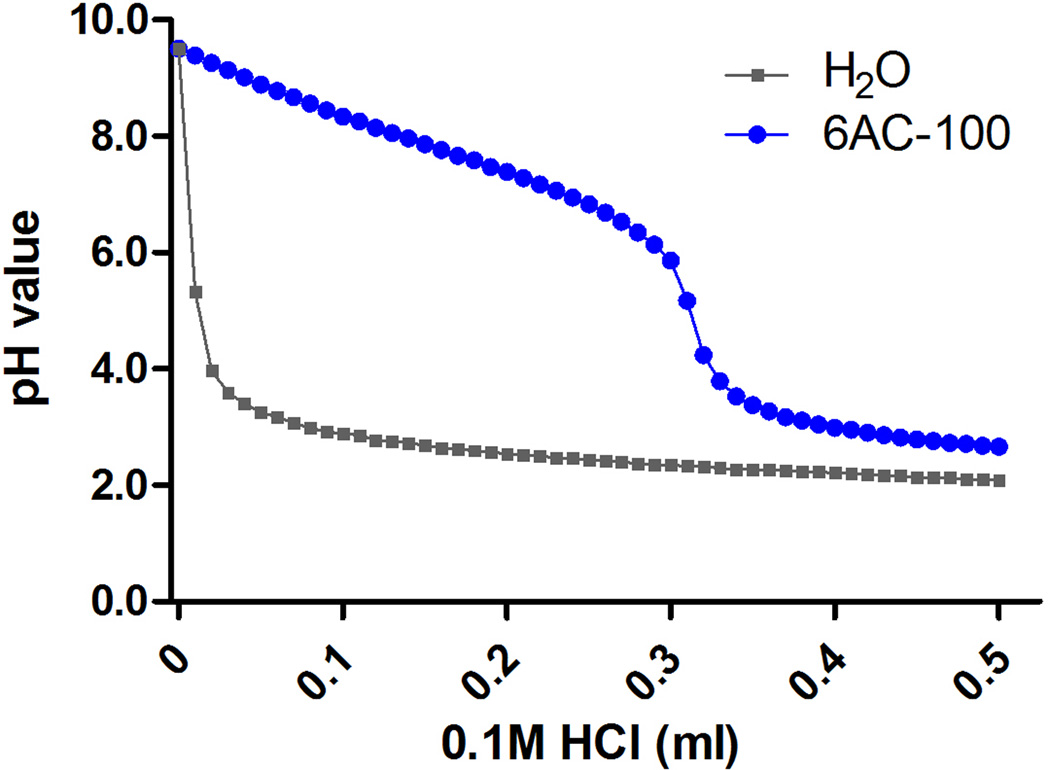
The buffering capacity of 6AC-100 was determined by acid-base titration. The pH of a 6AC-100 solution (10 mL, 1 mg/mL) was adjusted to 9.5 by adding NaOH solution. The 6AC-100 solution was then titrated with 0.1 M HCl in 10 mL increments at room temperature and the pH values were measured using OHAUS Starter 300C pH meter (Parsippany, NJ, USA).
2.4. Cell culture, cytotoxicity measurement and siRNA transfection
Human cancer cell lines (HeLa cells, A549 cells, HepG2 cells, H727 cells, and HCT116 cells, ATCC, Manassas, VA, USA) were maintained at 37 °C with 5% CO2 in DMEM supplemented with 10% fetal bovine serum (HyClone), 100 U mL−1 penicillin and 100 µg mL−1 streptomycin. Human leukemia cell line THP-1 was cultured in RPMI1640 containing 5 µM 2-mercaptomethanol supplemented with 10% fetal bovine serum (HyClone). CCE mouse embryonic stem cells stably expressing eGFP were maintained on gelatin-coated dishes in DMEM supplemented with 2 mM glutamine, 0.001% βmercaptoethanol, 1x nonessential amino acids, 15% FBS and leukemia inhibitory factor (10 ng/mL) (Stem Cell Technologies, Vancouver, Canada).
For cytotoxicity assay, cells were plated onto 96-well plates at 5,000 cells per well in 100 µl of culture medium. Twenty-four hours after plating, 100 µl of 6AC solutions to be tested, prepared as described above, was added to the cells (in triplicate) and incubated as described above. After a 24 h-exposure period, cell viability was assayed using the MTT test.
For in vitro transfection experiment, siGenome Non-Targeting siRNA Control, siGenome Human GAPDH siRNA and siGenome Mouse Trp53 siRNA (Dharmacon, Lafayette, CO, USA) were used. SiRNA duplex targeting GFP was designed as previously described (Chiu & Rana, 2002). Cells (2.5x105 cells per well) were seeded into 12-well plates at the day before transfection. Cells were transfected with 0.5 ml per well of siRNA (25 nM) complexed to 6AC polymers (3 µg/ml) for 3.0 h at 37 °C. To determine siRNA levels in cell culture after siRNA treatment, total RNA was extracted with TRIZOL (Invitrogen, Carlsbad, CA, USA). The expression of mRNA was measured using iScript™ Reverse Transcription Supermix for RT-qPCR and iTaq™ Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) for quantitative PCR. The sequences of primers used for RT-qPCR were as following: human β-actin, forward: 5’-ccaaccgcgagaagatga-3’, reverse: 5’-ccagaggcgtacagggatag-3’, human GAPDH, forward: 5’-agccacatcgctcagacac-3’, reverse: 5’-gcccaatacgaccaaatcc-3’.
