Abstract
Background: Evidence suggests nonelderly adults with cancer are likely to receive aggressive treatment in their last month of life and less likely to receive hospice and/or palliative services. Young adults with cancer (18–39 years) are a unique population, and little is known about the characteristics of their end-of-life care trajectories when they die in the hospital.
Objective: The purpose of this descriptive pilot study was to explore the characteristics of death among young adults with cancer who died in a tertiary academic hospital in order to elucidate their end-of-life trajectories.
Methods: A retrospective chart review was conducted among hospitalized young adults with a primary cancer diagnosis who died in the hospital within a 10-year period. Study variables were abstracted for quantification and medical record notes were reviewed for validation.
Results: A review of 61 patient records indicate that young adults commonly received cancer treatment within weeks of death and that do-not-resuscitate orders were frequently written only when death appeared imminent. Palliative care teams were frequently consulted for management of physical symptoms but often within days of death and most commonly on the day of death.
Conclusions: Findings suggest palliative care was initiated late in the care trajectory for young adults with cancer who died in the hospital. This study highlights the need for further inquiry into end-of-life care for young adults with cancer so that interventions can be developed to meet the physical, emotional, social, and spiritual needs of this unique group of patients, their families, and friends.
Introduction
The current focus on young adult oncology is less than a decade old and highlights the burden of disease and risks of morbidity and mortality in young adults with cancer.1,2 Caught between the worlds of pediatric and adult medical providers, young adults with cancer who are 18–39 years of age are less likely to access optimal medical and psychosocial services, compared to other age groups.3 While the 5-year survival rates for some common young adult malignancies such as thyroid and testicular cancer exceed 80%, the survival rates for diseases such as leukemia remain less than 60%, with survival rates for some solid tumors even lower.4
An emerging trend in the literature suggests that nonelderly adults are more likely to receive aggressive treatment in the last month of their life (chemotherapy, intensive care unit [ICU] admission, cardiopulmonary resuscitation [CPR], intubation, and mechanical ventilation), but much of what we know is based on Medicare utilization in adults over 65.5–10 Nonelderly adults are also less likely to receive palliative and/or hospice services prior to death.6,11 From the pediatric perspective, the majority of children who die from cancer die in the initial treatment phase, in the hospital, in an ICU, while still receiving aggressive curative therapies (e.g., chemotherapy).12–17 Preliminary evidence from adolescents at the end of life suggests that they prefer to die at home, yet the vast majority die in a hospital setting.12 Furthermore, nearly 90% of children and adolescents who die from cancer do so while experiencing two to eight troubling symptoms.18
In general, little is known about the care trajectories of young adults with cancer as they near the end of life.19 The purpose of this descriptive pilot study was to explore the characteristics of death among young adults with cancer who died in a tertiary academic hospital in order to elucidate the characteristics of end-of-life care specific to this age group. We also wanted to explore differences among characteristics of death between various diagnostic groups (hematologic malignancies, solid tumors, central nervous system [CNS] malignancies) as well as compare those patients who had an ICU admission preceding their death with those who did not have an ICU admission. To the authors' knowledge, characteristics of care trajectories among young adults who die of cancer have not been previously described.
Materials and Methods
Study design and subject overview
This retrospective study received Institutional Review Board exemption prior to study initiation. The University of Virginia's Clinical Data Repository (CDR) was used for subject identification based on a single query. Patients were identified using: death between ages 18–39, primary diagnosis of cancer of any origin (based on malignant neoplasm ICD-9 codes), who died while admitted to the hospital within a 10-year span (2001–2011). Therefore, all patients who met inclusion criteria were included in this study. Once patients were identified, a retrospective chart review was conducted, and a set of objective variables were abstracted using the electronic medical record and CDR. Annotated notes were reviewed to confirm and validate variables of interest that included analysis of: discharge summaries (which described death), progress notes, nursing notes, procedure notes, social work consults, palliative care consults, and other specialty consult notes. The final admission preceding the death was the admission of primary focus for this study, but all notes following diagnosis were reviewed to determine palliative care involvement. Several variables including cost, charges, and insurance status were supplemented from the CDR.
