Abstract
Objective: The purpose of this study was to assess the time of onset and time course of efficacy over 12.0 hours of extended-release multilayer bead formulation of methylphenidate (MPH-MLR) compared with placebo in children 6–12 years of age with attention-deficit/hyperactivity disorder (ADHD) in a laboratory school setting.
Methods: This randomized double-blind placebo-controlled study included children 6–12 years of age with ADHD. Enrolled children went through four study phases: 1) Screening period (≤4 weeks) and a 2 day medication washout period; 2) open-label period with dose initiation of MPH-MLR 15 mg daily and individual dose optimization treatment period (2–4 weeks); 3) double-blind crossover period in which participants were randomized to sequences (1 week each) of placebo and the optimized MPH-MLR dose given daily; and 4) follow-up safety call. Analog classroom time course evaluations were performed at the end of each double-blind week. The primary efficacy end-point was the mean of the on-treatment/postdose Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP)-Total scores over time points collected 1.0–12.0 hours after dosing. End-points were evaluated using a mixed-effects analysis of covariance.
Results: The evaluable population included 20 participants. The least-squares mean postdose SKAMP-Total score was higher for placebo than for MPH-MLR (2.18 vs. 1.32, respectively; p=0.0001), indicating fewer symptoms with MPH-MLR therapy than with placebo. No difference in SKAMP-Total score between participants who received sequence 1 or sequence 2 was noted. From each of hours 1.0–12.0, least-squares mean SKAMP-Total score was significantly lower for those receiving MPH-MLR than for those receiving placebo (p≤0.0261). Neither serious adverse events nor new or unexpected safety findings were noted during the study.
Conclusions: MPH-MLR showed a significant decrease in SKAMP scores compared with placebo in children with ADHD 6–12 years of age, indicating a decrease in ADHD symptoms. The estimated onset was observed within 1.0 hour, and duration was measured to 12.0 hours postdose.
Trial registration: ClinicalTrials.gov Identifier: NCT01269463
Introduction
Stimulant agents, including methylphenidate (MPH), are recommended as first-line pharmacotherapy treatment options for children and adolescents with attention-deficit/hyperactivity disorder (ADHD) (National Institute for Health and Clinical Excellence 2008; Wolraich et al. 2011). The short elimination half-life of MPH (2–3 hours) (Kimko et al. 1999) enables clinicians to tailor doses of MPH immediate-release (IR) tablets at different times of the day to meet the specific needs of their patients (Markowitz et al. 2003). However, the adherence and privacy issues associated with IR formulations have led to the development of extended-release (ER) formulations with similar efficacy (Faraone 2009).
Many ER formulations of MPH are currently available. Each offers a unique MPH delivery profile with absorption characteristics similar to two or three times daily dosing with MPH IR tablets (Maldonado 2013). The IR components of the ER formulations include 20% (Quillivant XR™, NextWave Pharmaceuticals, Inc., Cupertino CA), 22% (Concerta®, Janssen Pharmaceuticals, Inc., Titusville, NJ), 30% (Metadate CD®, UCB, Inc., Smyrna, GA), and 50% (Ritalin LA, Novartis Pharmaceuticals Corporation, East Hanover, NJ) options. Although as a group these ER formulations have been effective, no single formulation is ideal for all children with ADHD.
A novel formulation of ER MPH in encapsulated multilayer beads (MPH-MLR; Rhodes Pharmaceuticals L.P., Coventry, RI) provides a different initial release of MPH: 37% of the total MPH dose (Quinn et al. 2007; Reiz et al. 2008; Adjei et al. 2014b). After morning administration of MPH-MLR, a small drop in plasma MPH concentration occurs ∼4 hours postdosing, followed by a gradual increase in MPH concentrations, producing a second attenuated peak at ∼7 hours after dosing and then a gradual decline throughout the evening and nighttime hours (Adjei et al. 2014a,b). Phase 2 studies of MPH-MLR in children, adolescents, and adults across a variety of settings demonstrated that once-daily administration produces significant improvements in behavioral and cognitive measures (Jain et al. 2007; Weiss et al. 2007; Schachar et al. 2008). The MPH-MLR capsule is ingested orally or opened for its contents to be sprinkled on food; the two administration modes are bioequivalent (Adjei et al. 2014b). The present phase 3 study was designed to assess the time of onset and time course of efficacy of MPH-MLR compared with placebo over 12.0 hours in the laboratory school setting.
Methods
Study conduct
This single-center randomized double-blind placebo-controlled crossover design study (ClinicalTrials.gov identifier, NCT01269463) was registered December 2010. The study protocol (protocol number RP-BP-EF001) and amendments, as well as the informed consent form, were reviewed and approved by the local investigational review board of the University of California, Irvine. The study was undertaken in compliance with the Good Clinical Practice guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, the United States Code of Federal Regulations dealing with clinical studies, and the principles of the Declaration of Helsinki.
