Abstract
Abnormal changes in skin color induce significant cosmetic problems and affect quality of life. There are two groups of abnormal change in skin color; hyperpigmentation and hypopigmentation. Hyperpigmentation, darkening skin color by excessive pigmentation, is a major concern for Asian people with yellow–brown skin. A variety of hypopigmenting agents have been used, but treating the hyperpigmented condition is still challenging and the results are often discouraging. Panax ginseng has been used traditionally in eastern Asia to treat various diseases, due to its immunomodulatory, neuroprotective, antioxidative, and antitumor activities. Recently, several reports have shown that extract, powder, or some constituents of ginseng could inhibit melanogenesis in vivo or in vitro. The underlying mechanisms of antimelanogenic properties in ginseng or its components include the direct inhibition of key enzymes of melanogenesis, inhibition of transcription factors or signaling pathways involved in melanogenesis, decreasing production of inducers of melanogenesis, and enhancing production of antimelanogenic factor. Although there still remain some controversial issues surrounding the antimelanogenic activity of ginseng, especially in its effect on production of proinflammatory cytokines and nitric oxide, these recent findings suggest that ginseng and its constituents might be potential candidates for novel skin whitening agents.
Keywords: ginsenoside, melanogenesis, Panax ginseng, skin whitening
1. Introduction
Panax ginseng (ginseng) has been used traditionally in eastern Asia over thousands of years. It has been used orally to treat various diseases including hypertension, diabetes mellitus, liver and kidney dysfunction, mental disorders, and postmenopausal disorders. In addition, topical applications have also been used to heal wounds and reduce skin inflammation [1]. In the past few decades, it has been proved that ginseng extracts actually show a wide range of effects against human diseases. Their potential therapeutic effects have been mainly attributed to its immunomodulatory [2], [3], neuroprotective [4], [5], antioxidative [6], antitumor [7], and hepatoprotective activities [8].
Ginseng contains a number of active ingredients including ginsenosides, polysaccharides, phytosterols, peptides, polyacetylenes, fatty acids, and polyacetylenic alcohols, which have different effects on carbohydrate and lipid metabolism, cognition, angiogenesis, and the neuroendocrine, immune, cardiovascular, and central nervous systems [9], [10]. Among the active constituents of ginseng, ginsenosides are known to be the major biologically active components of ginseng and the most widely studied. Several studies have shown that ginsenosides play important roles in the pharmacological effects of ginseng [11]. So far, over 30 different ginsenosides have been isolated and identified from ginseng [11]. However, ginseng contains other constituents, including ginsenoyne, phenolic compounds, polyacetylenes, sesquiterpenes, methoxypyrazine, alkylpyrazine derivatives, sesquiterpene alcohols, panasinsanols, and β-carboline [12], [13]. The biological functions of these compounds are being investigated by a number of researchers.
There are two traditional preparations of ginseng; white ginseng and red ginseng. White ginseng is peeled, dried, ginseng root and red ginseng is produced by steaming fresh ginseng root at 98–100°C for 2–3 h, and then drying until the moisture content is <15% [14]. Red and white ginseng have both been shown to have immunomodulatory [15], [16], anti-inflammatory [17], antioxidant [18], and antiatopic activities [19]. Moreover, red ginseng has been reported to have more potent pharmacological activities than white ginseng in some respects [20], [21], [22]. The differences in biological activities between red and white ginseng are caused by the chemical changes of ginsenosides after the steaming process [23]. Steaming partially converts the original ginsenosides to deglycoslated derivatives [24]. As a result, the species and amounts of ginsenosides are quite different based on the processing method used. Chu et al [25] showed that a total of 53 and 43 compounds were tentatively identified in white ginseng and red ginseng samples, respectively. The featured compounds are mainly malonyl ginsenosides in white ginseng, and decarboxyl products of mal-ginsenosides and the dehydrated compounds from polar ginsenosides were characteristic in red ginseng [25].
It is interesting that ginsenosides show a wide variety of biological activities, although the absorption rates from orally administered intact ginsenosides are very low. In the human intestinal tract, ginsenosides are metabolized by intestinal bacteria and the metabolites are absorbed [26], [27]. Thus, the pharmacological actions of these ginsenosides have been closely related to their biotransformation by human intestinal bacteria [28]. In this context, fermentation strategies have been used to improve oral absorption and bioavailability. Several studies showed that the transformation of ginsenosides into deglycosylated ginsenosides is needed to increase ginseng's effectiveness in vivo [27].
