Abstract
Objective: This study explores the use of stimulant medication for parents with attention-deficit/hyperactivity disorder (ADHD) who also have adolescents with ADHD.
Methods: Five parents, diagnosed with ADHD, had their dose of lisdexamfetamine (LDX) titrated to optimal effect. Next, parents and their adolescents completed two interactions, once when parents were on placebo and once when parents were on optimal dose of LDX, to assess acute effects of parental medication on parenting during a neutral discussion (NeuDiss), a problem discussion (ProbDiss), and a homework task (HW).
Results: Parents demonstrated a significant decrease in the ratio of commands to total verbalizations during the NeuDiss on LDX compared with placebo. Although no other statistically significant effects emerged at the p<0.05 level, moderate to large effects of medication on some aspects of parenting related to the amount and timing of speech (i.e., total verbalizations, total commands, ratio of commands to total verbalizations, and responsiveness) emerged and varied by task. Parental stimulant medication did not appear to impact the content of parents' speech (i.e., use of negative talk or praise).
Conclusions: These results add to a growing literature suggesting that treatment for parental ADHD may impact parenting performance, and suggest that attention to parental ADHD in treatment for adolescents with ADHD may possibly enhance family functioning.
Introduction
Parent–child relationships are highly impaired among children with ADHD, and over time, may worsen and beget further impairment (e.g., Molina and Pelham 2014). Compared with adolescents without ADHD, adolescents with ADHD display more conflict, poorer communication quality, and more hostility with their parents (Edwards et al. 2001; Barkley 2006). In families with an ADHD teenager, pharmacological and psychosocial treatments targeting parent–adolescent relationships have generally yielded limited improvements (Sibley et al. 2014). However, these studies have typically focused on adolescent behavior and have not addressed parental characteristics that may impact the relationship. Parental ADHD, for example, which affects at least 25% of parents of children with ADHD, is associated with parenting difficulties (e.g., inconsistent discipline, harsh parenting) that directly impair parent–child interactions (Johnston et al. 2012). Among parents of elementary school aged children, parental stimulant medication treatment has yielded several observable parenting improvements (Evans et al. 1994; Chronis-Tuscano et al. 2008; Waxmonsky et al. 2014), but it is not known whether parental stimulant medication may also improve the parenting of parents of adolescents with ADHD. Effective parenting practices are critical in adolescence, as they mitigate risk for a range of adolescent problem behaviors (e.g., Patterson and Forgatch 2005; Molina and Pelham 2014). Therefore, the impact of pharmacotherapy of parental ADHD on parenting behaviors merits exploration.
This study explores the capacity of parental ADHD treatment with lisdexamfetamine (LDX) to improve observed parenting (i.e., verbalizations, commands, praise, negative talk, ratio of commands to total verbalizations, and responsiveness). It is hypothesized that stimulant medication will lead to improvements in the same domains observed in school-aged children, including reduced verbalizations, negative talk, and commands, and increased use of praise (Waxmonsky et al. 2014).
Methods
All procedures were approved by the Western Institutional Review Board. Parents provided written consent, and their children assented prior to enrollment. Parent–adolescent dyads were recruited from a larger trial of lisdexamfetamine (LDX) for parents with ADHD (see Waxmonsky et al. 2014 for a detailed summary of the trial and the results for parents of children ages 5–12 years). Parental ADHD was diagnosed using a clinical interview with an MD/PhD level clinician. Parents met full Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (American Psychiatric Association 1994) ADHD criteria with sufficient severity (i.e., ≥28 on the ADHD Rating Scale [ADHD-RS] with adult prompts [DuPaul et al. 1998], at least moderate severity on the Clinical Global Impressions [CGI] Severity subscale), and impairment in the participants' functioning within their family (i.e., ≥5 on the Sheehan Disability Scale (SDS) (Sheehan et al. 1996) family scale. Medical or psychiatric (e.g., mania, substance use disorders) conditions that could be worsened by stimulants or required psychotropic medication other than stimulants were exclusionary. Adolescents (ages 13–16) met DSM-IV criteria for ADHD using the parent-completed Disruptive Behavior Disorder Rating Scale (DBD) (Pelham et al. 1992). The Computerized Diagnostic Interview Schedule for Children (C-DISC) (Shaffer et al. 2000) confirmed ADHD and screened out for comorbidities. Adolescents needing psychotropic medication for disorders other than ADHD, Oppositional Defiant Disorder (ODD), or Conduct Disorder (CD) were not eligible.
