Abstract
Background
Many patients with glaucoma have difficulty using antiglaucoma eye drops because of dry eye symptom. In this prospective, randomized, double-blind, placebo-controlled study, we evaluated the effect of Korean Red Ginseng on dry eye syndrome in patients with glaucoma treated with antiglaucoma eye drops.
Methods
Forty-nine participants were allocated to the Korean Red Ginseng (3 g/day; n = 24) or placebo (n = 25) groups for 8 weeks. Tear film stability, fluorescein corneal staining, conjunctival hyperemia, tear production, grade of meibomian gland dysfunction, and dry eye questionnaire (Ocular Surface Disease Index) were evaluated at baseline and on completion of the treatment.
Results
Almost all patients displayed dry eye symptoms and signs at baseline. After the 8-week intervention, Korean Red Ginseng supplementation significantly improved the tear film stability and total Ocular Surface Disease Index score, as compared to placebo (p < 0.01).
Conclusion
Korean Red Ginseng supplementation may provide an additional treatment option for dry eye and patients with glaucoma using antiglaucoma eye drops.
Keywords: dry eye syndrome, glaucoma, Korean Red Ginseng, Panax ginseng
1. Introduction
Glaucoma is a chronic and progressive optic neuropathy characterized by visual field loss and is the second leading cause of global blindness after cataract [1]. The primary goal of glaucoma treatment is to reduce intraocular pressure (IOP) using antiglaucoma eye drops, laser treatment, or surgery [2,3]. Antiglaucoma eye-drop application is the most common therapy, and can significantly lower IOP and delay glaucoma progression [4,5]. However, patients with glaucoma who use antiglaucoma eye drops have been shown to have a higher prevalence of ocular surface disease than the normal population [6,7]. Irritation and conjunctival hyperemia induced by dry eyes are among the main problems when treating patients with glaucoma who require a lifetime management [8–10]. Dry-eye therapy has been solely symptomatic, mainly by the application of artificial tears. However, numerous recent studies have demonstrated that inflammation and apoptosis may play key roles in the development of dry eye syndrome (DES) [11–16].
Ginseng (the root of Panax ginseng Meyer) is a valuable folk medicine used in East Asian countries. The two kinds of ginseng, air-dried white ginseng and steamed red ginseng, harbor a variety of active components, including ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids, and its diverse pharmacological effects have been observed in the central nervous system and the cardiovascular, endocrine, and immune systems [17–25]. Korean Red Ginseng (KRG) is known to have more pharmacological effects than raw ginseng because of the change of its chemical components (such as Rg3 and Rh2) that are produced in the steaming process [26].
Because of chronic inflammation, conjunctival pathological changes, including squamous metaplasia and goblet cell loss, have been found on cytological analysis of dry eye disease and, thus, anti-inflammatory drugs, such as topical steroid and cyclosporine A, are effective agents for DES [27,28]. In an earlier study performed by the authors [29], participants stated that the discomfort caused by antiglaucoma eye drops was relieved by KRG intake. Furthermore, the symptoms and signs of dry eyes were improved in some of these patients.
In this randomized, double-blind, placebo-controlled study, we examined the effect of KRG supplementation on DES in patients with glaucoma.
2. Materials and methods
2.1. Ethical statement
This prospective, randomized, double-blind, placebo-controlled, parallel group study was performed at the glaucoma clinic of the Severance Hospital, Seoul, Korea. The study was conducted in accordance with the Declaration of Helsinki, and informed written consent was obtained from each participant. The Institutional Review Board of the Yonsei University Health System approved the study protocol.
2.2. Participants
Participants were enrolled prospectively between July 2013 and December 2013. Inclusion criteria were an age of 20–75 years, diagnosis of glaucoma, established topical hypotensive therapy using only travoprost, and presence of subjective dry eye symptoms, including a tear film breakup time (TBUT) < 10 seconds and a Schirmer I test < 15 mm in at least one eye. One eye of each patient was selected randomly when both eyes were eligible. Glaucomatous eyes were defined by a glaucoma specialist based on a glaucomatous visual field (VF) defect confirmed by two reliable VF tests and typical appearance of a glaucomatous optic nerve head including cup-to-disc ratio > 0.7, intereye cup asymmetry > 0.2, or neuroretinal rim notching, focal thinning, disc hemorrhage, or vertical elongation of the optic cup. Exclusion criteria included a history of any ocular surgery, evidence of acute or chronic infections, an inflammatory condition of the eye, a history of intolerance or hypersensitivity to any component of the study medications, women of childbearing age, and the presence of current punctal occlusion. Patients with media opacity or other diseases affecting the VF were also excluded. All participants were provided with the same artificial tears (1 mg sodium hyaluronate) to use as required during the study period, whereas individuals who were on medications for dry eye treatment other than artificial tears were excluded.