Variables included demographic characteristics (age, race, gender), payer status, primary cancer diagnosis (hematologic malignancy, solid tumor, brain/CNS tumor), place of death (acute care floor, ICU, emergency department), resuscitation status, cause of death, overall length of stay (LOS), ICU LOS, current active therapy (i.e., chemotherapy administration or radiation therapy 4 weeks prior to death), presence of advance directives (AD), CPR performed, palliative care consult, and documentation of family meeting.
Analysis
Descriptive statistics were calculated to describe all variables using mean/standard deviation for continuous variables and frequency/percentage for categorical variables. Significant differences in pertinent outcome variables were compared among diagnostic groups (hematologic malignancy, solid tumor, brain/CNS) and compared among those patients who had an ICU stay versus no ICU stay using either t tests for continuous variables with normal distributions and the χ2 statistic (and Fisher's exact test when n<10) for dichotomous variables. Kaplan-Meier survival curves were calculated to compare the three diagnostic classifications on their survival since diagnosis. During initial data abstraction, it was hypothesized that there may be significant differences among those patients who had palliative care consult more than 1 day prior to death compared to those who either had no palliative care consult, or a palliative care consult on the day of death for withdrawal of life-sustaining interventions. Therefore, this post hoc comparison was also performed using t tests and χ2/Fisher's exact tests. Finally, independent associations between palliative care consult more than 1 day and outcome variables were calculated. Odds ratios and confidence intervals were calculated to determine the impact of palliative care consult more than 1 day on patient-related outcomes. Statistics were calculated with SPSS version 20, (IBM SPSS, Armonk, NY).
Results
A total of 61 patients met inclusion criteria for this study. These young adults had a mean age of 30 years and an average survival of 15.2 months from time of diagnosis to death. The majority of patients had a solid tumor (51.6%), were single (53%), and white (77%). Only 8.2% had an AD upon admission, and 13.1 had a do-not-resuscitate (DNR) order documented prior to the admission. Thirteen patients (21%) were diagnosed with cancer during this same admission as their death. The remaining patient characteristics can be found in Table 1.
Table 1.
Patient Characteristics, n=61
| Characteristic n=61 | Number (%) | Mean (SD) |
|---|---|---|
| Age at diagnosis | 29.5 (6.5) Range 17–39 |
|
| Age at death | 30.7 (6.2) Range, 18–39 |
|
| Gender | ||
| Male | 30 (49.2) | |
| Female | 31 (50.8) | |
| Race | ||
| White | 48 (78.7) | |
| Black | 11 (18.0) | |
| Other | 2 (3.3) | |
| Marital status | ||
| Single | 33 (54.1) | |
| Married | 24 (39.3) | |
| Other | 4 (6.6) | |
| Disease type | ||