Study participants
All participants and/or legal guardians provided written informed consent before receiving pre-enrollment psychiatric and medical evaluations. Children (male or female) 6–12 years of age at the time of consent with any of the three subtypes of ADHD as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision were eligible for inclusion in the study (American Psychiatric Association 2000). The diagnosis of ADHD was supported by the parent/guardian completion of the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (Kaufman et al. 1997). Participants were required to have an ADHD Rating Scale, Version 4 (ADHD-RS-IV) total or subscale score≥90th percentile relative to the general population of children by age and sex (DuPaul et al. 1998), and they had to be naïve to treatment for ADHD or inadequately managed on their current treatment regimen. In addition, all participants were required to have negative illicit drug and alcohol test results at screening and at each visit to the research site.
Participants were excluded from study participation if their estimated Full Scale intellectual level was <80, using the four-subtest form of the Wechsler Abbreviated Scale of Intelligence®, Second Editon (WASI-II). Additional exclusion criteria were any severe psychiatric or significant comorbid condition, use of a monoamine oxidase inhibitor or any psychotropic medication with central nervous system effects ≤14 days of screening or any experimental drug or medical device ≤30 days of screening, a clinically significant electrocardiogram (ECG), or any laboratory abnormality. Any participant unable or unwilling to follow directions and complete study assessments or take oral capsules also was excluded from participation in the study.
Study treatments
MPH-MLR (15, 20, 30, or 40 mg; lot numbers A67958, A67827, A67871, and A67957, respectively) or placebo was supplied to participants in a 10 count bottle for a 1 week treatment duration. Participants received weekly supplies of medication beginning at the first open-label visit through visit 7. Beginning the day following baseline assessments, study medication was taken as an oral capsule once daily in the morning no later than 10:00 a.m. At the end of the week, before receiving the next week's supply, participants returned any unused medication to the study site.
Study design
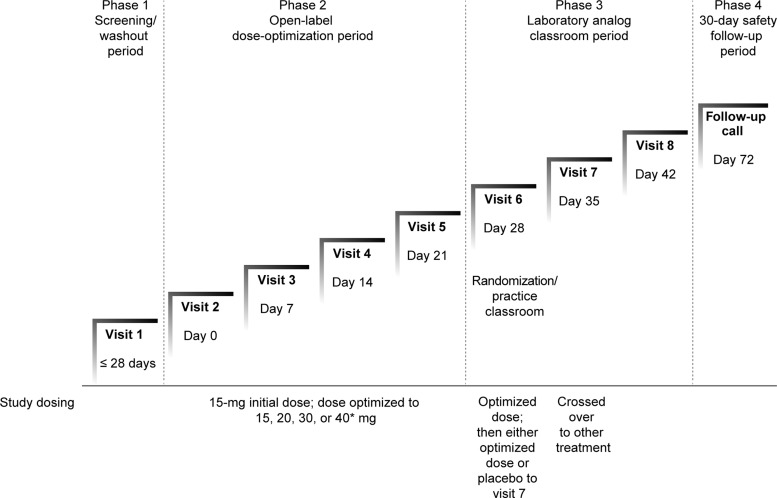
The study was conducted in four distinct phases (Fig. 1). The initial phase (screening) was conducted over ≤4 weeks and included screening assessments and, if the participant was already on medication for ADHD, a 2 day washout period (five medication half-lives). During the screening visit, questioning for medical and psychiatric history, physical examination, vital signs, 12 lead ECG, urine and blood collection for testing, K-SADS-PL, WASI-II, ADHD-RS-IV, Clinical Global Impressions Scale of Severity (CGI-S), and Columbia-Suicide Severity Rating Scale (Baseline/Screening version) were performed. A phone call during the initial phase provided parents of study participants with a review of study entry criteria, evaluation of concomitant medications, instructions for drug washout (if needed), and adverse events (AEs).
FIG. 1.
Study design. *Only in patients weighing >25 kg.
The second phase was an open-label dose-optimization phase (2–4 weeks). Participants returned to the study site, eligibility was confirmed, and baseline assessments (vital signs, weight, 12 lead ECG, urine pregnancy test, CGI-S, ADHD-RS-IV) were conducted. The Permanent Product Measure of Performance (PERMP) math pretest was administered to determine individual levels of performance, and participants received their initial supply of MPH-MLR 15 mg. At each subsequent visit (approximately weekly) over the next 2–4 weeks, investigators used AE information and ADHD-RS-IV and Clinical Global Impressions Scale of Improvement (CGI-I) scores to determine whether the current dose was acceptable or whether dose titration was required. For participants weighing >25 kg, MPH-MLR doses of 15, 20, 30, or 40 mg once daily were used. For participants weighing ≤25 kg, it was planned that only MPH-MLR doses of 15, 20, or 30 mg once daily would be permitted. However, there were two protocol deviations in which participants weighing <25 kg received the 40 mg dose. The optimal dose—the dose that produced the best clinical response without intolerable AEs—was identified for each participant and used in the double-blind crossover phase. If necessary, participants could receive their optimized MPH-MLR dose beyond the 4 week open-label period while the required number of participants for classroom study was assembled.