2.3. Study design
Participants were randomized to receive one of two treatment regimens for 8 weeks. The treatments were 1 g of KRG administered as two 500-mg powder capsules or placebo administered as two identically appearing capsules, taken three times daily in both groups. KRG powder was manufactured by the Korea Ginseng Corporation (Seoul, Republic of Korea) from roots of a 6-year-old KRG, Panax ginseng, harvested in the Republic of Korea. KRG was made by steaming fresh ginseng at 90–100°C for 3 hours and then drying at 50–80°C. KRG powder prepared from grinded red ginseng, and a capsule contained 500 mg of powder. KRG was analyzed by high-performance liquid chromatography. KRG extract contained major ginsenoside-Rb1: 5.61 mg/g, -Rb2: 2.03 mg/g, -Rc: 2.20 mg/g, -Rd: 0.39 mg/g, -Re: 1.88 mg/g, -Rf: 0.89 mg/g, -Rg1: 3.06 mg/g, -Rg2s: 0.15 mg/g, -Rg3s: 0.17 mg/g, -Rg3r: 0.08 mg/g, and other minor ginsenosides. Placebo capsules were also provided by the Korea Ginseng Corporation, and they were identical in size, weight, color, and taste. The participants were instructed to avoid taking other forms of KRG or any type of ginseng for the duration of the study.
Group assignment of the participants was determined prior to the initiation of the study. Block randomization, which was generated by our institutional biostatistics department using a computer-generated random sequence, was used to randomize the participants. Study investigators, participants, and their caregivers were blinded through the provision of the medication as identically appearing capsules in boxes, with neither the investigator providing the medication nor the participants aware of the allocated treatment.
We performed objective clinical measurements of all participants, including tear film stability (TBUT), ocular surface health (fluorescein ocular surface staining), conjunctival hyperemia, tear production (Schirmer I test), and the grade of meibomian gland dysfunction (MGD), at baseline and again 8 weeks after the daily oral administration of 3 g of KRG or placebo. Subjective assessment of DES using a questionnaire was also conducted at each visit.
2.4. Outcome measures
The TBUT was identified following the procedure reported by Lemp [30]. A fluorescein strip (Haag-Streit AG, Köniz, Switzerland) was moistened with a drop of saline solution, and placed on the inferior palpebral conjunctiva. The patients were asked to blink several times to mix the fluorescein with the tear film. They were instructed to open their eyes and not blink, and the time between eye opening and the appearance of the first dry spot was measured in seconds. This procedure was repeated three times, and the mean of the three measurements was recorded finally as TBUT.
After the measurement of the TBUT, fluorescein staining on the ocular surface was evaluated using the standardized methods recommended by the National Institutes of Health Symposium on Dry Eye [30]. Briefly, corneal staining was scored 3 minutes after fluorescein instillation by observing the cornea through a cobalt blue light. It was graded using a scale of 0–3 (absent to diffuse) and recorded for the five corneal sections (central, superior, temporal, nasal, and inferior.). The maximum score for each area was 3. The scores of the five areas were summed to obtain a total score for each eye, producing a maximum score of 15.
Conjunctival hyperemia was evaluated by the investigator based on a visual inspection. A standard five-point scoring system was used with the following descriptors based on photographic standards: 0 (none) = normal, bulbar conjunctival vessels easily observed; +0.5 (trace) = trace flush, reddish-pink color; +1 (mild) = mild flush, reddish color; +2 (moderate) = bright red color; and +3 (severe) = deep, bright, diffuse redness.
The Schirmer I test was performed under anesthesia. To obtain anesthetic conditions of all the ocular structures, more than three drops of topical anesthetic (proparacaine hydrochloride ophthalmic solution 0.5%) were applied to the conjunctiva and both lid margins. Then, Schirmer strip was placed on the lower lid 2 mm lateral to the lateral canthus. Patients sat in the dark with both eyes closed for 5 minutes. After the strip was removed, a length of the wet area of the strip was measured in millimeters.
The quality and quantity of meibomian gland secretions were evaluated using manual expression. The quantity was graded using a three-point scale: 0 = normal; 1 = delay; 2 = partially blocked; and 3 = blocked. The quality was also scored similarly: 0 = clear; 1 = cloudy; 2 = granular; and 3 = opaque solid.
To evaluate subjective symptoms of dry eye, the participants were asked to complete the Ocular Surface Disease Index (OSDI) prior to taking any clinical measurements. The OSDI is a disease-specific questionnaire used to quantify the specific effect of DES on vision-related quality of life [31]. It includes three subscales: ocular discomfort (OSDI-symptom); vision-related function (OSDI-function); and environmental triggers (OSDI-trigger). The patients answered the 12 items on the OSDI questionnaire that were graded on a scale of 0–4 (0: none of the time, 1: some of the time, 2: 50% of the time, 3: most of the time, and 4: all of the time). The OSDI score was calculated from (sum of the scores for all the questions answered) × 25/(the total number of the questions answered). Scores range over 0–100 for the overall score and in each category. A score of 0–12 indicates a normal eye, 13–22 a mild dry eye, 23–32 a moderate dry eye, and > 33 a severe dry eye. It should be noted that a decrease in the OSDI score indicates an improvement.