| Hematologic | 16 (26.2) | |
| Solid | 32 (52.5) | |
| CNS | 13 (21.3) | |
| Payer status | ||
| Public coverage | 19 (30.6) | |
| Private insurance | 27 (43.5) | |
| Self-pay | 9 (14.5) | |
| Other | 5 (8.1) | |
| (missing) | 1 (1.6) | |
| Relationship of hospital contact | ||
| Parents | 29 (47.5) | |
| Marital partner | 22 (36.1) | |
| Sibling | 5 (8.2) | |
| Other (friend/relative) | 5 (8.2) | |
| Survival (months) diagnosis to death | 15.2 (20.9) Range, 0.1–100 |
|
| LOS (days) | 15.1 (15.4) Range, 1–78 |
|
| Advanced directives on file prior to admission | ||
| None | 56 (91.8) | |
| Yes | 5 (8.2) | |
| Resuscitation status prior to admission | ||
| Full code | 53 (86.9) | |
| DNR | 8 (13.1) | |
| New diagnosis during admission | ||
| No | 48 (78.7) | |
| Yes | 13 (21.3) | |
| On active primary treatment for malignancy | ||
| No | 32 (52.5) | |
| Yes | 29 (47.5) | |
| Documented goals of care discussion at admission | ||
| No | 41 (67.2) | |
| Yes | 20 (32.8) | |
| Place of death | ||
| Floor | 39 (63.9) | |
| ICU | 22 (36.1) | |
| Cause of death | ||
| Disease-related | 51 (83.9) | |
| Complication-related (e.g., sepsis) | 10 (16.4) | |
| ICU stay | ||
| No | 32 (52.5) | |
| Yes | 29 (47.5) | |
| ICU LOS (days) | 13.9 (16.4) Range, 1–78 |
|
| CPR performed | ||
| No | 50 (82.0) | |
| Yes | 11 (18.0) | |
| Palliative care consult | ||
| No | 31 (50.8) | |
| Yes | 30 (49.2) | |
| Palliative care consult more than 1 day prior to death (of those with palliative care consult n=30) | ||
| No | 11 (36.7) | |
| Yes | 19 (63.3) | |
| Timing of palliative care consult (days prior to death) | 12.8 (25.1) Range, 0–120 |
|
| DNR order prior to death | ||
| No | 7 (11.5) | |
| Yes | 54 (88.5) | |
| Timing of DNR order (days prior to death) | 9.8 (23.8) Range, (1–60) |
|
| Documentation of family meeting | ||
| No | 14 (23.0) | |
| Yes | 47 (77.0) | |
| Hospital cost | $85,578.42 (95,156.66) Range, ($2850.00–371,726.00) |
|
SD, standard deviation; CNS, central nervous system; LOS, length of stay; DNR, do-not-resuscitate; ICU, intensive care unit; CPR, cardiopulmonary resuscitation.
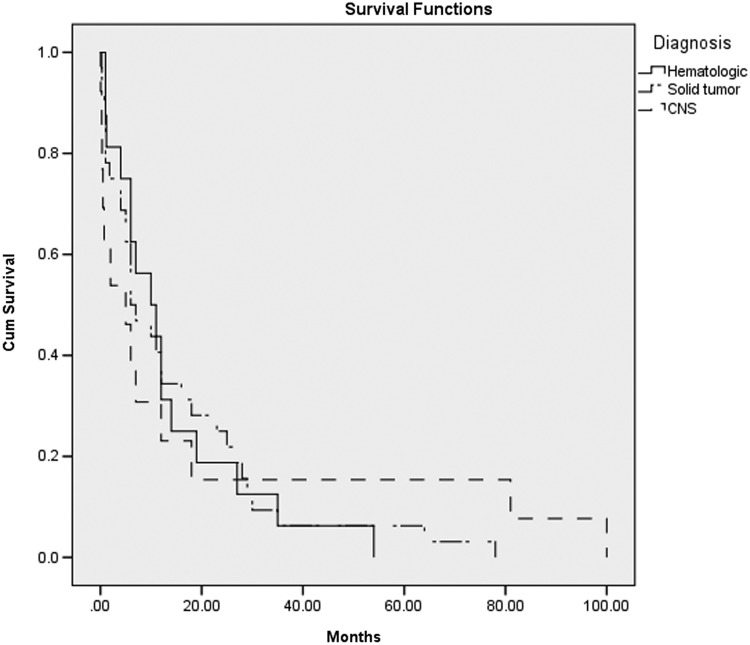
The Kaplan-Meier survival curve by disease type (Fig. 1) showed no significant difference in survival between those with a hematologic malignancy, solid tumor, or brain tumor/CNS disease. However, significant differences in care were found between diagnostic groups (Table 2). Those with hematologic malignancies had longer LOS (22.7 days versus 13.8 for solid tumor and 9.1 for CNS, p=0.044), were more likely to die in the ICU (56% versus 22% for solid tumor and 46% CNS, p=0.045), and had more costly hospitalization on the final admission ($140,000 versus $72,000 for solid tumor, $56,000 for CNS, p=0.029). Those with solid tumors were more likely to receive palliative care consults (66% versus 25% for hematologic and 38% CNS, p=0.02).
FIG. 1.
Kaplan-Meier survival curve by disease type (survival since diagnosis; p=0.994).
Table 2.