The third phase was the laboratory school period (Wigal and Wigal 2006). At the sixth study visit, participants took part in an open-label dose optimized analog classroom practice session and completed four PERMP practice tests (predose and at hours 1.0, 2.0, and 3.0 postdose). At this visit, participants were randomized in a 1:1 ratio to one of two treatment sequence groups and received a 1 week supply of the study drug. Randomization was determined by using a table of random numbers. The randomization tables were provided to the randomization monitor who created unblinding envelopes and packaged the drug with blinded labels. All sponsor representatives, investigators, participants, and independent raters remained blinded until after data lock. For treatment sequence 1, participants received placebo for 1 week and crossed over to their optimized MPH-MLR dose given once daily in the morning for 1 week. For treatment sequence 2, participants received their optimized dose of MPH-MLR for the first week and placebo for the second week, given once daily in the morning for 1 week.
At visit 7, participants arrived at the site at ∼7:00 a.m. for predose assessments (e.g., sitting vital signs, weight) and were given the last dose of their week-long study drug at ∼8:30 a.m., followed by breakfast, after which they participated in the double-blind analog classroom study day. Assessments during the laboratory classroom study day included Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Attention, Deportment, and Total scores and PERMP scores (predose and 1.0, 2.0, 3.0, 4.5, 6.0, 7.5, 9.0, 10.5, and 12.0 hours postdose), and clinician-rated ADHD-RS-IV inattention and hyperactivity/impulsivity scores (3.0 hours postdose). Participants were dispensed their other randomized week of double-blind study drug before leaving this visit. At visit 8, participants followed the same procedure for the second study treatment.
The fourth study phase was the final follow-up telephone call ∼30 days after completion of the double-blind phase when end-of-study information about AEs and any new medications was collected.
End-points
The primary efficacy end-point was the mean of the double-blind on-treatment postdose SKAMP-Total scores over time points 1.0, 2.0, 3.0, 4.5, 6.0, 7.5, 9.0, 10.5, and 12.0 hours. Trained observers use this validated 13 item scale (Wigal et al. 1998) to assess impairment of specific classroom behaviors. Each item is rated on a seven point scale of 0 (normal) to 6 (maximal impairment). When all item scores are averaged, the result is reported as the SKAMP-Total score. Averaging of items 1–4 produces a SKAMP-Attention subscale score, and averaging items 5–8 yields a SKAMP-Deportment subscale score.
The key secondary efficacy end-point was the duration of efficacy between MPH-MLR and placebo during the double-blind phase, using the SKAMP-Total score at each postdose time point. Other secondary efficacy end-points were SKAMP-Attention and -Deportment scores averaged over all postdose time points and at each postdose time point, PERMP math scores averaged over all postdose time points, and clinician-rated ADHD-RS-IV scores at 3.0 hours postdose each laboratory day. Safety, and tolerability assessments also were conducted.
The PERMP is a 10 minute skill-adjusted math test used as an objective measure of treatment efficacy (Wigal and Wigal 2006). Scoring includes number of math problems attempted and those answered correctly. The appropriate difficulty level for each participant was determined at the practice session.
The ADHD-RS-IV is an 18 item rating scale that incorporates each of the symptoms of ADHD (DuPaul et al. 1998). Scoring for each item uses the recommended four point scale where 0=never and 3=occurs very often. Individual item scores are totaled; possible scores range from 0 (no impairment) to 54 (maximal impairment).
The CGI-S was completed at visits 1 and 2 by clinicians assessing severity of illness; ratings range from 1 (normal or no impairment) through 7 (severely impaired, i.e. the most extreme, requiring inpatient services) (Busner and Targum 2007). The CGI-I was used at subsequent visits to denote changes from baseline (treatment initiation). Clinicians completing the CGI-I rated change in the patient's condition since visit 2 on a scale from 1 (very much improved) to 7 (very much worse, loss of functioning) (Busner and Targum 2007).
Statistical analysis
The primary efficacy analysis was based on the mean of the on-treatment/postdose SKAMP-Total score in the double-blind phase for the evaluable population (those participants who finished the study without a protocol deviation). It was estimated that ∼27 participants (assuming a 10% dropout rate) would be needed to detect a difference of 1.0 in the mean of the double-blind on-treatment postdose SKAMP-Total scores over time points 1.0, 2.0, 3.0, 4.5, 6.0, 7.5, 9.0, 10.5, and 12.0 hours between MPH-MLR and placebo, with 80% power and a two sided significance level of 0.05. None of the participants dropped out during the double-blind phase. No imputation was done for any scale in the evaluable population.
The SKAMP-Total scores collected during the double-blind period were tested in the following sequential order of time points 3.0, 4.5, 6.0, 7.5, 2.0, 1.0, 9.0, 10.5, and 12.0 hours. Testing was to stop if the difference between scores for MPH-MLR– and placebo-treated participants was not statistically significant. A mixed-effects analysis of covariance was used to evaluate the end-points with fixed terms for treatment, sequence, period, random term for participant within sequence, and covariate term for the predose value.