2.5. Statistical analysis
The basic characteristics were compared between the two groups using an independent t test for continuous variables or the Chi-square test for categorical variables. The comparisons of outcome measures between the baseline and 8-week visits in each group were performed using a paired t test and the differences in the degree of change were compared between the two groups using an independent t test. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered significant.
3. Results
A total of 54 participants were included in this study and were randomly assigned to two groups prior to the study initiation, the KRG and placebo groups, of whom 49 participants (24 participants and 25 participants in the KRG and placebo groups, respectively) successfully completed the study (Fig. 1). No significant side effect related to the KRG or placebo was found. The two groups were comparable in their basic characteristics: the mean ages were 59.5 years and 62.0 years (KRG and placebo, respectively); there were slightly more women than men in both groups; and mean IOP was ∼12 mmHg in both groups (Table 1).
Fig. 1.
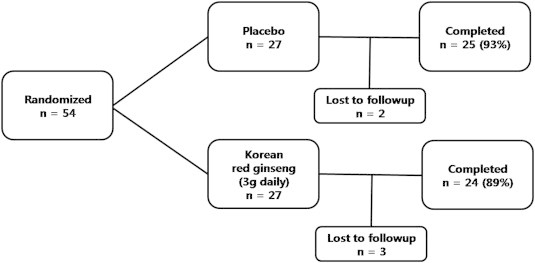
Participants' enrollment flowchart.
Table 1.
Baseline characteristics of the study participants
| KRG group | Placebo group | p1) | |
|---|---|---|---|
| No. | 24 | 25 | |
| Mean age, y ± SD | 59.5 ± 10.5 | 62.0 ± 9.4 | 0.38 |
| Female sex | 13 (54) | 16 (64) | 0.48 |
| Mean BCVA, Snellen ± SD | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.68 |
| Mean IOP, mmHg ± SD | 12.9 ± 2.8 | 12.2 ± 2.3 | 0.36 |
| Diagnosis | |||
| Open-angle glaucoma | 21 (88) | 21 (84) | 0.52 |
| Angle-closure glaucoma | 1 (4) | 3 (12) | |
| Secondary glaucoma | 2 (8) | 1 (4) | |
Data are presented as n (%), unless otherwise indicated
BCVA, best corrected visual acuity; IOP, intraocular pressure; KRG, Korean Red Ginseng; SD, standard deviation
p values were derived from an independent t test for continuous data and the Chi-square test for categorical data
3.1. Clinical assessment
Compared to the baseline, there was no statistically significant change after 8 weeks in the placebo group using a paired t test, whereas in the KRG group the mean TBUT score (range from 4.21 ± 1.53 to 6.63 ± 1.64, p < 0.01), conjunctival hyperemia (range from 1.02 ± 0.60 to 0.63 ± 0.45, p = 0.01), and MGD quantity grade (range from 1.58 ± 0.97 to 1.04 ± 0.55, p = 0.04) showed significant improvement. Of these, the change in the TBUT was significantly greater in the KRG group than in the placebo group when the difference in the degree of change between the two groups was analyzed using an independent t test (p < 0.01) (Table 2, Fig. 2).
Table 2.
Comparison of the clinical assessment for dry eye
| KRG group (n = 24) |
Placebo group (n = 25) |
p2) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8-wk visit | p1) | Baseline | 8-wk visit | p1) | ||
| Corneal staining (0–15) | 1.63 ± 1.41 | 1.40 ± 1.41 | 0.35 | 1.64 ± 1.60 | 1.92 ± 2.18 | 0.28 | 0.16 |
| TBUT (s) | 4.21 ± 1.53 | 6.63 ± 1.64 | < 0.01** | 3.84 ± 1.60 | 4.24 ± 1.33 | 0.41 | < 0.01** |
| Schirmer I test (mm) | 5.00 ± 4.45 | 5.46 ± 4.46 | 0.35 | 7.56 ± 4.24 | 6.88 ± 6.65 | 0.53 | 0.34 |
| Conjunctival hyperemia (0–3) | 1.02 ± 0.60 | 0.63 ± 0.45 | 0.01* | 0.84 ± 0.50 | 0.76 ± 0.58 | 0.43 | 0.06 |
| MGD, quality (0–3) | 1.38 ± 0.65 | 1.17 ± 0.56 | 0.14 | 1.56 ± 0.51 | 1.48 ± 0.59 | 0.57 | 0.51 |
| MGD, quantity (0–3) | 1.58 ± 0.97 | 1.04 ± 0.55 | 0.04* | 1.80 ± 0.87 | 1.64 ± 0.70 | 0.33 | 0.20 |
Data are expressed as mean ± standard deviation, unless otherwise indicated
*p < 0.05
**p < 0.01
KRG, Korean Red Ginseng; MGD, meibomian gland dysfunction; TBUT, tear breakup time
p values were derived from a paired t test between the baseline and 8-wk visits in each group
p values were derived from an independent t test between changes in the KRG and placebo groups
Fig. 2.