3. Results and discussion
In an attempt to develop a safe, bio-originated vehicle for siRNA delivery we undertook amination of curdlan to create a polymeric carrier, and investigated the efficiency and biocompatibility of resulting amine-bearing curdlan. The goal of amination is (1) to provide cationic functional groups for increased affinity to siRNA, and (2) to enhance the hydrophilicity for efficient formulation with siRNA. To create an efficient carrier with potential applications for oral or systemic delivery of therapeutic RNAs including siRNAs to achieve enhanced cell penetration, biocompatibility as well as tissue specificity, we chemically modified natural polysaccharide curdlan so as the original hydroxyl group (−OH) at C6 position of glucose units is replaced by an amino group (−NH2) (Scheme 1) (Borjihan, 2003). By controlling the reaction time and ratio of reactants, we synthesized curdlan with different number of 6-azido substitution, which were subsequently reduced to give three types of C6 amine functionalized curdlan with different degree of substitution (DS), i.e. 100% amine substitution (6AC-100), 70% amine substitution (6AC-70) and 50% amine substitution (6AC-50), as estimated from 13C NMR analysis. C6 of glucose units, which gives signal at 60.81 ppm in unmodified curdlan, shifted to 40.56 ppm in 6AC polymers (Figure 2), indicating that 6-amino-curdlan was successfully synthesized. All 6AC polymers showed excellent solubility in dilute hydrochloride acid, which resulted in a neutral solution of cationic polysaccharide containing amine hydrochloride. The weight average molecular weight (Mw) of 6AC-100 was 50117, as measured by size exclusion chromatography (SEC), which is in the range of reported Mw of unmodified curdlan in sodium hydroxide solution (Nakata, Kawaguchi, Kodama & Konno, 1998). The accurate molecular weight for 6AC-70 and 6AC-50 was not determined by SEC due to the aggregation of the polymers in the column caused by decreased solubility in the elution system.
Scheme 1.
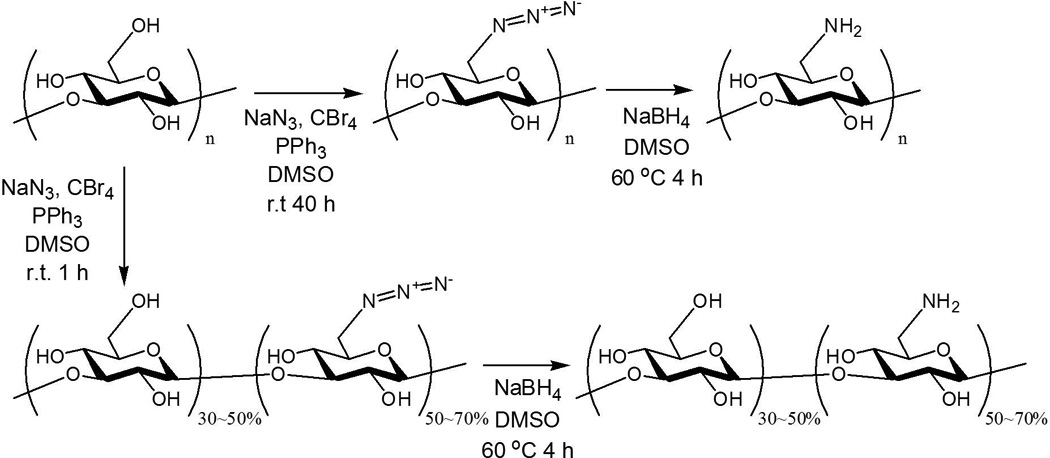
Synthetic scheme for synthesis of 6AC polymers.
Figure 2.
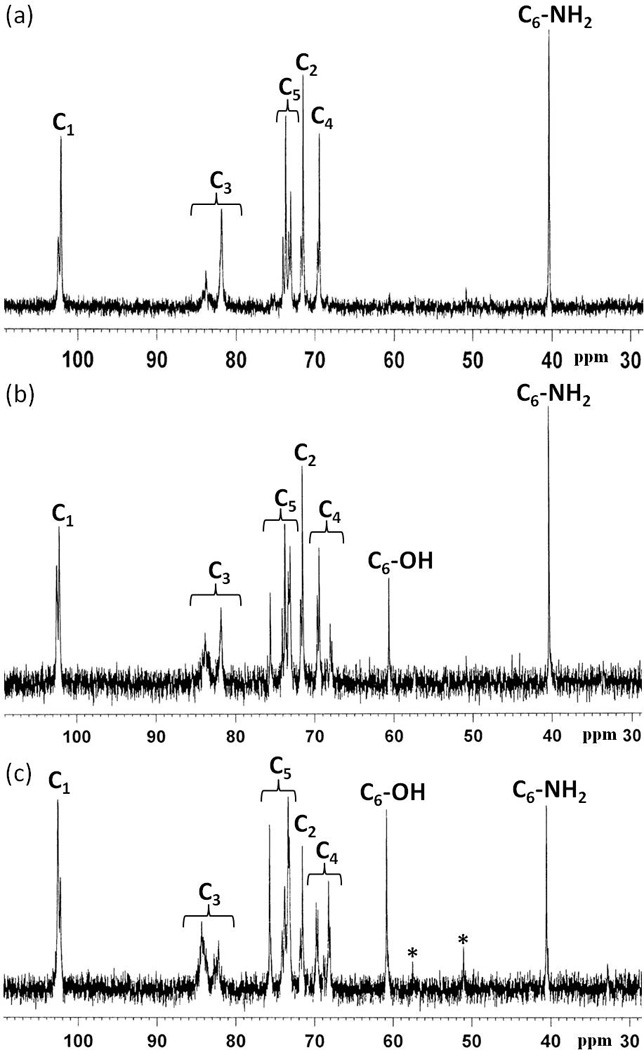
13C NMR spectra of (a) 6AC-100, (b) 6AC-70 and (c) 6AC-50 in D2O.