Results
Participant disposition and population demographics
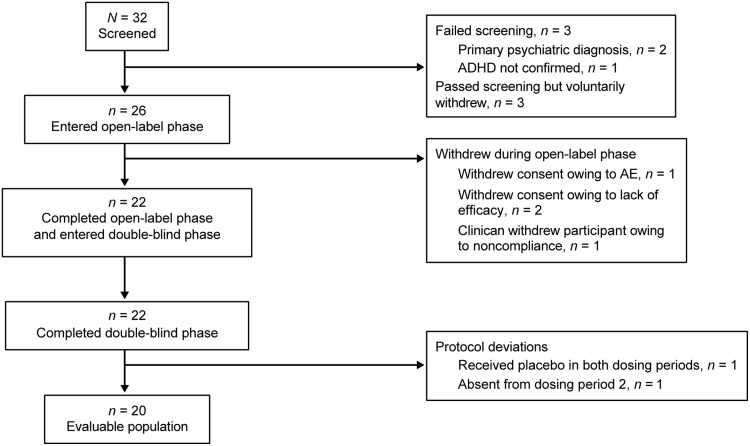
Thirty-two participants were screened, 26 participants were enrolled in the open-label phase and received at least one dose of study drug (safety population), and 22 participants entered (intent-to-treat [ITT] population) and completed the double-blind phase (Fig. 2). Of these, two participants were excluded because of protocol deviations (one participant received placebo in both dosing periods because of a packaging error, and one participant who received placebo in dosing period 1 was absent from dosing period 2 because of illness, and, therefore, did not have SKAMP assessments performed); thus, the evaluable population included 20 participants.
FIG. 2.
Participant disposition. ADHD, attention-deficit/hyperactivity disorder; AE, adverse event.
The mean age of the 26 participants included in the safety population was 8.7 years compared with 8.8 years in the ITT population (Table 1). Approximately half (n=12; 46%) of enrolled participants had a diagnosis of inattentive ADHD, three participants had hyperactive/impulsive ADHD, and 11 participants had combined ADHD. A previous or current psychiatric diagnosis (generalized anxiety disorder, enuresis, oppositional defiant disorder, chronic motor or vocal tic disorder, transient tic disorder) was noted for 11 participants. Full Scale (WASI-II) intellectual scores in this study ranged from 86 to 133. At visit 2, mean (standard deviation [SD]) ADHD-RS-IV total score was 40.85 (6.35) for the safety population (range, 29–54). Mean (SD) ADHD-RS-IV subscale scores were 22.46 (3.48) for inattention (range, 10–27) and 18.38 (5.71) for hyperactivity/impulsivity scores (range 8–27). Mean (SD) score for the CGI-S was 4.73 (0.45) and ranged from 4–5, where 4 indicated moderate severity and 5 indicated marked severity and impairment.
Table 1.
Baseline Demographics
| Characteristic | Safety population n=26 | ITT population sequence 1 placebo/MPH-MLR n=11 | ITT population sequence 2 MPH-MLR/placebo n=11 | ITT population n=22 |
|---|---|---|---|---|
| Mean (SD) age, y | 8.7 (1.9) | 8.9 (1.9) | 8.7 (1.9) | 8.8 (1.9) |
| Male, n (%) | 14 (54) | 6 (55) | 6 (55) | 12 (55) |
| Race, n (%) | ||||
| White | 21 (81) | 9 (82) | 9 (82) | 18 (82) |
| Black | 3 (12) | 2 (18) | 0 (0) | 2 (9) |
| Asian | 1 (4) | 0 (0) | 1 (9) | 1 (5) |
| Other | 1 (4) | 0 (0) | 1 (9) | 1 (5) |
| Hispanic or Latino ethnicity, n (%) | 6 (23) | 3 (27) | 2 (18) | 5 (23) |
| Mean (SD) weight, kg | 33.7 (12.0) | 37.9 (15.1) | 30.7 (8.9) | 34.3 (12.6) |
| Mean (SD) height, cm | 135.9 (12.8) | 139.2 (13.7) | 133.4 (11.2) | 136.3 (12.5) |
ITT, intent-to-treat; MPH-MLR, extended-release multilayer bead methylphenidate.
Compliance
Compliance with treatment was verified at scheduled study visits by study personnel who examined documentation of drug dispensed, drug consumed, and remaining drug, and recorded the information on the drug reconciliation form. Compliance with prescribed study drug was calculated to be >82% throughout the study, and highest (96.1%) during the double-blind phase.
Efficacy
In the evaluable population, the least-squares (LS) mean postdose SKAMP-Total score was lower (1.32 vs. 2.18; p=0.0001) for participants receiving MPH-MLR versus placebo, respectively, indicating fewer symptoms in classroom behavior, written work, and general behavior with MPH-MLR therapy (Table 2). The covariate predose SKAMP-Total score was significant (p=0.0003), indicating that the predose score helped predict the postdose score. There was no significant difference in SKAMP-Total scores between the treatment sequences or treatment periods. A similar response was observed in the ITT population.
Table 2.
Primary Efficacy End-Point Analysis: SKAMP-Total Score Averaged Over All Postdose Time Points for the Evaluable (n=20) and ITT (n=22) Populations
| LS mean* | p value* | |||||
|---|---|---|---|---|---|---|
| Total score | Placebo | MPH-MLR | Treatment | Covariate | Sequence | Period |
| Evaluable population | 2.18 | 1.32 | 0.0001 | 0.0003 | 0.5279 | 0.0714 |
| ITT population | 2.05 | 1.32 | 0.0005 | 0.0006 | 0.8824 | 0.2570 |
Mixed-effects analysis of covariance, fixed terms for treatment, sequence, period, random term for participant within sequence, and covariate term for the predose value.