Eight parents of adolescents began a 3 week open label LDX trial (see Table 1for participant characteristics). LDX, provided by Shire Pharmaceuticals, was started at 30 mg, and could increase to 50 mg for week 2, and to 70 mg for week 3. Clinician-rated CGI Severity and ADHD-RS, as well as side effects and vital signs, were collected weekly. Optimal dose was defined as a physically tolerable dose that produced an ADHD-CGI of 1 or 2 (much/very much improved) plus≥30% symptom reduction on the ADHD-RS. Two mothers and one father, all stimulant treatment naïve, dropped out because of adverse events (i.e., jitteriness) all with the initial 30 mg dose. Therefore, this study henceforth describes the results from the remaining five parents, three who were optimized on 30 mg, and one each on 50 mg and 70 mg.
Table 1.
Parent and Adolescent Demographic and Baseline Characteristics (N=8)
| Measure | Parent | Adolescent |
|---|---|---|
| Age | 43.63 (6.05) | 14.13 (0.83) |
| Gender (% male) | 37.50 | 87.50 |
| Ethnicity (% Hispanic or Latino) | 75.00 | 87.50 |
| Race (% Caucasian) | 62.50 | 75.00 |
| Prior ADHD diagnosis | 12.50 | 50.00 |
| Prior ADHD med usage | 12.50 | 50.00 |
| ADHD diagnosis | ||
| Combined type | 50.00 | 50.00 |
| Inattentive type | 50.00 | 50.00 |
| ODD diagnosis | – | 37.50 |
| CD diagnosis | – | 0.00 |
| ADHD-RS Total Score | 38.13 (7.14) | – |
| DBD ADHD severity | – | 33.75 (9.53) |
| IQ | – | 106.00 (6.00) |
| Academic achievement | ||
| Reading | – | 110.33 (8.33) |
| Math | – | 99.33 (16.65) |
| Spelling | – | 101.33 (4.51) |
| Marital status (% married) | 62.50 | – |
| Number of children | 2.13 (0.64) | – |
| Education | ||
| High school graduate | 12.50 | – |
| Partial college (at least 1 year) | 37.50 | – |
| College or university graduate | 50.00 | – |
| Weight at baseline (kg) | 78.04 (14.64) | – |
| Weight at parent–child task (kg) | 74.26 (17.02) | – |
Values in table are means (standard deviations in parentheses) or percentages, and describe the intent-to-treat sample with the exception of weight at parent–child task, which includes only the five parents completing the trial. DBD ADHD Severity scores were calculated from adding all DSM-IV ADHD items rated on a 0–3 Likert scale, producing a score comparable to the ADHD-RS total score. Number of children includes participating adolescent. Standard scores from the Wechsler Individual Achievement Test – 2nd edition are reported for academic achievement. ADHD, attention-deficit/hyperactivity disorder; ODD; oppositional defiant disorder; CD, conduct disorder, ADHD-RS, ADHD-Rating Scale; DBD, disruptive behavior disorder; IQ, intelligence quotient; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition.
Beginning the week after dose optimization, two separate parent–adolescent interactions were conducted under double-blind conditions, once with parents on placebo and once with parents on the optimal LDX dose. Assessments were completed 2–10 hours post dosing, when LDX has been demonstrated to be efficacious (Wigal et al. 2010). Between assessments (average duration of 22 days), participants and adolescents, if medicated at the onset of the trial, could use their medication. Medication order (placebo vs. LDX) was counterbalanced across participants. Adolescents were not medicated during the tasks, to increase the likelihood of challenging behaviors.
The interaction consisted of three tasks: plan a family activity for 5 minutes (neutral discussion [NeuDiss]); discuss two family problems for 20 minutes (problem discussion [ProbDiss]); and complete a developmentally appropriate writing prompt (Homework task [HW]), based on the adolescent's intelligence quotient (IQ) and achievement level (Wechsler 2002, 2005) for 5 minutes (Dishion et al. 2004; Barkley 2006). Total parental verbalizations and commands, as well as percent of praise (i.e., number of praise statements divided by total verbalizations), negative talk (i.e., number of negative statements divided by total verbalizations), ratio of commands to total verbalizations, and responsiveness (i.e., number of child requests that were answered by the parent divided by the total number of child requests and questions) were coded. Interactions were recorded and coded by a trained observer, and a second observer coded 19% of videos from the larger study. Coders were blind to parental treatment status and obtained adequate reliability (Waxmonsky et al. 2014).