Cationic polymers bind to nucleic acids through electrostatic interaction, the strength of which can be assessed by electrophoretic mobility shift assay. To test the binding ability of 6AC polymers with nucleic acid, we incubated siRNA with 6AC-100, 6AC-70 or 6AC-50 in PBS buffer, respectively, and performed agarose gel electrophoresis. 6AC-100 and 6AC-70 readily bind to siRNA in neutral buffer (pH7.4) at the phosphate:amine (P/N) ratio of 1:3 and 1:4, respectively, while 6AC-50 did not show significant mobility shift, even at a high P/N ratio of 1:7 (Figure 3).
Figure 3.
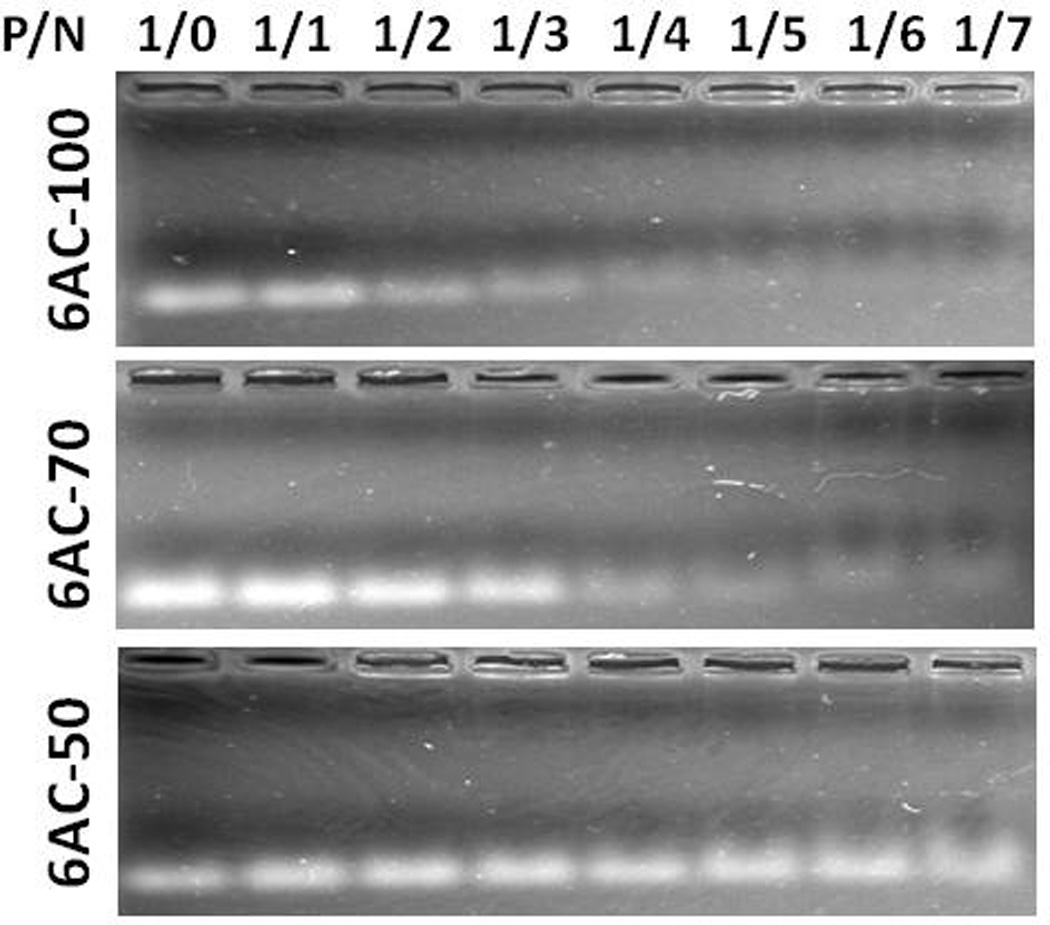
6AC-100 readily binds to short nucleic acids. Gel shift assay of 6AC-100 complexed with a short single strand DNA at phosphate:amine (P/N) ratio ranging from 1/0 to 1/7;
Effective endosomal escape of nanoparticles and subsequent cargo release could be achieved by nanoparticles with high buffering capacity. The buffering capacity of 6AC-100 was verified by the acid–base titration profiles (Figure 4). A broad buffering range (~pH 6.0) was observed, indicating that 6AC-100 has proton sponge effects which may enable endosomal escape of siRNA/6AC-100 complex and subsequent release of siRNA in the cytoplasm
Figure 4.