Abnormal changes in skin color induce significant cosmetic problems with a negative effect on quality of life. There are two groups of pigmentary disorders: disorders of the quantitative and qualitative distribution of normal pigment and the abnormal presence of exogenous or endogenous pigments in the skin. The first group includes hyperpigmentation and hypopigmentation (leukodermia). Hyperpigmentation is darkening of the skin color due to excessive pigmentation. Usually, hyperpigmentation issues are major concerns for people of color [29]. Hyperpigmentation-related diseases include melasma, lentigines, nevus, ephelis, freckles, postinflammatory hyperpigmentation, and age spots [30]. Postinflammatory hyperpigmentation appears in many skin conditions, including acne, eczema, and contact dermatitis. Meanwhile, hypopigmentation is lightening of the skin by insufficient pigmentation [31]. Skin color is determined by various factors including melanin content, oxygenation state of hemoglobin in capillary vessels, carotenoid content, water content, and organization of collagen fibers in the dermis. Among these factors, melanin is the major determinant of skin color [32]. In this context, understanding the mechanisms involved in melanogenesis is of great interest pharmaceutically and cosmetically.
Melanogenesis is a biochemical pathway responsible for melanin synthesis that is controlled by complex regulatory mechanisms [33]. Melanogenesis occurs in melanocytes confined in separate cytoplasmic organelles called melanosomes, which contain key enzymes of melanogenesis. Differences in skin color are related to the size, number, shape, and distribution of melanosomes, whereas melanocyte density typically remains relatively constant [34]. Although tyrosinase is the key regulatory enzyme of melanogenesis, tyrosinase-related protein (TRP)-1, dopachrome tautomerase (DCT/TRP2), and melanosomal matrix proteins (Pmel17, MART-1) carry out important roles in regulating melanogenesis [35]. The genes of tyrosinase, TRP-1, and DCT contain common transcription starting sites, the microphthalmia-associated transcription factor (MITF) binding sites. MITF plays a critical role in the transcriptional regulation of melanogenesis [36]. The intracellular signal transduction pathways of protein kinase C, cyclic AMP (cAMP), and nitrogen oxide are involved in the regulation of melanogenesis [34]. Various endogenous and exogenous factors, such as estrogen and ultraviolet (UV) radiation, affect melanogenesis via signal transduction pathways. These endogenous/exogenous factors exert their actions directly on melanocytes or indirectly via surrounding skin cells [36].
Melanocytes, keratinocytes, dermal fibroblasts, and other skin cells communicate with each other by factors that are secreted and cell–cell contacts [37]. It has been shown that the interactions between keratinocytes and melanocytes are critical in the regulation of melanogenesis [38]. Keratinocytes control melanocyte growth and activity through various soluble factors and cell adhesion molecules [39], [40]. In addition, dermal factors have been found to be involved in the regulation of melanogenesis [41].
At the same time, stimulated melanocytes secret a number of signal molecules targeting not only keratinocytes but also skin immune cells [42], [43]. Soluble factors released by melanocytes include proinflammatory cytokines and chemokines such as interleukin (IL)-1α/1β, IL-6, IL-8 IL-10, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, catecholamines, eicosanoids, serotonin, α-melanocyte stimulating factor (α-MSH), and nitric oxide (NO) [42], [43].
A variety of hypopigmenting agents including hydroquinone, arbutin, tretinoin, kojic acid, azelaic acid, vitamin C, N-acetylglucosamine, niacinamide, linoleic acid, ellagic acid, methimazole, dioic acid, soy extract, licorice extract, rucinol, and glycolic acid have been used alone or in combination to treat abnormal hyperpigmentation [29], [31]. These agents can interfere with the pigmentation process at several different steps of skin pigmentation. However, the treatment of hyperpigmented conditions still remains challenging and the results are often discouraging. Thus there is a need for novel skin-whitening agents that are highly effective and tolerable.
In this article, we review recent reports investigating the skin-whitening effect of ginseng and its components and the underlying mechanisms of action, and then discuss their potential as candidates for novel skin-whitening agents.
2. Effects of ginseng and its components on melanogenesis
P. ginseng is one of the most widely used medicinal plants in traditional oriental medicine. Over thousands of years, it has been used to improve the overall condition of skin, as well as to treat a wide variety of diseases. However, genuine scientific approaches to clarify the efficacy of ginseng in skin have only been made in recent years. Several reports have shown that ginseng extract, powder, or some other constituents could inhibit melanogenesis in vitro and in vivo. Table 1 summarizes the direct effects of ginseng and its components on skin color and key enzymes involved in melanogenesis. Song et al reported that red ginseng powder improved melasma in a human clinical trial [44]. They orally administered Korean Red Ginseng powder for 24 weeks to female patients with melasma. After 24 weeks, the melasma area and severity index score decreased and melasma quality of life scale showed improvement in 91% of patients. The mean level of pigmentation and erythema levels also decreased. In addition, 74% of the patients showed some improvement on the patient- and investigator-rated global improvement scales [44].