ITT, intent-to-treat; LS, least-squares; MPH-MLR, extended-release multilayer bead methylphenidate; SKAMP, Swanson, Kotkin, Agler, M-Flynn, and Pelham.
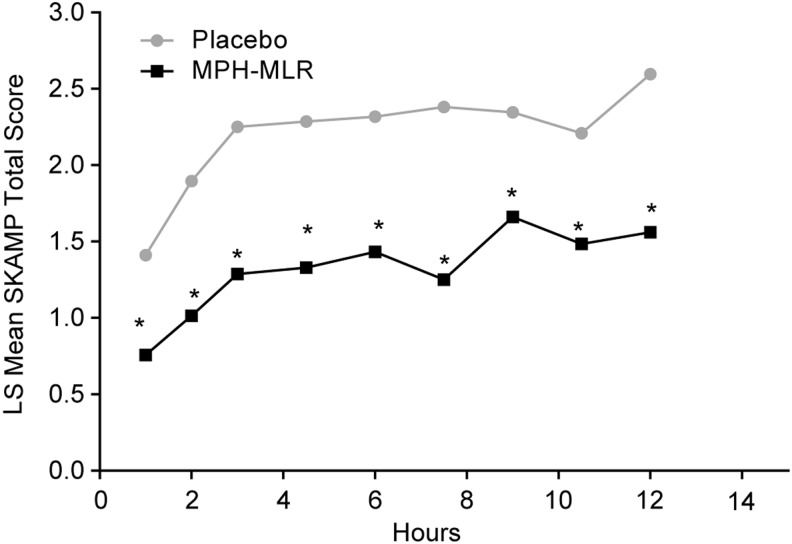
For the key secondary analysis in the evaluable population, at each of hours 1.0–12.0, the mean LS SKAMP-Total scores were significantly lower for participants receiving MPH-MLR than for those receiving placebo (p≤0.0261; Fig. 3). Sequence was only significant at hour 3.0 (p=0.0397); at all other time points, the difference between sequences was nonsignificant (p≥0.3266). Overall, the results were similar when the ITT population was used; however, neither sequence nor period was ever statistically significant.
FIG. 3.
Least-squares (LS) mean† SKAMP-Total scores over time (evaluable population, n=20). MPH-MLR, extended-release multilayer bead methylphenidate; SKAMP, Swanson, Kotkin, Agler, M-Flynn, and Pelham. *p≤0.0261. †Mixed-effects analysis of covariance with fixed terms for treatment, sequence, period, random term for participant within sequence, and covariate term for the predose value.
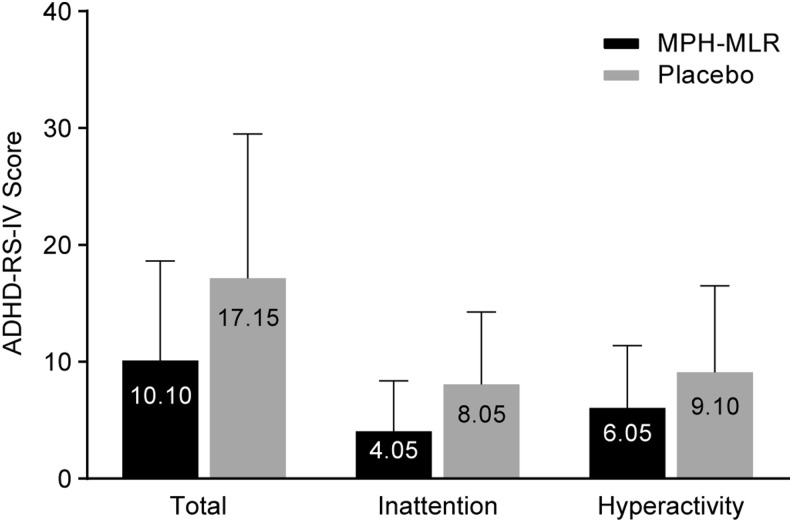
Results for additional secondary efficacy analyses in the evaluable population are summarized in Table 3. SKAMP-Attention and -Deportment scores averaged over all postdose time points were significantly lower for participants receiving MPH-MLR than for those receiving placebo (SKAMP-Attention: 1.05 vs. 1.81, respectively; p=0.0001; SKAMP-Deportment: 0.78 vs. 1.64, respectively; p=0.0008). As with the SKAMP-Total score, the covariate predose score was statistically significant for SKAMP attention and deportment (p≤0.0108), indicating that the predose score helped predict the postdose score. Increased classroom productivity was demonstrated by PERMP math test scores averaged over all postdose time points, which were higher for participants receiving MPH-MLR than for those receiving placebo. Clinician ratings of the ADHD-RS-IV hyperactivity/impulsivity, inattention, and total scores at 3.0 hours were lower in participants receiving MPH-MLR than in those receiving placebo, indicating reduced symptoms of ADHD with MPH-MLR therapy (Fig. 4). Treatment with MPH-MLR produced significantly lower LS mean ADHD-RS-IV total (10.27 vs. 17.64; p=0.0019), inattention (4.20 vs. 8.42; p=0.0003), and hyperactivity/impulsivity (6.08 vs. 9.22, p=0.0391) scores compared with placebo.