Characteristics by Disease Type (Mean or Percentage)
| Characteristic | Hematologic (n=16) | Solid tumor (n=32) | CNS (n=13) | p value |
|---|---|---|---|---|
| Age at diagnosis | 27.44 | 31.31 | 27.54 | 0.110 |
| Age at death | 28.75 | 32.28 | 29.08 | 0.105 |
| Survival (months) | 13.73 | 14.79 | 17.92 | 0.862 |
| LOS (days) | 22.69 | 13.75 | 9.08 | 0.044a |
| New diagnosis at admission | 18.75% (3/16) | 15.63% (5/32) | 38.46% (5/13) | 0.38 |
| Advanced directives at admission | 6.25% (1/16) | 9.38% (3/32) | 7.69 (1/13) | 0.93 |
| DNR at admission | 6.25% (1/16) | 15.63% (5/32) | 15.38% (2/13) | 0.64 |
| Active primary therapy | 62.5% (10/16) | 53.12% (17/32) | 15.38% (2/13) | 0.027a |
| Goals of care discussion at admission | 12.5% (2/16) | 34.38% (11/32) | 53.85% (7/13) | 0.06 |
| ICU as place of death | 56.25% (9/16) | 21.88% (7/32) | 46.15% (6/13) | 0.045a |
| ICU LOS (days) | 11.92 | 20.69 | 10.25 | 0.396 |
| CPR used | 18.75% (3/16) | 15.63% (5/32) | 23.08% (3/13) | 0.837 |
| Palliative care consult received | 25% (4/16) | 65.63% (21/32) | 38.46% (5/13) | 0.02a |
| Timing of palliative care consult before death (days) | 2.60 | 17.33 | 3.80 | 0.351 |
| DNR order prior to death | 93.75% (15/16) | 87.5% (28/32) | 84.61%(11/13) | 0.719 |
| Timing of DNR order prior to death (days) | 3.87 | 5.50 | 2.55 | 0.668 |
| Hospital cost | $140,170.27 | $71,677.94 | $55,724.77 | 0.029a |
Denotes significance at 0.05 level.
LOS, length of stay; DNR, do-not-resuscitate; ICU, intensive care unit; CPR, cardiopulmonary resuscitation.
When comparisons were made between patients who had an ICU stay during the final admission and patients with no ICU stay, patients who had an ICU stay had significantly longer (19.1 days versus 11.5 days, p=0.038) and more expensive hospitalizations ($133,000 versus $43,000, p=0.023). Patients who did not have an ICU stay were significantly more likely to have documented AD (16% versus 0, p=0.026), a discussion about goals of care at admission (47% versus 17%, p=0.014), to have a palliative care consult (66% versus 31%, p=0.007), and to have the palliative care consult earlier (16.9 versus 4.2 days, p=0.038; Table 3).
Table 3.
Characteristics by ICU Stay Status (Mean or Percentage)
| Characteristic | ICU stay (n=29) | No ICU stay (n=32) | p value |
|---|---|---|---|
| Age at diagnosis (years) | 27.90 | 30.94 | 0.444 |
| Age at death (years) | 28.86 | 32.31 | 0.493 |
| Survival (months) | 11.45 | 18.57 | 0.309 |
| LOS (days) | 19.10 | 11.47 | 0.038a |
| New diagnosis at admission | 37.93% (11/29) | 6.25% (2/32) | 0.003a |
| Advanced directives at admission | 0 (0/29) | 15.63% (5/32) | 0.026a |
| DNR at admission | 0 (0/29) | 25% (8/32) | 0.004a |
| Active primary therapy | 43.75% (14/29) | 46.88% (15/32) | 0.913 |
| Goals of care discussion at admission | 17.24% (5/29) | 46.88% (15/32) | 0.014a |
| CPR used | 31.03% (9/29) | 6.25% (2/32) | 0.012a |
| Palliative care consult received | 31.03% (9/29) | 65.63% (21/32) | 0.007a |
| Timing of palliative care consult before death (days) | 4.20 | 16.86 | 0.038a |
| DNR status prior to death | 82.76% (24/29) | 93.75% (30/32) | 0.179 |
| Timing of DNR prior to death (days) | 2.33 | 6.13 | 0.086 |
| Hospital cost | $132,763.82 | $42,959.35 | 0.023a |
Denotes significance at 0.05 level.
ICU, intensive care unit; LOS, length of stay; DNR, do-not-resuscitate; CPR, cardiopulmonary resuscitation.