Table 1.
Direct effects of ginseng and its components on melanogenesis in vivo and in vitro
| Reagent | Experimental model | Dose | Effects on melanogenesis | Ref. |
|---|---|---|---|---|
| Powder of KRG | Melasma lesion of female patients | 3 g/d oral 24 wk |
MASI: decreased MELASQoL: improved Patient- and investigator-rated global improvement scale: improved |
[44] |
| Ethanol extract of ginseng seed | Melan-a cells cultured with phorbol-12 myristate 13-acetate | 100 ppm 3 d |
Melanin content: decreased tyrosinase activity: decreased |
[45] |
| Extract of KRG and FKRG | Mushroom tyrosinase | Tyrosinase activity: decreased | [58] | |
| Aglycone of ginsenoside Rh4 | B16 cells stimulated by α-MSH | 20–50μM 5 d |
Melanin content: decreased Tyrosinase activity: decreased MITF expression: decreased |
[49] |
| p-Coumaric acid | Mushroom tyrosinase | Tyrosinase activity: decreased | [46] | |
| Ginsenoside Rb1 | B16 cells stimulated by α-MSH | 125–500μM 2 d |
Melanin content: decreased Tyrosinase activity: decreased |
[51] |
| Ginsenoside F1 | Human skin artificially tanned by UV irradiation | 0.1% cream topical 8 wk |
Luminosity values: increased | [75] |
α-MSH, α-melanocyte stimulating factor; FKRG, fermented Korean Red Ginseng; KRG, Korean Red Ginseng; MASI, melasma area and severity index; MELASQoL, melasma quality of life scale; MITF, micropthalmia-associated transcription factor
Most of reports investigating the antimelanogenic effect of ginseng were conducted in vitro used purified tyrosinase or melanocyte cell lines. In melan-a cells treated with ethanol extract of ginseng seeds, melanin content and tyrosinase activity was reduced [45]. In addition to the crude extract or powder, several studies tested the effects of specific constituents of ginseng. The phenol compounds inhibited tyrosinase activity while ginsenoside prevents UVB-induced intracellular increase of reactive oxygen species [46], [47], [48]. In some reports, ginsenosides alone exerted antimelanogenic effects. Aglycone of ginsenoside Rh4 inhibited melanin synthesis in B16 melanoma cells, possibly by involvement of protein kinase A pathway [49]. Ginsenoside Rh4 is one of the components isolated from Korean Red Ginseng [50]. It significantly reduced melanin content and tyrosinase activity in α-MSH and forskolin-stimulated B16 melanoma cells. It reduced the cAMP level and cAMP response-element binding protein level in B16 melanoma cells, and this might be responsible for the downregulation of MITF and tyrosinase [49]. In addition, ginsenoside Rb1 inhibited melanogenesis through the inhibition of tyrosinase activity in α-MSH-stimulated B16 cells in a dose-dependent manner [51].
The antimelanogenic effect of ginseng does not arise from ginsenosides only. The crude methanol extract of the fresh leaves of P. ginseng showed inhibitory activity on mushroom tyrosinase, and p-coumaric acid was characterized as the principal tyrosinase inhibitor in the extract [47]. p-Coumaric acid inhibited melanogenesis in B16F10 melanoma cells stimulated by α-MSH, and was suggested to interfere with melanogenesis by its structural similarity with tyrosine [52]. Interestingly, p-coumaric acid showed weaker inhibition against mushroom tyrosinase but more strongly inhibited human or murine tyrosinase in comparison with kojic acid and arbutin [53]. Enzyme kinetics analysis indicate that p-coumaric acid is a mixed type (for tyrosine) or competitive inhibitor (for DOPA) of human tyrosinase. In addition, p-coumaric acid potently inhibits melanogenesis in human epidermal melanocytes exposed to UVB [53]. Cinnamic acid, one of the major components of Cinnamomum cassia Blume, is found in the root and seed of P. ginseng [54], [55]. Cinnamic acid is reported to have inhibitory activity on mushroom tyrosinase [56], [57]. Cinnamic acid significantly reduced melanin production, tyrosinase activity, and tyrosinase expression in the melan-a cells [56]. In addition, cinnamic acid showed depigmenting activity on the UVB-tanned skin of brown guinea pigs [57].