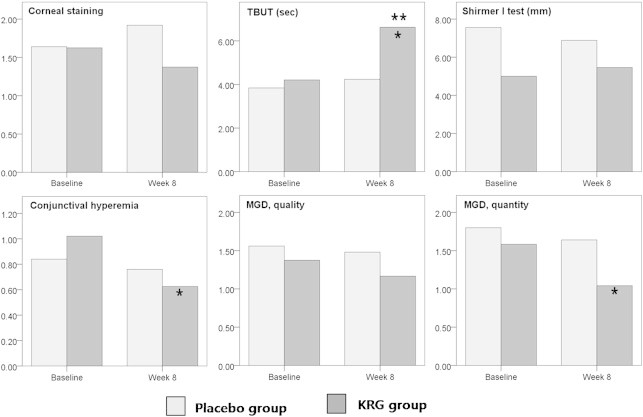
Changes in the clinical assessment for dry eye before and after the intervention. *p < 0.05 by a paired t test in the KRG and placebo groups. **p < 0.05 by an independent t test between changes in the KRG and placebo groups. KRG, Korean Red Ginseng; MGD, meibomian gland dysfunction; TBUT, tear breakup time.
3.2. Subjective symptoms
Table 3 presents the results of the OSDI scores at the baseline and 8-week visits. The mean baseline total OSDI score was 36.22 ± 17.90 and 36.56 ± 19.58 in the KRG and placebo groups, respectively. Virtually all the participants had abnormal OSDI scores. After the 8-week intervention, the total OSDI score in the KRG group was significantly improved from 36.22 ± 17.90 to 27.77 ± 21.68 (p = 0.01), and it differed significantly from the placebo group (p = 0.04). In the KRG group, the OSDI-symptom subtotal improved the most, from 35.42 ± 16.42 to 23.40 ± 18.65 (p < 0.01), which was thought to affect the greater part of the total OSDI score improvement. Compared to the baseline, six of the 12 items were significantly improved in the KRG group after the 8-week supplementation: three items (painful eye, blurred vision, and poor vision) of the OSDI-symptom; two items of OSDI-function (driving at night and working with a computer); and one item (feeling uncomfortable in air-conditioned areas). In addition, five of these items, except blurred vision, displayed significant differences between the KRG and placebo groups.
Table 3.
Changes in subjective dry eye symptoms using the OSDI score
| KRG group (n = 24) |
Placebo group (n = 25) |
p2) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8-wk visit | p1) | Baseline | 8-wk visit | p1) | ||
| Light-sensitive eyes (0–4) | 1.27 ± 1.20 | 0.95 ± 1.40 | 0.19 | 1.26 ± 1.21 | 1.09 ± 0.90 | 0.76 | 0.40 |
| Gritty eyes (0–4) | 1.23 ± 0.81 | 1.00 ± 0.74 | 0.21 | 1.14 ± 0.99 | 1.21 ± 0.98 | 0.06 | 0.04* |
| Painful or sore eyes (0–4) | 1.90 ± 1.07 | 1.00 ± 0.69 | < 0.01** | 1.74 ± 1.56 | 1.29 ± 1.46 | 0.33 | < 0.01** |
| Blurred vision (0–4) | 1.05 ± 1.00 | 0.52 ± 0.99 | 0.01* | 1.55 ± 1.43 | 1.73 ± 1.58 | 0.84 | 0.12 |
| Poor vision (0–4) | 1.55 ± 0.74 | 0.82 ± 0.85 | < 0.01** | 1.22 ± 1.17 | 1.67 ± 1.58 | 0.05 | < 0.01** |
| OSDI symptom subtotal (0–100) | 35.42 ± 16.42 | 23.40 ± 18.65 | < 0.01** | 32.80 ± 19.36 | 34.70 ± 22.13 | 0.43 | < 0.01** |
| Difficulty in | |||||||
| Reading (0–4) | 1.68 ± 1.17 | 1.46 ± 1.10 | 0.16 | 1.56 ± 1.36 | 1.54 ± 1.53 | 0.65 | 0.24 |
| Driving at night (0–4) | 1.94 ± 1.34 | 1.06 ± 1.06 | 0.01* | 1.69 ± 1.80 | 1.69 ± 1.70 | 0.34 | 0.05 |
| Working with a computer or a bank machine (0–4) | 1.50 ± 1.15 | 0.95 ± 0.94 | 0.01* | 1.56 ± 1.50 | 1.67 ± 1.32 | 0.46 | 0.03* |
| Watching TV (0–4) | 1.32 ± 1.32 | 1.04 ± 1.16 | 0.48 | 1.39 ± 1.53 | 1.00 ± 1.27 | 0.44 | 0.83 |
| OSDI vision-related function subtotal (0–100) | 34.55 ± 30.06 | 30.73 ± 25.97 | 0.28 | 35.17 ± 27.91 | 36.17 ± 32.10 | 0.80 | 0.36 |
| Feel uncomfortable in | |||||||
| Windy conditions (0–4) | 1.09 ± 0.97 | 0.86 ± 0.83 | 0.55 | 1.76 ± 1.54 | 1.25 ± 1.22 | 0.14 | 0.48 |
| Places with low humidity (0–4) | 1.41 ± 1.01 | 1.21 ± 1.06 | 0.43 | 1.60 ± 1.22 | 1.26 ± 1.29 | 0.01* | 0.43 |
| Air-conditioned areas (0–4) | 1.54 ± 1.22 | 1.32 ± 1.04 | 0.04* | 1.26 ± 1.25 | 1.45 ± 1.28 | 0.33 | 0.03* |
| OSDI trigger subtotal (0–100) | 34.72 ± 22.74 | 30.90 ± 23.11 | 0.39 | 38.67 ± 30.96 | 34.33 ± 27.75 | 0.13 | 0.92 |
| Total OSDI (0–100) | 36.22 ± 17.90 | 27.77 ± 21.68 | 0.01* | 36.56 ± 19.58 | 35.89 ± 22.05 | 0.67 | 0.04* |
Data are expressed as mean ± standard deviation
*p < 0.05
**p < 0.01
KRG = Korean Red Ginseng; OSDI = ocular surface disease index
p values were derived from a paired t-test between the baseline and 8-week visits in each group
p values were derived from an independent t-test between changes in the KRG and placebo groups
4. Discussion