Finally, patients who had palliative care consults more than 1 day before they died were significantly less likely to die in the ICU (11% versus 48%, p=0.005), more likely to have documentation of a family meeting (95% versus 69%, p=0.036), have DNR orders documented days prior to their death (9.9 days versus 1.9, p=0.001), and exhibit a longer overall survival (20 months versus 13, p=0.019; Table 4). When odds ratios were calculated, those with no palliative care consult/palliative care consult received on the day of death were nearly three times as likely to have an ICU stay during the admission of death compared to those who received a palliative care consult more than 1 day prior to death (odds ratio 2.83, confidence interval 1.143–6.994).
Table 4.
Characteristics by Receiving Palliative Care Consult More Than One day (Mean or Percentage)
| Characteristic | Palliative care consult>1 day before death (n=19) | No palliative care consult/palliative care consult on day of death (n=42) | p value |
|---|---|---|---|
| Age at diagnosis | 31.32 | 28.67 | 0.346 |
| Age at death | 33.11 | 29.57 | 0.288 |
| Survival (months) | 20.02 | 12.99 | 0.019a |
| LOS | 14.37 | 15.43 | 0.121 |
| New diagnosis at admission | 21.05 (4/19) | 21.42 (9/42) | 0.974 |
| Advanced directives at admission | 15.79 (3/19) | 4.76 (2/42) | 0.146 |
| DNR at admission | 21.05 (4/19) | 9.52 (4/42) | 0.217 |
| Active primary therapy | 47.37 (9/19) | 47.62 (20/42) | 0.986 |
| Goals of care discussion at admission | 42.11 (8/19) | 28.57 (12/42) | 0.297 |
| ICU as place of death | 10.53 (2/19) | 47.62 (20/42) | 0.005a |
| ICU LOS | 15.25 | 13.64 | 0.979 |
| CPR used | 15.79 (3/19) | 19.05 (8/42) | 0.759 |
| Documentation of family meeting | 94.73 (18/19) | 69.05 (8/42) | 0.036a |
| DNR order prior to death | 89.47 (17/19) | 88.10 (37/42) | 0.876 |
| Timing of DNR order prior to death (days) | 9.94 | 1.92 | 0.001a |
| Hospital cost | $81,373.47 | $87,575.30 | 0.925 |
Denotes significance at 0.05 level.
LOS, length of stay; DNR, do-not-resuscitate; ICU, intensive care unit; CPR, cardiopulmonary resuscitation.
Discussion
This study provides preliminary evidence demonstrating opportunities that exist for early goal-directed discussions after a young adult is diagnosed with cancer. Findings from this descriptive pilot data are hypothesis-generating because they highlight differences in care outcomes between those who receive early palliative care. In our review, those with palliative care consults more than 1 day prior to death exhibited significantly longer overall survival, greater occurrence of documentation of family meetings, DNR orders written more days prior to death, and less occurrence of death in the ICU. Only half of this study population received a palliative care consult prior to death. Our data suggests that a palliative care consult was often initiated on the day of death to help the primary team transition the dying patient to comfort measures. More research is needed to understand the barriers of earlier palliative care involvement among this unique population while also elucidating the reason for palliative care consult (e.g., transition to comfort measures versus comprehensive symptom management). The earlier use of palliative care would have great utility in providing symptom-based, psychosocial and spiritual support in this unique population.20
These data are limited because it is a single academic center, with a small sample size, included all cancer types, and relied solely on what was available through retrospective chart review so it lacks generalizability. Additionally, the sample itself was limited because it did not include those who died in their homes or in hospice care settings. Even so, this study provides preliminary descriptive evidence highlighting the care trajectories and progression in acute care and ICU settings of young adults with cancer who died in the hospital. Larger prospective studies are needed to further investigate end-of-life care needs among young adults with cancer.
Acknowledgments
Funding for this research was provided by University of Virginia School of Nursing Oscar and Ruth Lanford Research Award (J.K.-M.) and the Nightingale Research Award (J.M.E.). Analysis and dissemination of this article was financially supported while the lead author (J.K.-M.) was a postdoctoral research fellow at Department of Social Sciences & Health Policy, Wake Forest School of Medicine under NIH 5R25CA122061-05.