It is already known that the pharmacological actions of these ginsenosides have been closely related to their biotransformation by human intestinal bacteria [28]. Although the contents of total ginsenosides in red ginseng and fermented red ginseng using Lactobacillus brevis were not significantly different, the ginsenoside metabolite content was higher in fermented red ginseng compared to red ginseng [58]. The tyrosinase inhibitory activity of fermented red ginseng extract was more potent compared with red ginseng extract in a test using mushroom tyrosinase [58].
3. Effects of ginseng and its components on factors related to melanogenesis
As reviewed above, crude extract or some components of ginseng and its components showed antimelanogenic activities by direct inhibition on key enzymes of melanogenesis, such as tyrosinase. Moreover, ginseng and its components could exert antimelanogenic activity via action on the several factors related in melanocyte physiology.
Among a large number of soluble factors produced from melanocytes, keratinocytes, fibroblasts, and immune cells in skin, adrenocorticotropic hormone (ACTH), α-MSH, endothelin-1, prostaglandin E2, prostaglandin F2α, NO, and histamine are well-known stimulators of melanogenesis [37], [59], [60], [61], [62], [63]. By contrast, the effects of cytokines on melanogenesis are more complicated. IL-1α/1β and granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulate melanogenesis, while IL-6, TGF-β1, and TNF-α downregulate melanin production [36], [64].
GM-CSF is produced and released from UV-irradiated keratinocytes [65]. GM-CSF has been reported to be involved in regulating the proliferation and differentiation of epidermal melanocytes [39], [66]. Treatment of melan-a cells with conditioned media from UV-irradiated SP-1 keratinocytes increased melanocyte proliferation, and the proliferative effect of the conditioned media was blocked by anti-GM-CSF antibody treatment [66]. When UV-irradiated SP-1 keratinocytes were treated with red ginseng extract or saponin of red ginseng, the increased melanocyte proliferation by the conditioned media was blocked [67]. In that report, red ginseng extract or saponin of red ginseng treatment decreased the expression of GM-CSF induced by UV-B irradiation in SP-1 keratinocytes [67]. As mentioned above, inflammatory cytokines such as IL-1 and TNF-α take part in the regulation of melanogenesis. Ginseng extracts and ginsenosides have been reported to have anti-inflammatory activities in several different studies. Ginsenosides inhibit different inducer-activated signaling protein kinases and transcription factor nuclear factor (NF)-κB, and then decrease the production of proinflammatory cytokines and mediators of inflammation [68]. Korean Red Ginseng extracts decreased TNF-α and IL-8 production in lipopolysaccharide (LPS)-stimulated HaCaT keratinocytes and show radical scavenging and antioxidant activity in human dermal fibroblasts [69]. These findings suggest that ginseng extracts and ginsenosides might affect melanogenesis through their anti-inflammatory activities.
The effect of ginseng on NO production is still questionable. Several reports showed that ginseng reduces NO production [70], [71], [72]. Sun Ginseng, a new processed ginseng prepared by steaming at high temperature, reduced UV-B-induced cell damage and decreased NO production by inhibition of inducible NO synthase mRNA synthesis in HaCaT keratinocytes and human dermal fibroblasts [70]. Red ginseng marc oil inhibited inducible NO synthase and cyclooxygenase-2 via NF-κB and p38 pathways in LPS-stimulated RAW264.7 cells [71]. In addition, ginsenjilinol, a protopanaxatriol-type saponin obtained from the roots of P. ginseng, shows inhibitory activity on NO production in LPS-stimulated RAW264.7 cells [72]. By contrast, there are some controversial reports that ginseng extract enhanced NO production or NO signaling [73], [74]. Hong et al reported that ginseng extract administration stimulated nongenomic endothelial NO synthase activation and enhanced NO production in spontaneously hypertensive rats [73]. In another report, water extract of Korean Red Ginseng exerted vasoprotective effects through augmentation of NO production by inhibiting arginase [74]. Therefore, the effect of ginseng on melanogenesis via NO signaling remains to be clarified by further study.
Human skin tissue does not consist only of melanocytes, keratinocytes, and fibroblasts. Considerable numbers of immune cells including Langerhans cells, macrophages, mast cells, and T cells are working actively in skin tissue. Because the immunostimulatory activities of many ginsenosides are known, it is not surprising that ginsenosides could enhance the reactivity of skin immune cells. In a recent paper, a cream containing 0.1% ginsenoside F1 (a metabolite of ginsenoside Rg1) showed a significant whitening effect on artificially tanned human skin [75]. However, ginsenoside F1 did not directly inhibit mRNA expression of tyrosinase or DCT in normal human epidermal melanocytes. Instead, ginsenoside F1 enhanced production of IL-13 from human epidermal γδ T cells, and IL-13 significantly reduced the mRNA expression and protein amount of both tyrosinase and DCT resulting in visible brightening of normal human epidermal melanocyte pellets [75]. These results suggest that ginsenosides might be able to regulate melanogenesis via their effect on skin immune cells.