Patients with full-blown glaucoma suffer from the disease itself. However, most patients, particularly those in the early to moderate stages of glaucoma, complain more about their dry eye symptoms caused by topical glaucoma medication until the disease progressed. Many earlier studies reported that patients with glaucoma suffer a higher prevalence of ocular surface disease than the normal population [7–10]. Leung et al [10] found that 59% of patients with primary open-angle glaucoma (OAG) and ocular hypertension (OHT) reported dry eye symptoms, whereas severe symptoms were noted by 27% of these patients. The authors concluded that a large proportion of the patients with OAG or OHT had signs and/or symptoms of dry eye, and that the presence of dry eye and the use of benzalkonium chloride (BAK)-containing medications may affect quality of life. Our study similarly demonstrated that dry eye is prevalent in patients treated for glaucoma by showing that almost all the participants had OSDI scores consistent with the presence of dry eye symptoms.
The cause of DES in patients with glaucoma is thought to be multifactorial and may include an active ingredient and a preservative, most commonly BAK [9,32]. Several previous studies reported that BAK may cause inflammation and potentially other ocular diseases, including allergy, blepharitis, DES, and anatomical eyelid abnormalities [33,34]. The prolonged use of preserved topical drugs is an extrinsic cause of increased tear evaporation, which induces a toxic response from the ocular surface. BAK has a well-known dose-dependent toxicity and is most commonly used as a preservative in ophthalmic solutions, particularly in antiglaucoma eye drops [33,35]. Its cellular toxicity has been demonstrated experimentally in in vitro studies of conjunctiva-derived and corneal cells [36,37]. BAK induces the expression of inflammatory cell markers at the ocular surface [38] and causes epithelial cell damage, apoptotic cell death, and a decrease in goblet cell density, resulting in tear film instability and tear hyperosmolarity [39,40].
In recent years, the immune system has been thought to play an increasingly important role in the pathogenesis of dry eye. Studies have demonstrated an infiltration of the conjunctival epithelia with inflammatory cells, particularly lymphocytes [41–43]. Furthermore, changes in the expression of immune system stimulation markers, including the intracellular adhesion molecule I antigen and the human leukocyte antigen D receptor (HLA-DR), which induce T-cell homing and antigen presentation, were observed in the context of dry eye [44]. Several studies reported alterations in the protein expression profiles of cytokines in the tears of patients with DES. This suggests that dry eye is the result of inflammatory reactions, which are caused by cytokines, resulting in an autoimmune response [45]. Moreover, recent studies have shown the positive effect of oral omega-3 and -6 essential fatty acid supplementation in DES with an inflammatory component [46–48]. Reduced dry eye symptoms were reported as well as an improvement in objective signs, including corneal staining and decreased conjunctival HLA-DR expression. Oral omega-6 supplementation also increased tear production and reduce dry eye symptoms after photorefractive keratectomy [49].
Ginsenosides, unique saponins contained in the Panax species, are believed to be responsible for most of the pharmacological actions of ginseng, which include anti-inflammatory, -stress, and -oxidant activities [50–53]. Many studies have reported the anti-inflammatory effects of ginseng extracts and ginsenosides on cellular responses triggered by various inducers, including endotoxin, tumor necrosis factor-α, and interferon-γ [54–56]. Ginseng extracts and ginsenosides, including Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, and Rg2 have been reported to have anti-inflammatory properties in different forms of inflammation [57]. Ginsenosides inhibit various inducer-activated signaling protein kinases and nuclear factor kappa-light-chain-enhancer of activated B cells transcription factor, resulting in decreased production of cytokines and inflammation mediators [58,59]. Based on these studies, we hypothesized that the anti-inflammatory property of KRG may have a positive effect on the ocular surface. This KRG anti-inflammatory effect improved tear film instability, and consequently the TBUT was increased. Additionally, there were significant improvements in conjunctival hyperemia and MGD quantity after KRG supplementation, although these were not significantly different from the placebo group. These results strongly support our hypothesis regarding the anti-inflammatory effects of KRG on dry eye. This hypothesis should be confirmed by additional in vitro and in vivo studies.