Buffering capacity titration. The concentrationof 6AC-100 was 1.0 mg/ml and was titrated by using 0.1 M HCl in 10 µl increments at room temperature.
The complex of nucleic acid and cationic macromolecules could be large aggregates or uniform particles. To further characterize siRNA/6AC-100 complex, we measured the particle size distribution and zeta potential of 6AC-100 complexed with siRNA at two different ratios (Table 1). At the P/N ratio of 1:14, the siRNA/6AC-100 complex formed nanoparticles with average particle size of 92.9 nm (± 3.3) and average zeta potential of 21.7 (± 0.1); as the content of siRNA in siRNA/6AC-100 complex increased (P/N ratio 1:7), the particle size increased to 182.7 nm and the zeta potential decreased to 17.5 (± 1.1) , indicating that we have successfully formulated a novel, natural polysaccharide-based nanoparticles with a range of particle sizes that could escape from renal clearance and potentially penetrate tissues.
Table 1.
Particle size and zeta potential of siRNA-loaded 6AC-100 nanoparticles
| Weight ratio (siRNA/6AC-100) |
Charge ratio (−/+) |
Particle size (nm) |
Polydispersity index ([(µ2)/Γ2]) |
Zeta potential (mV) |
|---|---|---|---|---|
| 1:10 | 1:14 | 92.9 ± 3.3 | 0.267 | 21.7 ± 0.1 |
| 1:5 | 1:7 | 182.7 ± 5.1 | 0.099 | 17.5 ± 1.1 |
Particle preparation conditions: 6AC-100 concentration: 1.0 mg/mL (PBS pH7.4), siRNA concentration: for weight ratio 10:1, 0.1 mg/mL (7.0 µM) and for weight ratio 5:1, 0.2 mg/ml (14 µM), T: 25 ± 2°C. ND: not determined; data shown are the mean ± standard deviation (n = 3).
With the novel curdlan-based nanoparticles in hand, we next investigated whether or not 6AC polymers deliver siRNA into cells. To do this, we first treated A549 cells with a Cy3-labeled RNA (Cy3-siRNA) complexed to 6AC-100 nanoparticles, and observed the cell internalization of the siRNA by fluorescence microscopy. Strong fluorescence signals were detected in the majority of cells treated with 6AC-100 nanoparticles four hours after transfection, demonstrating that 6AC-100 successfully delivered siRNA to the cytoplasm (Figure 5).
Figure 5.
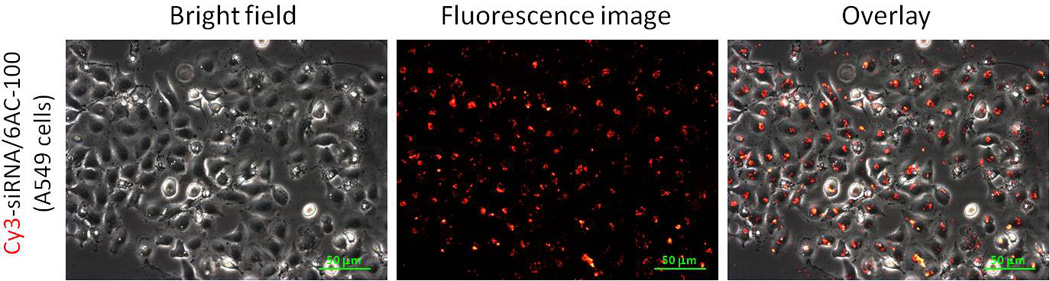
Internalization of siRNA in cancer cells by 6AC-100. A549 cells were transfected with Cy3-labeled siRNA complexed with 6AC-100 nanoparticles. Localization of dye-labeled siRNA after 4 h was monitored by fluorescence microscopy.
One of the major drawbacks of cationic macromolecules in biomedical application is their potential cytotoxicity. To test whether or not 6AC polymers are safe candidates for short RNA delivery, we treated HepG2 cells with different concentrations of 6AC polymers and measured the cytotoxicity by MTT assay after 24 hours. At a concentration of 5 µg/ml, all three 6AC polymers showed no apparent toxicity, giving more than 80% cell viability (Figure 6). Even at a high concentration of 30 µg/ml, which is a 10-times higher concentration than the working concentration, more that 75% cell viability was observed in the wells treated with 6AC-100 and 6AC-70, indicating that 6AC nanoparticles showed no major cytotoxicity at the range of working concentration.
Figure 6.