Table 3.
Secondary Efficacy End-Point Analysis (Evaluable Population, n=20)
| LS mean* | p value* | |||||
|---|---|---|---|---|---|---|
| Placebo | MPH-MLR | Treatment | Covariate | Sequence | Period | |
| Average SKAMP scores over all postdose time points | ||||||
| Attention | 1.81 | 1.05 | 0.0001 | 0.0002 | 0.4780 | 0.0770 |
| Deportment | 1.64 | 0.78 | 0.0008 | 0.0108 | 0.7346 | 0.1076 |
| Average PERMP math test scores over all postdose time points | ||||||
| PERMP-A | 83.15 | 113.75 | 0.0054 | <0.0001 | 0.7241 | 0.1270 |
| PERMP-C | 73.19 | 109.13 | 0.0006 | <0.0001 | 0.3286 | 0.0317 |
Mixed-effects analysis of covariance with fixed terms for treatment, sequence, period, random term for participant within sequence, and covariate term for the predose value.
LS, least-squares; MPH-MLR, extended-release multilayer bead methylphenidate; PERMP, Permanent Product Measure of Performance; PERMP-A, Permanent Product Measure of Performance, number of math problems attempted; PERMP-C Permanent Product Measure of Performance, number of math problems answered correctly; SKAMP, Swanson, Kotkin, Agler, M-Flynn, and Pelham.
FIG. 4.
Arithmetic mean ADHD-RS-IV scores (double-blind crossover phase evaluable population, n=20). ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale–IV; MPH-MLR, extended-release multilayer bead methylphenidate.
For the ITT population, results of the secondary analyses were generally similar to those of the evaluable population. LS mean SKAMP-Attention scores were significantly higher (indicating more inattention) for participants receiving MPH-MLR compared with those receiving placebo at hour 0.0. Over hours 1.0–12.0, scores of participants receiving MPH-MLR were significantly lower than those for participants receiving placebo; sequence was nonsignificant and period was significant only at hour 6.0. LS mean SKAMP-Deportment scores also were significantly higher for participants receiving MPH-MLR than for those receiving placebo at hour 0.0. Over hours 1.0–12.0, LS mean SKAMP-Deportment scores were significantly lower for those receiving MPH-MLR than for those receiving placebo, and neither sequence nor period were significant.
Safety
There were no new or unexpected safety results during the study (Table 4). The most common (>10%) treatment-related AEs in either the open-label or double-blind periods included decreased appetite, headache, irritability, cough, nasal congestion, rhinorrhea, pyrexia, otitis media, abdominal pain, vomiting, and insomnia. Overall, AEs were mild or moderate, and no serious AEs were reported. Cardiovascular events and other AEs were similar to those reported with other stimulant medications for ADHD and were self-limiting, with no required intervention.
Table 4.
Adverse Events Occuring in≥5% of Participants
| Double-blind dosing period | |||
|---|---|---|---|
| MedDRA System Preferred Term, n (%) | Open-label period n=26 | Placebo n=22 | MPH-MLR n=21 |
| Abdominal pain | 3 (11.5) | 1 (4.5) | 2 (9.5) |
| Vomiting | 3 (11.5) | 0 (0) | 1 (4.8) |
| Pyrexia | 4 (15.4) | 0 (0) | 2 (9.5) |
| Influenza | 2 (7.7) | 0 (0) | 0 (0) |
| Otitis media | 3 (11.5) | 0 (0) | 0 (0) |
| Respiratory tract infection | 2 (7.7) | 0 (0) | 0 (0) |
| Rhinitis | 2 (7.7) | 1 (4.5) | 1 (4.8) |
| Sinusitis | 2 (7.7) | 0 (0) | 0 (0) |
| Decreased appetite | 6 (23.1) | 0 (0) | 0 (0) |
| Headache | 6 (23.1) | 0 (0) | 3 (14.3) |
| Insomnia | 8 (30.8) | 1 (4.5) | 0 (0) |
| Somnolence | 2 (7.7) | 0 (0) | 0 (0) |
| Emotional distress | 2 (7.7) | 0 (0) | 1 (4.8) |
| Irritability | 5 (19.2) | 1 (4.5) | 0 (0) |
| Mood swings | 2 (7.7) | 0 (0) | 0 (0) |
| Cough | 4 (15.4) | 0 (0) | 0 (0) |
| Nasal congestion | 3 (11.5) | 0 (0) | 0 (0) |
| Rhinorrhea | 3 (11.5) | 0 (0) | 0 (0) |
| Double-blind dosing period | |||
|---|---|---|---|
| MedDRA System Organ Class, n (%) | Open-label period n=26 | Placebo n=22 | MPH-MLR n=21 |
| Rash | 2 (7.7) | 0 (0) | 1 (4.8) |
MedDRA, Medical Dictionary for Regulatory Activities; MPH-MLR, extended-release multilayer bead methylphenidate.