Data analytic plan
Planned comparisons (i.e., optimal dose LDX vs. placebo) assessed medication effects on parenting, and separate analyses were conducted by task (i.e., NeuDiss, ProbDiss, and HW). Effect sizes were computed as (Medication mean – Placebo mean)/pooled SD. Given the small sample size and exploratory nature of the study, we emphasize effect sizes when interpreting data.
Results
Means, standard deviations, and effect sizes are summarized and presented in Figure 1. As shown, in the NeuDiss, parents on LDX displayed a lower commands to verbalizations ratio (F=8.99, p=0.04), and although not statistically significant, LDX was also associated with a large decrease in the number of commands, and moderate increases in verbalizations and responsiveness. Medication effects did not emerge for praise or negative talk. No statistically significant effects emerged during the ProbDiss or HW (i.e., Fs<3.51). However, during the ProbDiss, LDX was associated with a large increase in verbalizations and a moderate decrease in the ratio of commands to verbalizations. During HW, LDX was associated with an increase in total commands with little change in verbalizations, resulting in an increase in the ratio of commands to total verbalizations.
FIG. 1.
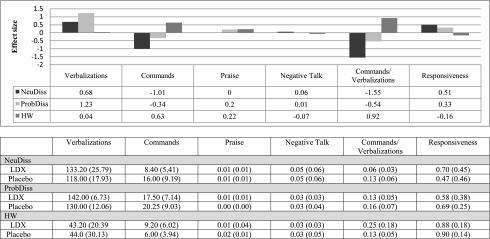
Medication effect sizes of observed parenting behaviors. Bars in the top half represent medication effects (i.e., mean standard differences) by task. Parent behavior codes are based on the coding scheme used for reporting results in the trial of parents of children with attention-deficit/hyperactivity disorder (ADHD) (Waxmonsky et al. 2014). Values in the bottom half represent means, and standard deviations are presented in parentheses. Verbalizations and commands represent total frequency counts, whereas all remaining parent behaviors are calculated as a percentage score. NeuDiss, neutral discussion; ProbDiss, problem discussion; HW, homework.
Discussion
This small study explored the effect of parental stimulant medication on the observed behaviors of parents of adolescents with ADHD, and found evidence that treatment of parental ADHD may improve at least some aspects of parenting in some situations.
Medication effects were task specific. During NeuDiss, for which an exchange of parent and adolescent statements would be appropriate, parents displayed more verbalizations with medication versus placebo, suggesting that medication may enhance parental engagement. Although this could suggest that parents were more dominating of the conversation, given the large decrease in commands and increase in responsiveness also demonstrated with LDX, it is more likely that parents were more engaged in the conversation while being less domineering. In contrast, during HW, in which more directive parenting was expected, LDX was associated with increased commands, with little impact on total verbalizations. Results regarding ProbDiss, which demanded both directive and responsive behaviors, were less clear, although the increased verbalizations with medication may also suggest that parents were more engaged in the conversation.
These improvements are consistent with the effects observed among parents of children (ages 5–12; Waxmonsky et al. 2014), although substantially more medication effects emerged among parents of younger children (i.e., increased praise, and decreased negative talk, commands, and verbalizations). Several improvements demonstrated in the child sample emerged more clearly when parental stimulant treatment was administered for several weeks rather than 1 (Waxmonsky et al. 2014). Therefore, extended pharmacotherapy may provide incremental benefit for parents of adolescents. However, the relative lack of praise or negative talk demonstrated among parents of adolescents was surprising, as higher rates were demonstrated in the child sample (praise: 3.6%; negative talk: 9.6%; Waxmonsky et al. 2014). Although the low base rate of these behaviors may be a function of the coding scheme, which primarily assessed verbal interaction rather than emotional and behavioral responses, it also suggests that over time, the pattern of negative parent–child interactions typical of ADHD worsen such that parents emotionally disengage from their adolescent during conflict. Therefore, multimodal treatment for parent–adolescent ADHD dyads need further study.