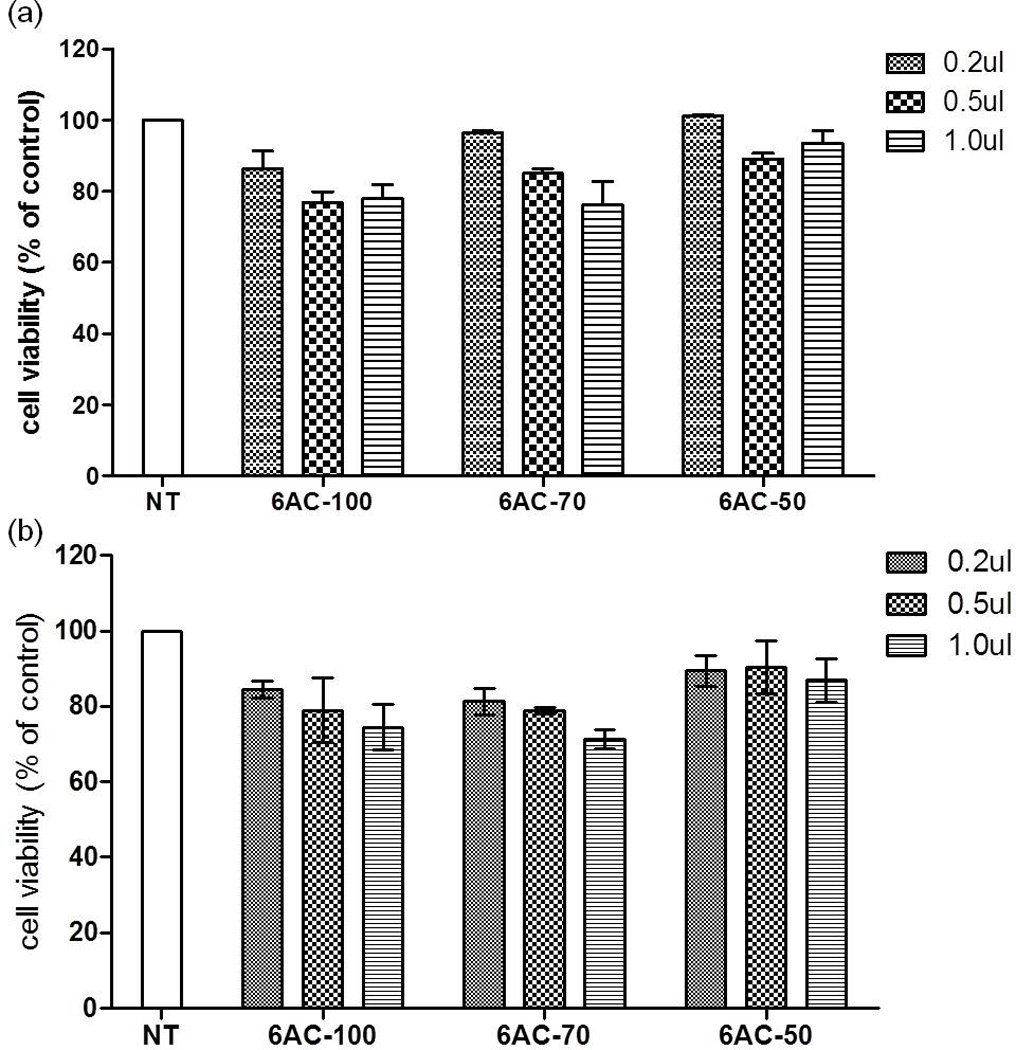
HepG2 cells (a) and HeLa cells (b) remain viable 24 h after treatment with 6AC-100. Cells were treated with 3 different concentrations of 6ACs. 0.2 µl, 0.5 µl or 1.0 µl of 6ACs stock solution (2mg/ml) were added to each well of 96-well plate. Cell toxicity levels are expressed as percent of control (no treatment).
The transfection efficiency of cationic polymers could be limited by their possible inability of endosomal escape and cargo release in the cytoplasm (ur Rehman, Hoekstra & Zuhorn, 2013). To determine siRNA transfection efficiency of 6AC polymers, we treated A549 cells (human lung adenocarcinoma epithelial cell line) with siRNA targeting GAPDH complexed to 6AC-100, 6AC-70 and 6AC-50, respectively, and compared their efficiency with commercially available transfection reagent lipofectamine 2000. 6AC-100 delivered siRNA to A549 cells and knocked down about 90% of endogenous GAPDH mRNA, a similar efficiency achieved by lipofectamine 2000, as confirmed by RT-qPCR (Figure 7a). Since 6AC-100 was more potent than 6AC-70 and 6AC-50, which caused only 15% and 5% decrease (data not shown) of mRNA level in cells, we focused on 6AC-100 in the following studies. A subsequent experiment on H727 cells (human lung neuroendocrine tumor cell line) and HCT116 cells (human colon cancer cell line) showed that 6AC-100 can efficiently deliver siRNA to these cancer cells and caused 70% to 90% decrease in expression of endogenous GADPH (Figure 7b, c). 6AC-100 not only delivered siRNA to cancer cells, but also delivered siRNA to THP-1 cell (human leukemia monocyte) derived macrophages and induced 80% knock down of GADPH mRNA (Figure 7d).
Figure 7.