4. Conclusion
Recently, several reports have shown that extract, powder, or some constituents of ginseng could inhibit melanogenesis in vivo or in vitro. The underlying mechanisms of the antimelanogenic effect of ginseng or its components included the direct inhibition of key enzymes of melanogenesis (tyrosinase and DCT), inhibition of transcription factors (MITF, NF-κB) or signaling pathways (protein kinase A pathway and protein kinase C pathway) involved in melanogenesis, decreasing the production of inducers of melanogenesis (cAMP, GM-CSF), and enhancing production of antimelanogenic factor (IL-13). Fig. 1 summarizes the effects of ginseng and its components on melanogenesis. Although issues surrounding the antimelanogenic activity of ginseng still remain controversial, especially in its effect on the production of proinflammatory cytokines and NO, these recent findings suggest that ginseng and its constituents might be potential candidates for novel skin-whitening agents.
Fig. 1.
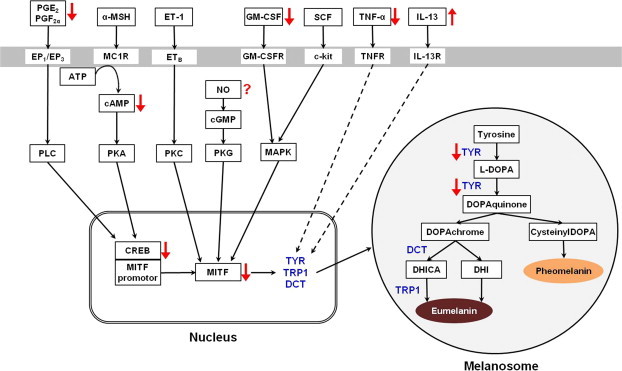
Schematic view of the effects of ginseng on melanogenesis. Black solid arrow indicates activation, black dashed arrow indicates inhibition, red upward arrow indicates increase by ginseng components, and red downward arrow indicates decrease by ginseng components. α-MSH, α-melanocyte stimulating factor; CREB, cAMP response element-binding protein; DCT, dopachrome tautomerase; DHI, 5,6-dihydroxyindole; DHICA, 5,6-dihydroxyindole-2-carboxylic acid; DOPA, 3,4-dihydroxyphenylalanine; EP1, prostaglandin E receptor 1; ET-1, endothelin-1; ETB , endothelin receptor type B; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-13, interleukin 13; MAPK, mitogen-activated protein kinases; MC1R, melanocortin 1 receptor; MITF, micropthalmia-associated transcription factor; NO, nitric oxide; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; PKA, protein kinase A; PKG, protein kinase G; PLC, phospholipase C; SCF, stem cell factor; TNF-α, tumor necrosis factor-α; TRP1, tyrosinase-related protein-1; TYR, tyrosinase.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This work was supported by the research fund of Dankook University in 2014.
References
- 1.Kimura Y., Sumiyoshi M., Sakanaka M. Effects of ginsenoside Rb1 on skin changes. J Biomed Biotechnol. 2012;2012:946242. doi: 10.1155/2012/946242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du X.F., Jiang C.Z., Wu C.F., Won E.K., Choung S.Y. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res. 2008;31:1153–1159. doi: 10.1007/s12272-001-1282-6. [DOI] [PubMed] [Google Scholar]
- 3.Shin J.Y., Song J.Y., Yun Y.S., Yang H.O., Rhee D.K., Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 4.Radad K., Gille G., Liu L., Rausch W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 5.Naval M.V., Gómez-Serranillos M.P., Carretero M.E., Villar A.M. Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. J Ethnopharmacol. 2007;112:262–270. doi: 10.1016/j.jep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Kang K.S., Yokozawa T., Kim H.Y., Park J.H. Study on the nitric oxide scavenging effects of ginseng and its compounds. J Agric Food Chem. 2006;54:2558–2562. doi: 10.1021/jf0529520. [DOI] [PubMed] [Google Scholar]
- 7.Xu L.L., Han T., Wu J.Z., Zhang Q.Y., Zhang H., Huang B.K., Rahman K., Qin L.P. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine. 2009;16:609–616. doi: 10.1016/j.phymed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa M., Morikawa T., Kashima Y., Ninomiya K., Matsuda H. Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J Nat Prod. 2003;66:922–927. doi: 10.1021/np030015l. [DOI] [PubMed] [Google Scholar]
- 9.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 10.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 11.Yue P.Y., Mak N.K., Cheng Y.K., Leung K.W., Ng T.B., Fan D.T., Yeung H.W., Wong R.N. Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin Med. 2007;2:6. doi: 10.1186/1749-8546-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun T.K. Update from Asia. Asian studies on cancer chemoprevention. Ann NY Acad Sci. 1999;889:157–192. doi: 10.1111/j.1749-6632.1999.tb08734.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee M., Sorn S., Baek S., Jang S., Kim S. Antioxidant and apoptotic effects of Korean white ginseng extracted with the same ratio of protopanaxadiol and protopanaxatriol saponins in human hepatoma HepG2 cells. Ann NY Acad Sci. 2009;1171:217–227. doi: 10.1111/j.1749-6632.2009.04918.x. [DOI] [PubMed] [Google Scholar]