Given the exploratory nature of this small sample study, replication is needed. Although the percentage of parents who did not tolerate the low dose of medication in this study appeared somewhat higher than that reported among parents and adults with ADHD in other stimulant medication trials (e.g., Chronis-Tuscano et al. 2008; Waxmonsky et al. 2014), it is not clear whether this result is a function of the small sample size or whether other characteristics (e.g., older age of parents, dosage, specific medications) are relevant clinical considerations. These results may not generalize to all parent–adolescent ADHD dyads. Furthermore, assessment of adolescent ADHD was based solely on parental report, without collecting ratings for collateral informants (e.g., teachers). The interaction task employed in this study was consistent with other assessments of parent–adolescent interaction for adolescents with ADHD (Edwards et al. 2001; Dishion et al. 2003; Sibley et al. 2014), although the coding scheme did not assess all relevant relationship features (e.g., disengagement, monitoring, encouragement of independence during homework completion). Despite these limitations, this study presents a worthwhile step in exploring how to improve parent–adolescent relationships in families in which both a parent and adolescent have ADHD.
Clinical Significance
Effective strategies are critical to mitigating adolescent-specific risk for adolescents with ADHD (Molina and Pelham 2014). However, interventions to improve parenting and the parent–adolescent relationship are limited, and generally focus on improving adolescent behavior (Sibley et al. 2014). Our study suggests that attention to parental ADHD may improve outcomes.
Disclosures
Over the past 3 years, Dr. Waxmonsky has received research funding from Shire (this study), Noven Pharmaceuticals, and Janssen (drug donation), as well as from the National Institute of Mental Health (NIMH), and has served on the advisory board for Noven and as a speaker for continuing medical education (CME) talks by Quintiles. Dr. Waschbusch has received research support from Shire (this study), and Dr. Pelham has served on the advisory board for Noven Pharmaceuticals. Both have also received funding from NIMH. All other authors have no disclosures to report.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Barkley RA: Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. Guildford Press: New York; 2006 [Google Scholar]
- Chronis-Tuscano AM, Seymour KE, Stein MA, Jones HA, Roomey ME, Conlon C, Efron LA, Wagner SA, Pian J, Robb AS: Efficacy of OROS methylphenidate for mothers with ADHD: Preliminary effects on ADHD symptoms and parenting. J Clin Psychiatry 69:1938–1947, 2008 [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Nelson SE, Bullock BM: Premature adolescent autonomy: Parent disengagement and deviant peer processes in the amplification of problem behavior. J Adolesc 27:515–530, 2004 [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York: Guilford Press; 1998 [Google Scholar]
- Edwards G, Barkley RA, Laneri M, Fletcher K, Metevia L: Parent–adolescent conflict in teenagers with ADHD and ODD. J Abnorm Child Psychol 29:557–572, 2001 [DOI] [PubMed] [Google Scholar]
- Evans SW, Vallano G, Pelham WE: Treatment of parenting behavior with psychostimulant: A case study of an adult with attention-deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 4:63–39, 1994 [Google Scholar]
- Johnston C, Mash EJ, Miller N, Ninowski JE: Parenting in adults with attention-deficit/hyperactivity disorder. Clin Psychol Rev 32:215–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE: Attention-deficit/hyperactivity disorder and risk for substance use disorder: Developmental considerations, potential pathways, and opportunities for research. Annu Rev Clin Psychol 10:607–639, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, Forgatch MS: Parents and Adolescents Living Together. Part 1: The Basics, 2nd ed. Champaign, IL: Research Press; 2005 [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R: Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry 31:210–218, 1992 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab–Stone ME: NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39:28–38, 2000 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA: The measurement of disability. Int Clin Psychopharmacol 11:89–95, 1996 [DOI] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH: Pharmacological and psychosocial treatments for adolescents with ADHD: An updated systematic review of the literature. Clin Psychol Rev 34:218–232, 2014 [DOI] [PubMed] [Google Scholar]
- Waxmonsky JG, Waschbusch DA, Babinski DE, Humphrey H, Alfonso A, Crum KI, Bernstein M, Slavec J, Augustus JN, Pelham WE: Does pharmacological treatment of ADHD in adults enhance parenting performance? Results of a double-blind randomized trial. CNS Drugs 28:665–677, 2014 [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test – Second Edition. San Antonio: Harcourt; 2002 [Google Scholar]
- Wechsler D: Wechsler Intelligence Scale for Children – Fourth Edition. San Antonio: The Psychological Corportation; 2005 [Google Scholar]
- Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J: Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: Novel findings using a simulated adult workplace environment design. Behav Brain Funct 6:34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