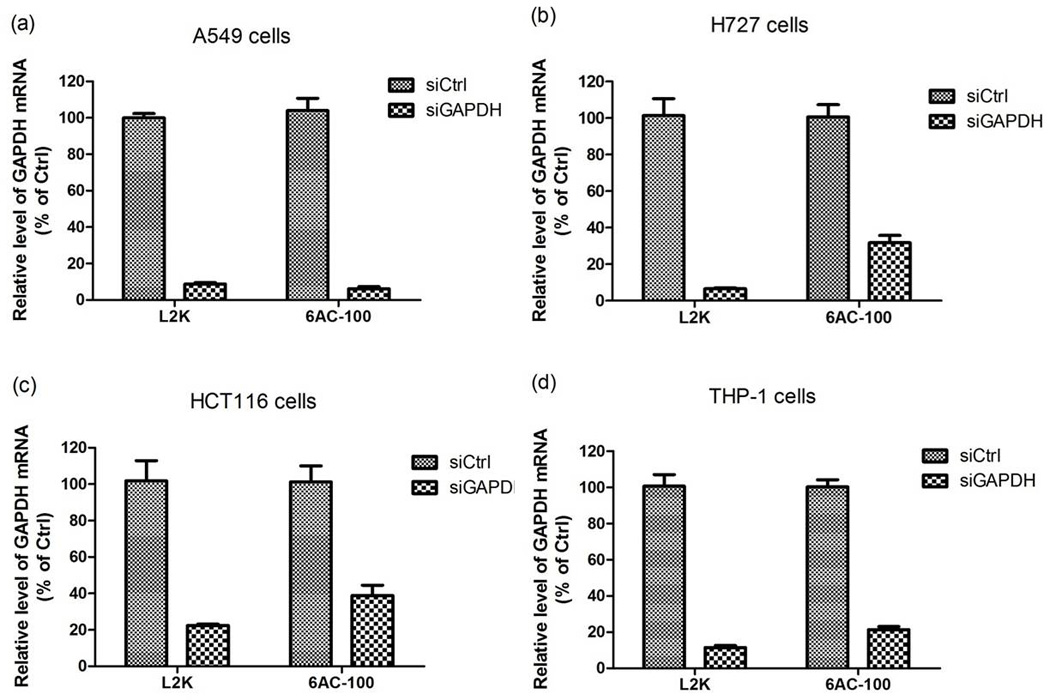
In vitro silencing of endogenous GAPDH in cancer cells by 6AC-100 nanoparticles complexed with siRNAs. Cells were treated for 3 h with either Lipofectamine 2000 or 6AC polymers containing non-targeting siRNA or siRNA targeting human GAPDH. mRNA levels are expressed as percent of control.
Curdlan binds to Dectin-1 expressed on the surface of some cells including macrophages and induces receptor-mediated endocytosis. However, since THP-1 cells express low levels of Dectin-1 (Rogers, Williams, Feng, Lewis & Wei, 2013), no enhancement in the transfection efficiency by 6AC-100 was observed in THP-1 cells compared to other cell lines such as A549. Moreover, the affinity of aminated curdlan to Dectin-1 receptor is yet to be further clarified. Therefore, the cellular uptake of siRNA/6AC-100 by THP-1 cells may have predominantly facilitated through membrane disruption induced by positive charges rather than receptor-mediate endocytosis.
To determine whether or not 6AC-100 deliver siRNA to primary cells and stem cells, we treated mouse MEF cells (mouse embryonic fibroblast cells) with 6AC-100 complexed to siRNA targeting mouse Trp53. 24 hours after transfection, 90% decrease of GAPDH mRNA was observed, indicating that 6AC-100 can efficiently delivery siRNA to primary cells including MEF cells (Figure 8). To measure the transfection efficiency of 6AC-100 on stem cells, we applied complex of 6AC-100 and siRNA targeting eGFP to mouse embryonic stem cells (mES cells) stably expressing eGFP. As shown in Figure 9, GFP signal was profoundly reduced 24 h after a single transfection with 25 nM siRNA complex to 6AC-100, demonstrating that 6AC-100 can efficiently deliver siRNA to stem cells, and knock down gene expression at protein level.
Figure 8.