In the current study, we also found an improvement in subjective dry eye symptoms determined using the OSDI questionnaire in the KRG group, as compared to the placebo group. Because the TBUT was significantly improved after 8 weeks in the KRG group, we can presume that this finding may be associated with the improvement in the total OSDI score, including the subtotal OSDI-symptom score. The improvement in tear film stability was thought to play an important role in making the patients feel more comfortable. This is consistent with previous studies, which reported that the TBUT is related to the dry eye symptoms [60,61].
This study has several limitations. First, its limited duration did not allow us to predict how long the effects of KRG administration would persist. The duration of the effect and optimal administration schedule for KRG treatment requires further investigation in patients with glaucoma. Second, because this study was performed only with Korean participants, we could not exclude any possible ethnic-related differences. Third, we did not evaluate the systemic effects of KRG, although no adverse events were noted during the study period. Checking vital signs, including systemic blood pressure, or performing blood tests to evaluate the inflammatory state would have enhanced our study. Despite these limitations, this is the first placebo-controlled study reporting the effect of KRG supplementation on the ocular surface and dry eye symptoms.
In conclusion, our results indicated that daily supplementation of 3 g of KRG for 8 weeks significantly improved the TBUT score and subjective dry eye symptoms, as compared to placebo. This improvement in dry eye was presumed to be induced by the anti-inflammatory property of KRG. Although further studies are required to identify a detailed mechanism, the use of KRG as a nutritional supplement is expected to be a clinically valuable additional option for dry eye and patients with glaucoma using antiglaucoma eye drops.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Acknowledgments
The authors are grateful to Hye Sun Lee (Department of Research Affairs, Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea) for her help with the statistics. This work was supported by the 2010 grant from the Korean Society of Ginseng funded, Seoul, Korea.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Resnikoff S., Pascolini D., Etya'ale D., Kocur I., Pararajasegaram R., Pokharel G.P., Mariotti S.P. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Pizzarello L., Abiose A., Ffytche T., Duerksen R., Thulasiraj R., Taylor H., Faal H., Rao G., Kocur I., Resnikoff S. VISION 2020: the Right to Sight: a global initiative to eliminate avoidable blindness. Arch Ophthal. 2004;122:615–620. doi: 10.1001/archopht.122.4.615. [DOI] [PubMed] [Google Scholar]
- 3.Gordon M.O., Beiser J.A., Brandt J.D., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.L., Miller J.P., Parrish R.K., 2nd, Wilson M.R. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthal. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 4.Higginbotham E.J., Schuman J.S., Goldberg I., Gross R.L., VanDenburgh A.M., Chen K., Whitcup S.M. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthal. 2002;120:1286–1293. doi: 10.1001/archopht.120.10.1286. [DOI] [PubMed] [Google Scholar]
- 5.Camras C.B. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology. 1996;103:138–147. doi: 10.1016/s0161-6420(96)30749-5. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Feijoo J., Sampaolesi J.R. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–446. doi: 10.2147/OPTH.S29158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fechtner R.D., Godfrey D.G., Budenz D., Stewart J.A., Stewart W.C., Jasek M.C. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 8.Rossi G.C., Tinelli C., Pasinetti G.M., Milano G., Bianchi P.E. Dry eye syndrome-related quality of life in glaucoma patients. Eur J Ophthalmol. 2009;19:572–579. doi: 10.1177/112067210901900409. [DOI] [PubMed] [Google Scholar]
- 9.Stewart W.C., Stewart J.A., Nelson L.A. Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res. 2011;36:391–398. doi: 10.3109/02713683.2011.562340. [DOI] [PubMed] [Google Scholar]
- 10.Leung E.W., Medeiros F.A., Weinreb R.N. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan S., Miller W.L., McDermott A.M. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest Ophthalmol Vis Sci. 2006;47:2445–2450. doi: 10.1167/iovs.05-1364. [DOI] [PubMed] [Google Scholar]