Discussion
This study demonstrated that there was a significant difference between placebo and MPH-MLR in the LS mean SKAMP-Total score that began at hour 1.0, and persisted through all measured time points to hour 12.0. Similar results were found with the SKAMP-Attention and -Deportment subscales. These findings confirmed that the biphasic pharmacokinetic profile of MPH-MLR that previously has been reported (Adjei et al. 2014b) translates into a significant ADHD symptom reduction in children and adolescents that is evident as early as hour 1.0 and persists through ≥12 hours postdose.
MPH-MLR is not the first long-acting MPH formulation to demonstrate time-response efficacy in an analog classroom study. Onset and duration of ADHD symptom reduction in this setting have been measured for other long-acting MPH formulations (Pelham et al. 2001; McGough et al. 2006; Swanson et al. 2004; Wigal et al. 2011). Differences in study design make cross-study comparisons of onset of action and duration of effect difficult.
The safety profile of MPH-MLR as identified in this study resembles the known safety profile of MPH. The most common AEs associated with MPH-MLR reported in this study are consistent with the reported AE profile of other MPH formulations. No serious drug-related AEs were reported. Reported AEs did not require intervention and were self-limiting.
Limitations
There were some limitations to this study. The study set out to include 27 randomized participants; however, only 22 completed the double-blind phase. The study was slightly underpowered; yet, the findings showed a statistically significant treatment effect. The exclusion criteria used in this study produced a study population that may not be reflective of the general population of children and adolescents with ADHD. In addition, the classroom setting, although useful for research purposes, is not a direct replication of a typical elementary school classroom. Rather, it is a tightly controlled environment, includes fewer children than are found in a normal classroom, and includes only students diagnosed with ADHD.
Conclusions
In this study, MPH-MLR administered to children 6–12 years of age demonstrated a significant decrease in SKAMP scores compared with placebo. The onset of action in this population is ∼1 hour, and duration of efficacy is sustained to ≥12 hours postdose. Future studies that include measurement of efficacy earlier than hour 1.0 and extend beyond hour 12.0 would add clarity to the precise onset and duration of clinical efficacy.
Clinical Significance
Once-daily MPH-MLR represents a new treatment option for the management of ADHD that will add to the clinician's ability to individualize treatment for patients. This study adds to the adult pharmacokinetic data that have already been published, and shows that MPH-MLR is efficacious in children and adolescents 6–12 years of age, as evaluated in a laboratory classroom setting. In this study, the treatment benefit of MPH-MLR was evident by hour 1.0 and continued to ≥12 hours postdose. The unique release profile (IR component, 37%, with a biphasic release profile) coupled with the ability to administer as intact capsules or sprinkle on food may address a previously unmet need for patients with ADHD.
Acknowledgments
A.A., R.K., and S.W. designed the study and created the study protocol. E.N. led the statistical effort including data analysis, and A.S. provided statistical support. All authors analyzed and interpreted the study data, contributed to the content of the manuscript, critically reviewed drafts, and approved the final version for submission. The authors acknowledge the assistance of Wei-Wei Chang, PhD, of NuTec Incorporated for her assistance with protocol development and audit of the data. Medical writing support was provided by Linda Wagner, PharmD, medical writer at Excel Scientific Solutions.
Disclosures
Dr. Wigal is an advisory board member/consultant/speakers bureau member for Eli Lilly, Ironshore, Neos Therapeutics Inc., NextWave Pharmaceuticals, Noven, NuTec Incorporated, Pfizer Inc, Purdue Pharma L.P., Rho, Rhodes Pharmaceuticals L.P., Shionogi Inc., Shire, and Vernalis, and has received grant and research support from Eli Lilly, Forest Laboratories, Ironshore, the National Institutes of Health (NIH), NextWave Pharmaceuticals, Noven, NuTec Incorporated, Rhodes Pharmaceuticals L.P., Shire, Sunovion, and Tris Pharma. Dr. Greenhill has received research support from the National Institute on Drug Abuse (NIDA)/NIH and Shire and is on the advisory board for BioBehavioral Diagnostics. Dr. Nordbrock is a consultant for Rhodes Pharmaceuticals L.P. Dr. Connor receives grant support from and is an ADHD consultant for Rhodes Pharmaceuticals L.P. and Shire. He receives additional support from the National Institute of Mental Health and the state of Connecticut. He receives royalties from the Guilford Press and W.W. Norton. Dr. Kollins has received research support and/or consulting fees from the following sources: Akili Interactive, Alcobra Pharmaceuticals, Arbor Pharmaceuticals, Atentiv, Environmental Protection Agency, NIH (NIDA, National Institute of Environmental Health Sciences), Neos Pharmaceuticals, Otsuka Pharmaceuticals, Pfizer Pharmaceuticals, Purdue Pharma LP, Rhodes Pharmaceuticals LP, Shire, and Tris Pharma. Dr. Adjei is the executive director of product development at Rhodes Pharmaceuticals L.P. and was study director for this study. Dr. Childress is an advisory board member/consultant/speakers bureau member for Bristol-Myers Squibb, GlaxoSmithKline, Ironshore, NextWave Pharmaceuticals, Novartis Pharmaceutical Corporation, Pfizer Inc, Shionogi, Inc., and Shire, and has received research support from Abbott Laboratories, Bristol-Myers Squibb, Forest, Ironshore, Johnson & Johnson Pharmaceutical Research & Development, Lilly USA, NextWave Pharmaceuticals, Nexus, Novartis Pharmaceutical Corporation, Noven, Ortho-McNeill Janssen Scientific Affairs, Otsuka, Pfizer Inc, Rhodes Pharmaceuticals, L.P., Sepracor, Inc., Shionogi, Inc., Shire, Somerset Pharmaceuticals, Sunovion, and Tris Pharma. Dr. Kupper is an employee of Rhodes Pharmaceuticals L.P.