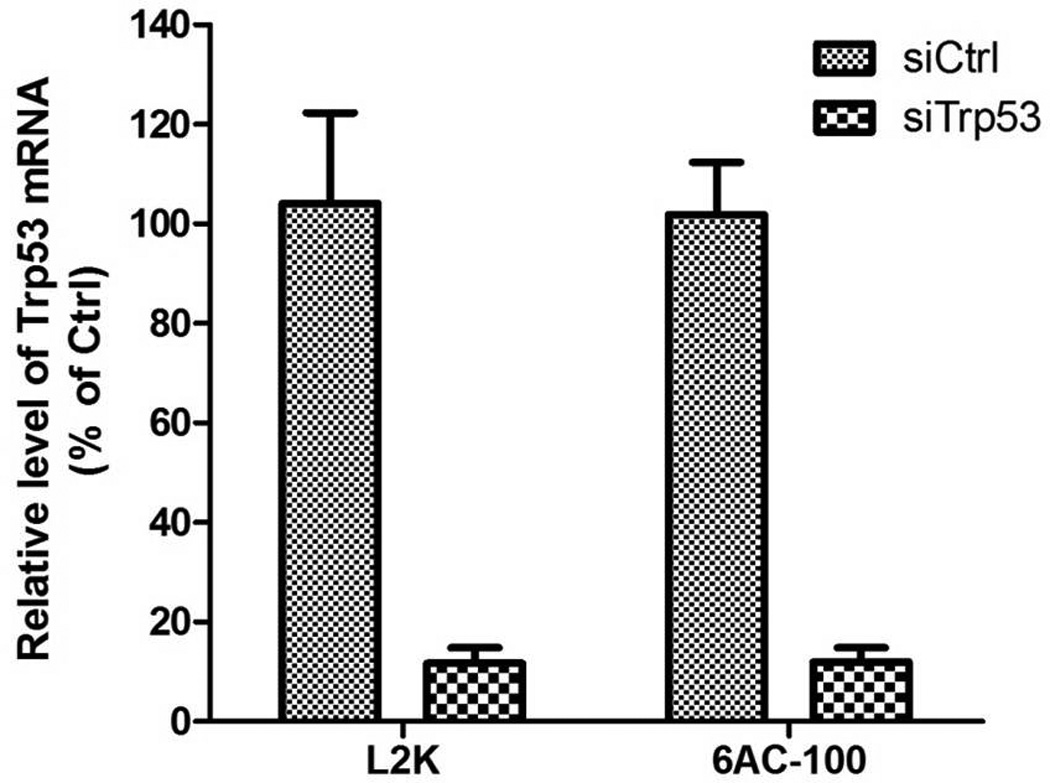
In vitro silencing of endogenous mRNA by 6AC-100 nanoparticles in mouse primary cells. 6AC-100 nanoparticles containing siRNA targeting mouse p53 silences p53 mRNA. MEF cells were treated for 3 h with 6AC-100 containing siRNAs or a scrambled single strand RNA. mRNA levels are expressed as percent of control.
Figure 9.
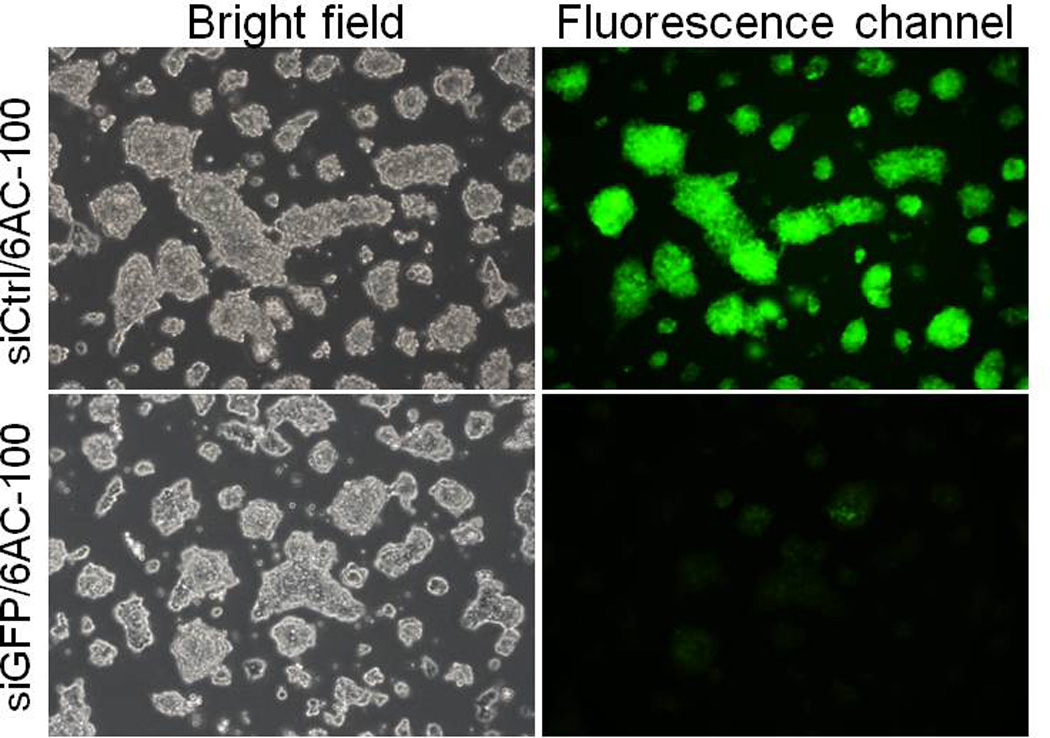
In vitro silencing of endogenous mRNA by 6AC-100 nanoparticles in mouse embryonic stem cells. Fluorescence microscope image of mES cells stably expressing eGFP treated with non-targeting siRNA complexed with 6AC-100 nanoparticles, or siRNA targeting GFP complexed with 6AC-100 nanoparticles. mRNA levels are expressed as percent of control
4. Conclusions
In the present study, we show that amine functionalized curdlan can be formulated to prepare nanoparticles with high solubility, biocompatibility as well as high siRNA delivery efficiency. The novel 6AC based nanoparticles delivered several unmodified siRNAs to cancer cells, primary mouse cells and mouse stem cells with high efficiency. In conclusion, our data suggest that 6AC based nanoparticles provide a promising platform for in vitro delivery of siRNA. Further functionalization and formulation of 6AC nanoparticles for in vitro and in vivo application is currently undergoing in our laboratories.