- 14.Gui Y., Ryu G.H. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder) J Ginseng Res. 2014;38:146–153. doi: 10.1016/j.jgr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang S.K., Kim J.H., Chung Y.S., Ahn D.C., Kang M.J., Lee D.G., Kim S.H. An experimental study on the effect of immunopotential and the anticancer effect of red ginseng extract. Korean J Ginseng Sci. 1994;18:151–159. [Google Scholar]
- 16.Wang Y., Wang B.X., Liu T.H., Minami M., Nagata T., Ikejima T. Metabolism of ginsenoside Rg1 by intestinal bacteria. II. Immunological activity of ginsenoside Rg1 and Rh1. Acta Pharmacol Sin. 2000;21:792–796. [PubMed] [Google Scholar]
- 17.Choi J.H., Oh D.H. Effect of white and red ginseng extract on the immunological activities in lymphocytes isolated from sasang constitution blood cells. J Ginseng Res. 2009;33:33–39. [Google Scholar]
- 18.Kim E.H., Rhee D.K. Anti-oxidative properties of ginseng. J Ginseng Res. 2009;33:1–7. [Google Scholar]
- 19.Cho E., Cho S.H. Effects of Korean red ginseng extract on the prevention of atopic dermatitis and its mechanism on early lesions in a murine model. J Ethnopharmacol. 2013;145:294–302. doi: 10.1016/j.jep.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Sohn S.H., Kim S.K., Kim Y.O., Kim H.D., Shin Y.S., Yang S.O., Kim S.Y., Lee S.W. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res. 2013;37:442–450. doi: 10.5142/jgr.2013.37.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 22.Takaku T., Kameda K., Matsuura Y., Sekiya K., Okuda H. Studies on insulin-like substances in Korean red ginseng. Planta Med. 1990;56:27–30. doi: 10.1055/s-2006-960877. [DOI] [PubMed] [Google Scholar]
- 23.Zheng P.H., Li C.Y., Pang S.F., Guan Y.M., Shao C., Xu S.Q., Liu J.Y., Hou W., Hou W., Lei X.J. Effects of steaming on chemical composition of Panax ginseng hairy roots and Panax ginseng. J Med Plants Res. 2012;6:2812–2815. [Google Scholar]
- 24.Yun T.K., Yun Y.S., Han I.W. Anticarcinogenic effect of long-term oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detect Prev. 1983;6:515–525. [PubMed] [Google Scholar]
- 25.Chu C., Xu S., Li X., Yan J., Liu L. Profiling the ginsenosides of three ginseng products by LC-Q-TOF/MS. J Food Sci. 2013;78:C653–C659. doi: 10.1111/1750-3841.12102. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 27.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 28.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20 (S)-and 20 (R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 29.Lee A.Y., Noh M. The regulation of epidermal melanogenesis via cAMP and/or PKC signaling pathways: insights for the development of hypopigmenting agents. Arch Pharm Res. 2013;36:792–801. doi: 10.1007/s12272-013-0130-6. [DOI] [PubMed] [Google Scholar]
- 30.Costin G.E., Hearing V.J. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 31.Woolery-Lloyd H., Kammer J.N. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171–175. doi: 10.1016/j.sder.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Ortonne J.P. Normal and abnormal skin color. Ann Dermatol Venereol. 2012;139:S125–S129. doi: 10.1016/S0151-9638(12)70123-0. [DOI] [PubMed] [Google Scholar]
- 33.Simon D.J., Peles D., Wakamatsu K., Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology and function. Pigment Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin J.Y., Fisher D.E. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 35.Hirobe T. How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Res. 2011;24:462–478. doi: 10.1111/j.1755-148X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 36.Cichorek M., Wachulska M., Stasiewicz A., Tymińska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol. 2013;30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizoguchi M. Melanocyte development: with a message of encouragement to young women scientists. Pigment Cell Res. 2004;17:533–544. doi: 10.1111/j.1600-0749.