The authors want to thank Jonathon D. Truwit, MD and Elayne K. Phillips, PhD, RN, for their thoughtful review of the findings.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bleyer WA: Cancer in older adolescents and young adults: Epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol 2002;38:1–10 [DOI] [PubMed] [Google Scholar]
- 2.Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC: Health status of adolescent and young adult cancer survivors. Cancer 2012;118:4884–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyer A, Barr R: Cancer in young adults 20 to 39 years of age: Overview. Semin Oncol 2009;36:194–206 [DOI] [PubMed] [Google Scholar]
- 4.Bleyer A: Latest estimates of survival rates of the 24 most common cancers in adolescent and young adult Americans. J Adolesc Young Adult Oncol 2011;1:37–42 [DOI] [PubMed] [Google Scholar]
- 5.Bird CE, Shugarman LR, Lynn J: Age and gender differences in health care utilization and spending for medicare beneficiaries in their last years of life. J Palliat Med 2002;5:705–712 [DOI] [PubMed] [Google Scholar]
- 6.Levinsky NG: Influence of age on Medicare expenditures and medical care in the last year of life. JAMA 2001;286:1349–1355 [DOI] [PubMed] [Google Scholar]
- 7.Levinsky NG, Ash AS, Yu W, Moskowitz MA: Patterns of use of common major procedures in medical care of older adults. J Am Geriatr Soc 1999;47:553–558 [DOI] [PubMed] [Google Scholar]
- 8.Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS: Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med 2003;138:639–643 [DOI] [PubMed] [Google Scholar]
- 9.Reich O, Signorell A, Busato A: Place of death and health care utilization for people in the last 6 months of life in Switzerland: A retrospective analysis using administrative data. BMC Health Serv Res 2013;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teno JM, Gozalo PL, Bynum JPW, Leland NE, Miller SC, Morden NE, Scupp T, Goodman DC, Mor V: Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013;309:470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack JW, Chen K, Boscoe FP, Gesten FC, Roohan PJ, Weeks JC, Schymura MJ, Schrag D: Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol 2013;31:2569–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell CJ, Skiles J, Pradhan K, Champion VL: End-of-life experiences in adolescents dying with cancer. Support Care Cancer 2010;18:827–835 [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw G, Hinds PS, Lensing S, Gattuso JS, Razzouk BI: Cancer-related deaths in children and adolescents. J Palliat Med 2005;8:86–95 [DOI] [PubMed] [Google Scholar]
- 14.Klopfenstein K, Hutchison C, Clark C: Variables influencing end-of-life care in children and adolescents with cancer. J Pediatr Hematol Oncol 2001;23:481–486 [DOI] [PubMed] [Google Scholar]
- 15.Feudtner C, Christakis D, Zimmerman F, Muldoon J, Neff J, Koepsell T: Characteristics of deaths occurring in children's hospitals: Implications for supportive care services. Pediatrics 2002;109:887–893 [DOI] [PubMed] [Google Scholar]
- 16.Feudtner C, Digiuseppe DL, Neff JM: Hospital care for children and young adults in the last year of life: A population-based study. BMC Med 2003;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavoshi N, Miller T, Siden H: Resource utilization among individuals dying of pediatric life-threatening diseases. J Palliat Med 2013;16:1210–1214 [DOI] [PubMed] [Google Scholar]
- 18.Hinds PS, Pritchard M, Harper J: End-of-life research as a priority for pediatric oncology. J Pediatr Oncol Nurs 2004;21:175–179 [DOI] [PubMed] [Google Scholar]
- 19.Coccia PF, Altman J, Bhatia S, Gesten FC, Roohan PJ, Weeks JC, Schymura MJ, Schrag D: Adolescent and young adult oncology. J Natl Compr Cancer Netw 2012;10:1112–1150 [DOI] [PubMed] [Google Scholar]
- 20.Zhukovsky DS, Herzog CE, Kaur G, Palmer JL, Bruera E:. The impact of palliative care consultation on symptom assessment, communication needs, and palliative interventions in pediatric patients with cancer. J Palliat Med 2009;12:343–349 [DOI] [PubMed] [Google Scholar]