- 12.Pflugfelder S.C., Jones D., Ji Z., Afonso A., Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 13.Massingale M.L., Li X., Vallabhajosyula M., Chen D., Wei Y., Asbell P.A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 14.Yoon K.C., Jeong I.Y., Park Y.G., Yang S.Y. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–437. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 15.Lam H., Bleiden L., de Paiva C.S., Farley W., Stern M.E., Pflugfelder S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pflugfelder S.C. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Jeon B.H., Kim C.S., Kim H.S., Park J.B., Nam K.Y., Chang S.J. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]
- 18.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 19.Sung J., Han K.H., Zo J.H., Park H.J., Kim C.H., Oh B.H. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28:205–216. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- 20.Bae E.A., Hyun Y.J., Choo M.K., Oh J.K., Ryu J.H., Kim D.H. Protective effect of fermented red ginseng on a transient focal ischemic rats. Arch Pharm Res. 2004;27:1136–1140. doi: 10.1007/BF02975119. [DOI] [PubMed] [Google Scholar]
- 21.Kwak Y.S., Kyung J.S., Kim J.S., Cho J.Y., Rhee M.H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 22.Hwang S.Y., Son D.J., Kim I.W., Kim D.M., Sohn S.H., Lee J.J., Kim S.K. Korean red ginseng attenuates hypercholesterolemia-enhanced platelet aggregation through suppression of diacylglycerol liberation in high-cholesterol-diet-fed rabbits. Phytother Res. 2008;22:778–783. doi: 10.1002/ptr.2363. [DOI] [PubMed] [Google Scholar]
- 23.Oh K.J., Chae M.J., Lee H.S., Hong H.D., Park K. Effects of Korean red ginseng on sexual arousal in menopausal women: placebo-controlled, double-blind crossover clinical study. J Sex Med. 2010;7:1469–1477. doi: 10.1111/j.1743-6109.2009.01700.x. [DOI] [PubMed] [Google Scholar]
- 24.Heo J.H., Lee S.T., Chu K., Oh M.J., Park H.J., Shim J.Y., Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur J Neurol. 2008;15:865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 25.Babayigit A., Olmez D., Karaman O., Bagriyanik H.A., Yilmaz O., Kivcak B., Erbil G., Uzuner N. Ginseng ameliorates chronic histopathologic changes in a murine model of asthma. Allergy Asthma Proc. 2008;29:493–498. doi: 10.2500/aap.2008.29.3137. [DOI] [PubMed] [Google Scholar]
- 26.Baek N.I., Kim D.S., Lee Y.H., Park J.D., Lee C.B., Kim S.I. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 27.Kunert K.S., Tisdale A.S., Stern M.E., Smith J.A., Gipson I.K. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthal. 2000;118:1489–1496. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 28.Sall K., Stevenson O.D., Mundorf T.K., Reis B.L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 29.Kim N.R., Kim J.H., Kim C.Y. Effect of Korean red ginseng supplementation on ocular blood flow in patients with glaucoma. J Ginseng Res. 2010;34:237–245. [Google Scholar]
- 30.Lemp M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 31.Schiffman R.M., Christianson M.D., Jacobsen G., Hirsch J.D., Reis B.L. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthal. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 32.Jaenen N., Baudouin C., Pouliquen P., Manni G., Fiqueiredo A., Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Opthalmol. 2007;17:341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 33.Baudouin C., Labbe A., Liang H., Pauly A., Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Noecker R.J., Herrygers L.A., Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–496. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 35.Guenoun J.M., Baudouin C., Rat P., Pauly A., Warnet J.M., Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:2444–2450. doi: 10.1167/iovs.04-1331. [DOI] [PubMed] [Google Scholar]
- 36.Debbasch C., Brignole F., Pisella P.J., Warnet J.M., Rat P., Baudouin C. Quaternary ammoniums and other preservatives' contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001;42:642–652. [PubMed] [Google Scholar]
- 37.Okada Y. Effects of topical antiglaucoma medications on corneal epithelium as evaluated by gene expression patterns. Cornea. 2007;26:S46–S54. doi: 10.1097/ICO.0b013e31812f6a71. [DOI] [PubMed] [Google Scholar]
- 38.Baudouin C., Pisella P.J., Fillacier K., Goldschild M., Becquet F., De Saint Jean M., Bechetoille A. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106:556–563. doi: 10.1016/S0161-6420(99)90116-1. [DOI] [PubMed] [Google Scholar]
- 39.Pisella P.J., Debbasch C., Hamard P., Creuzot-Garcher C., Rat P., Brignole F., Baudouin C. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45:1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 40.Xiong C., Chen D., Liu J., Liu B., Li N., Zhou Y., Liang X., Ma P., Ye C., Ge J. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 2008;49:1850–1856. doi: 10.1167/iovs.07-0720. [DOI] [PubMed] [Google Scholar]