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 38.Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment Cell Res. 2004;18:2–12. doi: 10.1111/j.1600-0749.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 39.Haass N.K., Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Invest Dermatol Symp Proc. 2005;10:153–163. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee A.Y. Role of keratinocytes in the development of vitiligo. Ann Dermatol. 2012;24:115–125. doi: 10.5021/ad.2012.24.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirobe T., Hasegawa K., Furuya R., Fujiwara R., Sato K. Effects of fibroblast-derived factors on the proliferation and differentiation of human melanocytes in culture. J Dermatol Sci. 2013;71:45–57. doi: 10.1016/j.jdermsci.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y., Zhu W.Y., Tan C., Yu G.H., Gu J.X. Melanocytes are potential immunocompetent cells: evidence from recognition of immunological characteristics of cultured human melanocytes. Pigment Cell Res. 2002;15:454–460. doi: 10.1034/j.1600-0749.2002.02065.x. [DOI] [PubMed] [Google Scholar]
- 43.Tam I., Stępień K. Melanocytes—immunocompetent pigment cells. Postep Derm Alergol. 2007;4:188–193. [Google Scholar]
- 44.Song M., Mun J.H., Ko H.C., Kim B.S., Kim M.B. Korean Red Ginseng powder in the treatment of melasma: an uncontrolled observational study. J Ginseng Res. 2011;35:170–175. doi: 10.5142/jgr.2011.35.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y., Kim K.T., Kim S.S., Hur J., Ha S.K., Cho C.W., Choi S.Y. Inhibitory effects of ginseng seed on melanin biosynthesis. Pharmacogn Mag. 2014;10(Suppl. 2):S272–S275. doi: 10.4103/0973-1296.133271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim J.Y., Ishiguro K., Kubo I. Tyrosinase inhibitory p-coumaric acid from ginseng leaves. Phytother Res. 1999;13:371–375. doi: 10.1002/(sici)1099-1573(199908/09)13:5<371::aid-ptr453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 47.Lee J.H., Lee B.S., Yang M.S., Byun B.S., Kim W.G., Kim B.H., Lee S.J. Prevention of photoaging and wrinkle formation in hairless mice dorsal skin by AP3-03. Korean J Food Sci Technol. 2005;37:989–996. [Google Scholar]
- 48.Hwang E.Y., Kong Y.H., Lee Y.C., Kim Y.C., Yoo K.M., Jo Y.O., Choi S.Y. Comparison of phenolic compounds contents between white and red ginseng and their inhibitory effect on melanin biosynthesis. J Ginseng Res. 2006;30:82–87. [Google Scholar]
- 49.Jeong Y.M., Oh W.K., Tran T.L., Kim W.K., Sung S.H., Bae K., Lee S., Sung J.H. Aglycone of Rh4 inhibits melanin synthesis in B16 melanoma cells: possible involvement of the protein kinase A pathway. Biosci Biotechnol Biochem. 2013;77:119–125. doi: 10.1271/bbb.120602. [DOI] [PubMed] [Google Scholar]
- 50.Baek N.I., Kim D.S., Lee Y.H., Park J.D., Lee C.B., Kim S.I. Ginsenoside Rh4, a genuine dammarane glycoside from Korean Red Ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 51.Wang L., Lu A.P., Yu Z.L., Wong R.N., Bian Z.X., Kwok H.H., Yue P.Y., Zhou L.M., Chen H., Xu M. The melanogenesis-inhibitory effect and the percutaneous formulation of ginsenoside Rb1. AAPS Pharm Sci Tech. 2014;15:1252–1262. doi: 10.1208/s12249-014-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An S.M., Lee S.I., Choi S.W., Moon S.W., Boo Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by alpha-melanocyte stimulating hormone. Br J Dermatol. 2008;159:292–299. doi: 10.1111/j.1365-2133.2008.08653.x. [DOI] [PubMed] [Google Scholar]
- 53.An S.M., Koh J.S., Boo Y.C. p-Coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother Res. 2010;24:1175–1180. doi: 10.1002/ptr.3095. [DOI] [PubMed] [Google Scholar]
- 54.Kong Y.H., Jo Y.O., Cho C.W., Son D., Park S., Rho J., Choi S.Y. Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol Pharm Bull. 2008;31:946–948. doi: 10.1248/bpb.31.946. [DOI] [PubMed] [Google Scholar]
- 55.Lee M.H., Kim S.S., Cho C.W., Choi S.Y., In G., Kim K.T. Quality and characteristics of ginseng seed oil treated using different extraction methods. J Ginseng Res. 2013;37:468–474. doi: 10.5142/jgr.2013.37.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H.S. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem. 2002;50:1400–1403. doi: 10.1021/jf011230f. [DOI] [PubMed] [Google Scholar]