References
- Adjei A, Kupper RJ, Teuscher NS, Wigal S, Sallee FR, Childress A, Kollins S, Greenhill L: Steady-state bioavailability of methyphenidate extended-release (MPH-MLR) capsule versus methylphenidate immediate-release tablets (Ritalin®) in healthy adult volunteers. Clin Drug Invest in press, 2014a [DOI] [PubMed] [Google Scholar]
- Adjei A, Teuscher NS, Kupper RJ, Chang W-W, Greenhill L, Newcorn J, Connor DF, Wigal S: Single-dose pharmacokinetics of methylphenidate extended-release administered as intact capsule or sprinkles versus methylphenidate immediate-release tablets (Ritalin®) in healthy adult volunteers. J Child Adolesc Psychopharmacol, 2014b [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Busner J, Targum SD: The Clinical Global Impressions Scale: Applying a research tool in clinical practice. Psychiatry (Edgmont) 4:28–37, 2007 [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale–IV: Checklist, Norms, and Clinical Interpretation. New York: Guilford Press; 1998 [Google Scholar]
- Faraone SV: Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T 34:678–683, 694, 2009 [PMC free article] [PubMed] [Google Scholar]
- Jain U, Hechtman L, Weiss M, Ahmed TS, Reiz JL, Donnelly GAE, Harsanyi Z, Darke AC: Efficacy of a novel biphasic controlled-release methylphenidate formula in adults with attention-deficit/hyperactivity disorder: Results of a double-blind, placebo-controlled crossover study. J Clin Psychiatry 68:268–277, 2007 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kimko HC, Cross JT, Abernethy DR: Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet 37:457–470, 1999 [DOI] [PubMed] [Google Scholar]
- Maldonado R: Comparison of the pharmacokinetics and clinical efficacy of new extended-release formulations of methylphenidate. Expert Opin Drug Metab Toxicol 9:1001–1014, 2013 [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Straughn AB, Patrick KS: Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: Focus on methylphenidate formulations. Pharmacotherapy 23:1281–1299, 2003 [DOI] [PubMed] [Google Scholar]
- McGough JJ, Wigal SB, Abikoff H, Turnbow JM, Posner K, Moon E: A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord 9:476–485, 2006 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence: Attention deficit hyperactivity disorder: Diagnosis and management of ADHD in children, young people and adults. Available at http://publications.nice.org.uk/attention-deficit-hyperactivity-disorder-cg72 Accessed accessed July17, 2014
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD: Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics 107:e105, 2001 [DOI] [PubMed] [Google Scholar]
- Quinn D, Bode T, Reiz JL, Donnelly GAE, Darke AC: Single-dose pharmacokinetics of multilayer-release methylphenidate and immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Pharmacol 47:760–766, 2007 [DOI] [PubMed] [Google Scholar]
- Reiz JL, Donnelly GA, Michalko K: Comparative bioavailability of single-dose methylphenidate from a multilayer-release bead formulation and an osmotic system: A two-way crossover study in healthy young adults. Clin Ther 30:59–69, 2008 [DOI] [PubMed] [Google Scholar]
- Schachar R, Ickowicz A, Crosbie J, Donnelly GAE, Reiz JL, Miceli PC, Harsanyi Z, Darke AC: Cognitive and behavioral effects of multilayer-release methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 18:11–24, 2008 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, Kollins S, Nguyen AS, DeCory HH, Hirshe Dirksen SJ, Hatch SJ: A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics 113:e206–e216, 2004 [DOI] [PubMed] [Google Scholar]
- Weiss M, Hechtman L, Turgay A, Jain U, Quinn D, Ahmed TS, Yates T, Reiz JL, Donnelly GA, Harsanyi Z, Darke AC: Once-daily multilayer-release methylphenidate in a double-blind, crossover comparison to immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 17:675–688, 2007 [DOI] [PubMed] [Google Scholar]
- Wigal SB, Gupta S, Guinta D, Swanson JM: Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull 34:47–53, 1998 [PubMed] [Google Scholar]
- Wigal SB, Wigal T, Schuck S, Brams M, Williamson D, Armstrong RB, Starr HL: Academic, behavioral, and cognitive effects of OROS® methylphenidate on older children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 21:121–131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Wigal TL: The laboratory school protocol: Its origin, use, and new applications. J Atten Disord 10:92–111, 2006 [DOI] [PubMed] [Google Scholar]
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]