- 41.Rivas L., Oroza M.A., Perez-Esteban A., Murube-del-Castillo J. Morphological changes in ocular surface in dry eyes and other disorders by impression cytology. Graefes Arch Clin Exp Ophthalmol. 1992;230:329–334. doi: 10.1007/BF00165940. [DOI] [PubMed] [Google Scholar]
- 42.Hikichi T., Yoshida A., Tsubota K. Lymphocytic infiltration of the conjunctiva and the salivary gland in Sjogren's syndrome. Arch Ophthal. 1993;111:21–22. doi: 10.1001/archopht.1993.01090010023009. [DOI] [PubMed] [Google Scholar]
- 43.Stern M.E., Gao J., Schwalb T.A., Ngo M., Tieu D.D., Chan C.C., Reis B.L., Whitcup S.M., Thompson D., Smith J.A. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 44.Tsubota K., Fujihara T., Saito K., Takeuchi T. Conjunctival epithelium expression of HLA-DR in dry eye patients. Ophthalmologica. 1999;213:16–19. doi: 10.1159/000027387. [DOI] [PubMed] [Google Scholar]
- 45.Grus F.H., Dick B., Augustin A.J., Pfeiffer N. Analysis of the antibody repertoire in tears of dry-eye patients. Ophthalmologica. 2001;215:430–434. doi: 10.1159/000050903. [DOI] [PubMed] [Google Scholar]
- 46.Rand A.L., Asbell P.A. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22:279–282. doi: 10.1097/ICU.0b013e3283477d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barabino S., Rolando M., Camicione P., Ravera G., Zanardi S., Giuffrida S., Calabria G. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Aragona P., Bucolo C., Spinella R., Giuffrida S., Ferreri G. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjogren's syndrome patients. Invest Ophthalmol Vis Sci. 2005;46:4474–4479. doi: 10.1167/iovs.04-1394. [DOI] [PubMed] [Google Scholar]
- 49.Macri A., Giuffrida S., Amico V., Iester M., Traverso C.E. Effect of linoleic acid and gamma-linolenic acid on tear production, tear clearance and on the ocular surface after photorefractive keratectomy. Graefes Arch Clin Exp Ophthalmol. 2003;241:561–566. doi: 10.1007/s00417-003-0685-x. [DOI] [PubMed] [Google Scholar]
- 50.Park B.G., Jung H.J., Cho Y.W., Lim H.W., Lim C.J. Potentiation of antioxidative and anti-inflammatory properties of cultured wild ginseng root extract through probiotic fermentation. J Pharm Pharmacol. 2013;65:457–464. doi: 10.1111/jphp.12004. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Wahhab M.A., Ahmed H.H. Protective effects of Korean Panax ginseng against chromium VI toxicity and free radical generation in rat. J Ginseng Res. 2004;28:11–17. [Google Scholar]
- 52.Lee S.H., Jung B.H., Kim S.Y., Lee E.H., Chung B.C. The antistress effect of ginseng total saponin and ginsenoside Rg3 and Rb1 evaluated by brain polyamine level under immobilization stress. Pharmacol Res. 2006;54:46–49. doi: 10.1016/j.phrs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Cho W.C., Chung W.S., Lee S.K., Leung A.W., Cheng C.H., Yue K.K. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173–179. doi: 10.1016/j.ejphar.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 54.Song S.B., Tung N.H., Quang T.H., Nqan N.T., Kim K.E., Kim Y.H. Inhibition of TNF-alpha-mediated NF-kappaB Transcriptional Activity in HepG2 Cells by Dammarane-type Saponins from Panax ginseng Leaves. J Ginseng Res. 2012;36:146–152. doi: 10.5142/jgr.2012.36.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ly S., Yi P.F., Shen H.Q., Zhang L.Y., Dong H.B., Wu S.C., Xia F., Guo X., Wei X.B., Fu B.D. Ginsenoside Rh2-B1 stimulates cell proliferation and IFN-gamma production by activating the p38 MAPK and ERK-dependent signaling pathways in CTLL-2 cells. Immunopharmacol Immunotoxicol. 2014;36:43–51. doi: 10.3109/08923973.2013.864669. [DOI] [PubMed] [Google Scholar]
- 56.Lee I.A., Hyam S.R., Jang S.E., Han M.J., Kim D.H. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem. 2012;60:9595–9602. doi: 10.1021/jf301372g. [DOI] [PubMed] [Google Scholar]
- 57.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 58.Kim W.K., Song S.Y., Oh W.K., Kaewsuwan S., Tran T.L., Kim W.S., Sung J.H. Wound-healing effect of ginsenoside Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. Eur J Pharmacol. 2013;702:285–293. doi: 10.1016/j.ejphar.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 59.Zang Y.X., Wang L., Xiao E.L., Li S.J., Chen J.J., Gao B., Min G.N., Wang Z.P., Wu Y.J. Ginsenoside-Rd exhibits anti-inflammatory activities through elevation of antioxidant enzyme activities and inhibition of JNK and ERK activation in vivo. Int Immunopharmacol. 2013;17:1094–1100. doi: 10.1016/j.intimp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Her Y., Lim J.W., Han S.H. Dry eye and tear film functions in patients with psoriasis. Jpn J Ophthalmol. 2013;57:341–346. doi: 10.1007/s10384-012-0226-4. [DOI] [PubMed] [Google Scholar]
- 61.Shimazaki-Den S., Dogru M., Higa K., Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013;32:1211–1218. doi: 10.1097/ICO.0b013e318295a2a5. [DOI] [PubMed] [Google Scholar]