- 57.Masamoto Y., Murata Y., Baba K., Shimoishi Y. Inhibitory effects of esculetin on melanin biosynthesis. Biol Pharm Bull. 2004;27:422–425. doi: 10.1248/bpb.27.422. [DOI] [PubMed] [Google Scholar]
- 58.Lee H.S., Kim M.R., Park Y., Park H.J., Chang U.J., Kim S.Y., Suh H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J Med Food. 2012;15:1015–1023. doi: 10.1089/jmf.2012.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillbro J.M., Olsson M.J. The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. Int J Cosmet Sci. 2011;33:210–221. doi: 10.1111/j.1468-2494.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang P., Liu W., Yuan X., Li D., Gu W., Gao T. Endothelin-1 enhances the melanogenesis via MITF-GPNMB pathway. BMB Rep. 2013;46:364–369. doi: 10.5483/BMBRep.2013.46.7.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wakabayashi Y., Nakajima H., Imokawa G. Abrogating effect of N-linked carbohydrate modifiers on the stem cell factor and endothelin-1-stimulated epidermal pigmentation in human epidermal equivalents. J Dermatol Sci. 2013;69:215–228. doi: 10.1016/j.jdermsci.2012.11.590. [DOI] [PubMed] [Google Scholar]
- 62.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 63.Roméro-Graillet C., Aberdam E., Clément M., Ortonne J.P., Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99:635–642. doi: 10.1172/JCI119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez-Esparza M., Solano F., García-Borrón J.C. Independent regulation of tyrosinase by the hypopigmenting cytokines TGF beta1 and TNF alpha and the melanogenic hormone alpha-MSH in B16 mouse melanocytes. Cell Mol Biol. 1999;45:991–1000. [PubMed] [Google Scholar]
- 65.Imokawa G., Yada Y., Kimura M., Morisaki N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. Biochem J. 1996;313:625–631. doi: 10.1042/bj3130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirobe T., Furuya R., Hara E., Horii I., Tsunenaga M., Ifuku O. Granulocyte-macrophage colony-stimulating factor (GM-CSF) controls the proliferation and differentiation of mouse epidermal melanocytes from pigmented spots induced by ultraviolet radiation B. Pigment Cell Res. 2004;17:230–240. doi: 10.1111/j.1600-0749.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- 67.Oh C.T., Park J.I., Jung Y.R., Joo Y.A., Shin D.H., Cho H.J., Ahn S.M., Lim Y.H., Park C.K., Hwang J.S. Inhibitory effect of Korean Red Ginseng on melanocyte proliferation and its possible implication in GM-CSF mediated signaling. J Ginseng Res. 2013;37:389–400. doi: 10.5142/jgr.2013.37.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee D.C., Lau A.S. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16:2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean Red Ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee H., Lee J.Y., Song K.C., Kim J., Park J.H., Chun K.H., Hwang G.S. Protective effect of processed Panax ginseng, Sun Ginseng on UVB-irradiated human skin keratinocyte and human dermal fibroblast. J Ginseng Res. 2012;36:68–77. doi: 10.5142/jgr.2012.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H.P., Yang X.B., Yang X.W., Liu J.X., Xu W., Zhang Y.B., Zhang L.X., Wang Y.P. Ginsenjilinol, a new protopanaxatriol-type saponin with inhibitory activity on LPS-activated NO production in macrophage RAW 264.7 cells from the roots and rhizomes of Panax ginseng. J Asian Nat Prod Res. 2013;15:579–587. doi: 10.1080/10286020.2013.787992. [DOI] [PubMed] [Google Scholar]
- 73.Hong S.Y., Kim J.Y., Ahn H.Y., Shin J.H., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J Agric Food Chem. 2012;60:3086–3091. doi: 10.1021/jf204447y. [DOI] [PubMed] [Google Scholar]
- 74.Shin W., Yoon J., Oh G.T., Ryoo S. Korean red ginseng inhibits arginase and contributes to endothelium dependent vasorelaxation through endothelial nitric oxide synthase coupling. J Ginseng Res. 2013;37:64–73. doi: 10.5142/jgr.2013.37.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han J., Lee E., Kim E., Yeom M., Kwon O.S., Yoon T.H., Lee T.R., Kim K. Role of epidermal γδ T-cell-derived interleukin 13 in the skin-whitening effect of ginsenoside F1. Exp Dermatol. 2014;23:860–862. doi: 10.1111/exd.12531. [DOI] [PubMed] [Google Scholar]