Highlights.
Water soluble curdlan derivatives were prepared and fully characterized.
The intracellular siRNA delivery efficiency depends on content of 6-amine units.
6-Amino curdlan/siRNA forms nanoparticles.
6-Amino curdlan efficiently delivered siRNA to cancer cells and stem cells.
We report a novel curdlan-based siRNA delivery system.
Acknowledgements
This research has been supported by in part by NIH grants to T.M.R., and in part by Program of Higher-level Talents of Inner Mongolia University (SPH-IMU, 30105-125145), the National Natural Science Foundation of China (21364006, 21375058) to H.B, and the Natural Sciences Foundation of Inner Mongolia (2011MS0207) to J.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458(7242):1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjihan G, Zhong G, Baigude H, Nakashima H, Uryu T. Synthesis and anti-HIV activity of 6-amino-6-deoxy-(1→ 3)-β-D-curdlan sulfate. Polym. Adv. Technol. 2003;14:326–329. [Google Scholar]
- Chiu YL, Rana TM. RNAi in human cells: basic structural and functional features of small interfering RNA. Mol Cell. 2002;10(3):549–561. doi: 10.1016/s1097-2765(02)00652-4. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9(9):1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detzer A, Overhoff M, Mescalchin A, Rompf M, Sczakiel G. Phosphorothioate-stimulated cellular uptake of siRNA: a cell culture model for mechanistic studies. Curr Pharm Des. 2008;14(34):3666–3673. doi: 10.2174/138161208786898770. [DOI] [PubMed] [Google Scholar]
- Eguchi A, Dowdy SF. Efficient siRNA delivery by novel PTD-DRBD fusion proteins. Cell Cycle. 2010;9(3):424–425. doi: 10.4161/cc.9.3.10693. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elmen J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, Wahren B, Liang Z, Orum H, Koch T, Wahlestedt C. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005;33(1):439–447. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Jagodzinski PP, Wiaderkiewicz R, Kurzawski G, Kloczewiak M, Nakashima H, Hyjek E, Yamamoto N, Uryu T, Kaneko Y, Posner MR, et al. Mechanism of the inhibitory effect of curdlan sulfate on HIV-1 infection in vitro. Virology. 1994;202(2):735–745. doi: 10.1006/viro.1994.1395. [DOI] [PubMed] [Google Scholar]
- Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- Li L, Gao FP, Tang HB, Bai YG, Li RF, Li XM, Liu LR, Wang YS, Zhang QQ. Self-assembled nanoparticles of cholesterol-conjugated carboxymethyl curdlan as a novel carrier of epirubicin. Nanotechnology. 2010;21(26):265601. doi: 10.1088/0957-4484/21/26/265601. [DOI] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh M, Stone BA, Stanisich VA. Curdlan and other bacterial (1-->3)-beta-D-glucans. Appl Microbiol Biotechnol. 2005;68(2):163–173. doi: 10.1007/s00253-005-1959-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Sakurai K. Dectin-1 targeting delivery of TNF-alpha antisense ODNs complexed with beta-1,3-glucan protects mice from LPS-induced hepatitis. J Control Release. 2011;151(2):155–161. doi: 10.1016/j.jconrel.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Na K, Park KH, Kim SW, Bae YH. Self-assembled hydrogel nanoparticles from curdlan derivatives: characterization, anti-cancer drug release and interaction with a hepatoma cell line (HepG2) J Control Release. 2000;69(2):225–236. doi: 10.1016/s0168-3659(00)00256-x. [DOI] [PubMed] [Google Scholar]
- Nakata M, Kawaguchi T, Kodama Y, Konno A. Characterization of curdlan in aqueous sodium hydroxide. Polymer. 1998;39(6–7):1475–1481. [Google Scholar]
- Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- Rogers H, Williams DW, Feng GJ, Lewis MA, Wei XQ. Role of bacterial lipopolysaccharide in enhancing host immune response to Candida albicans. Clin Dev Immunol. 2013;2013:320168. doi: 10.1155/2013/320168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, Hagstrom JE, Wolff JA. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007;104(32):12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- Snead NM, Escamilla-Powers JR, Rossi JJ, McCaffrey AP. 5' Unlocked Nucleic Acid Modification Improves siRNA Targeting. Mol Ther Nucleic Acids. 2013;2:e103. doi: 10.1038/mtna.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- ur Rehman Z, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano. 2013;7(5):3767–3777. doi: 10.1021/nn3049494. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu X, Zhang D, Yu W, Lv G, Xie H, Zheng J, Ma X. Chitosan/VEGF-sIRNA nanoparticle for gene silencing. J Control Release. 2011;152(Suppl 1):e160–e161. doi: 10.1016/j.jconrel.2011.08.062. [DOI] [PubMed] [Google Scholar]